Abstract
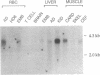
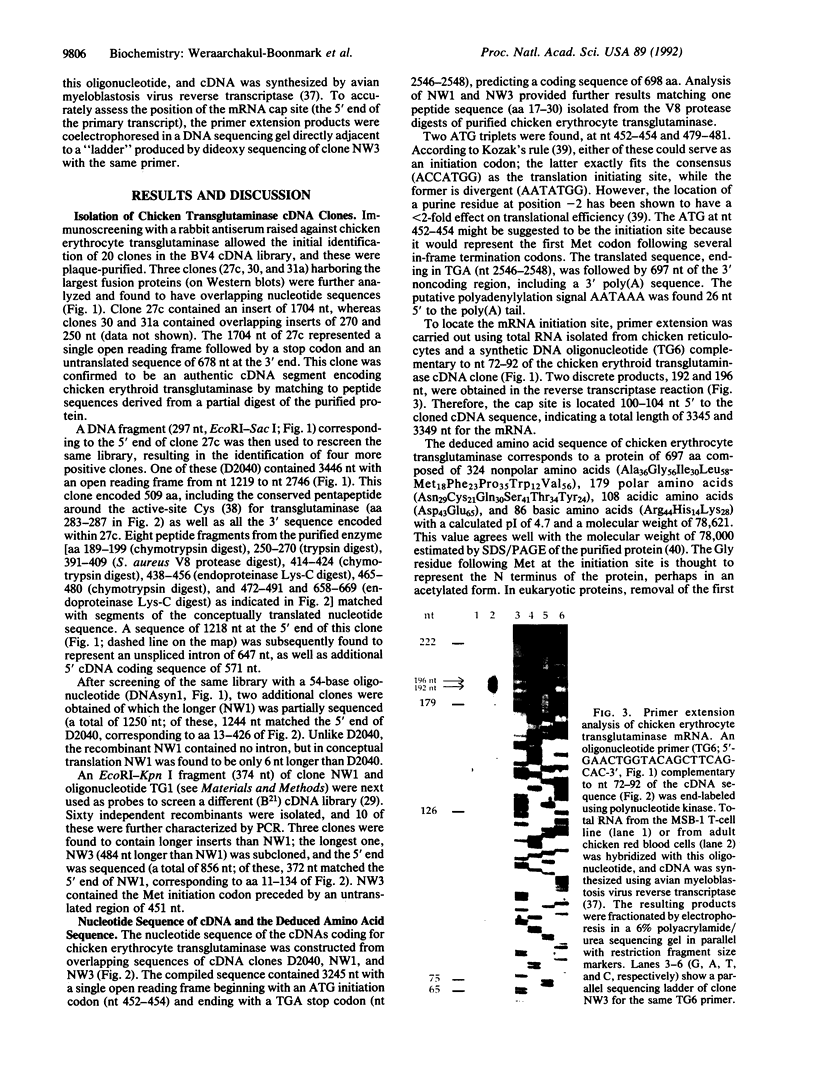
We report the sequences of cDNAs encoding chicken erythrocyte transglutaminase (EC 2.3.2.13). The complete mRNA consists of 3345/3349 nucleotides and predicts a single open reading frame. Nine peptide sequences derived from partial digests of the isolated protein agreed with the corresponding translation of the open reading frame. Approximately 60% identities between the avian protein and three related mammalian enzymes were found. Chicken erythrocyte transglutaminase mRNA is most abundant in red blood cells and kidney, and it accumulates during erythroid cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Boissel J. P., Kasper T. J., Shah S. C., Malone J. I., Bunn H. F. Amino-terminal processing of proteins: hemoglobin South Florida, a variant with retention of initiator methionine and N alpha-acetylation. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8448–8452. doi: 10.1073/pnas.82.24.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. C., Wold F. Human erythrocyte transglutaminase. Purification and properties. Biochim Biophys Acta. 1978 Jan 12;522(1):74–83. doi: 10.1016/0005-2744(78)90323-6. [DOI] [PubMed] [Google Scholar]
- CLARKE D. D., NEIDLE A., SARKAR N. K., WAELSCH H. Metabolic activity of protein amide groups. Arch Biochem Biophys. 1957 Sep;71(1):277–279. doi: 10.1016/0003-9861(57)90030-9. [DOI] [PubMed] [Google Scholar]
- Cariello L., Velasco P. T., Wilson J., Parameswaran K. N., Karush F., Lorand L. Probing the transglutaminase-mediated, posttranslational modification of proteins during development. Biochemistry. 1990 May 29;29(21):5103–5108. doi: 10.1021/bi00473a015. [DOI] [PubMed] [Google Scholar]
- Cariello L., Wilson J., Lorand L. Activation of transglutaminase during embryonic development. Biochemistry. 1984 Dec 18;23(26):6843–6850. doi: 10.1021/bi00321a087. [DOI] [PubMed] [Google Scholar]
- Chakravarty R., Rice R. H. Acylation of keratinocyte transglutaminase by palmitic and myristic acids in the membrane Anchorage region. J Biol Chem. 1989 Jan 5;264(1):625–629. [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dadabay C. Y., Pike L. J. Rapid increases in the transglutaminase activity of A431 cells following treatment with epidermal growth factor. Biochemistry. 1987 Oct 20;26(21):6587–6591. doi: 10.1021/bi00395a004. [DOI] [PubMed] [Google Scholar]
- Fesus L., Thomazy V., Autuori F., Ceru M. P., Tarcsa E., Piacentini M. Apoptotic hepatocytes become insoluble in detergents and chaotropic agents as a result of transglutaminase action. FEBS Lett. 1989 Mar 13;245(1-2):150–154. doi: 10.1016/0014-5793(89)80210-8. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Identification of a functional cysteine essential for the activity of guinea pig liver transglutaminase. J Biol Chem. 1966 Jul 10;241(13):3238–3240. [PubMed] [Google Scholar]
- Gentile V., Saydak M., Chiocca E. A., Akande O., Birckbichler P. J., Lee K. N., Stein J. P., Davies P. J. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991 Jan 5;266(1):478–483. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hsu K. H., Friedman H. Dimethyl sulfoxide-induced transglutaminase activity in murine-derived Friend erythroleukemia cells. J Natl Cancer Inst. 1983 May;70(5):965–969. [PubMed] [Google Scholar]
- Ichinose A., Davie E. W. Characterization of the gene for the a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5829–5833. doi: 10.1073/pnas.85.16.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Kim H. R., Yew N. S., Ansorge W., Voss H., Schwager C., Vennström B., Zenke M., Engel J. D. Two different mRNAs are transcribed from a single genomic locus encoding the chicken erythrocyte anion transport proteins (band 3). Mol Cell Biol. 1988 Oct;8(10):4416–4424. doi: 10.1128/mcb.8.10.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgren C., Lawler J., Lambert S., Speicher D., Cohen C. M. Complete amino acid sequence and homologies of human erythrocyte membrane protein band 4.2. Proc Natl Acad Sci U S A. 1990 Jan;87(2):613–617. doi: 10.1073/pnas.87.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- LORAND L., DOOLITTLE R. F., KONISHI K., RIGGS S. K. A NEW CLASS OF BLOOD COAGULATION INHIBITORS. Arch Biochem Biophys. 1963 Aug;102:171–179. doi: 10.1016/0003-9861(63)90168-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand J. B., Urayama T., Lorand L. Transglutaminase as a blood clotting enzyme. Biochem Biophys Res Commun. 1966 Jun 21;23(6):828–834. doi: 10.1016/0006-291x(66)90562-6. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Dailey J. E., Turner P. M. Fibronectin as a carrier for the transglutaminase from human erythrocytes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1057–1059. doi: 10.1073/pnas.85.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Hsu L. K., Siefring G. E., Jr, Rafferty N. S. Lens transglutaminase and cataract formation. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1356–1360. doi: 10.1073/pnas.78.3.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Michalska M., Murthy S. N., Shohet S. B., Wilson J. Cross-linked polymers in the red cell membranes of a patient with Hb-Koln disease. Biochem Biophys Res Commun. 1987 Sep 15;147(2):602–607. doi: 10.1016/0006-291x(87)90973-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mayr E. Evolution. Sci Am. 1978 Sep;239(3):46–55. doi: 10.1038/scientificamerican0978-46. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Young S., Chirivella J., Hawke D., Miyada C. G. Immunoglobulin variable-region-like domains of diverse sequence within the major histocompatibility complex of the chicken. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4377–4381. doi: 10.1073/pnas.88.10.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Stewart B. E., Qin Q., Chakravarty R., Floyd E. E., Jetten A. M., Rice R. H. Primary structure of keratinocyte transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9333–9337. doi: 10.1073/pnas.87.23.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Green H. Relation of protein synthesis and transglutaminase activity to formation of the cross-linked envelope during terminal differentiation of the cultured human epidermal keratinocyte. J Cell Biol. 1978 Mar;76(3):705–711. doi: 10.1083/jcb.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle R. D., Yamamoto M., Engel J. D. Expression of delta-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):792–796. doi: 10.1073/pnas.86.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARKAR N. K., CLARKE D. D., WAELSCH H. An enzymically catalyzed incorporation of amines into proteins. Biochim Biophys Acta. 1957 Aug;25(2):451–452. doi: 10.1016/0006-3002(57)90512-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Siefring G. E., Jr, Apostol A. B., Velasco P. T., Lorand L. Enzymatic basis for the Ca2+-induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry. 1978 Jun 27;17(13):2598–2604. doi: 10.1021/bi00606a022. [DOI] [PubMed] [Google Scholar]
- Stevens P. W., Dodgson J. B., Engel J. D. Structure and expression of the chicken ferritin H-subunit gene. Mol Cell Biol. 1987 May;7(5):1751–1758. doi: 10.1128/mcb.7.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L. A., Chien S., Chang L. S., Lambert K., Bliss S. A., Bouhassira E. E., Nagel R. L., Schwartz R. S., Rybicki A. C. Molecular cloning of human protein 4.2: a major component of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1990 Feb;87(3):955–959. doi: 10.1073/pnas.87.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8019–8023. doi: 10.1073/pnas.83.21.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Notides A. C., Pabalan S. S., Lorand L. Transamidase reactions involved in the enzymic coagulation of semen: isolation of -glutamyl- -lysine dipeptide from clotted secretion protein of guinea pig seminal vesicle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2322–2325. doi: 10.1073/pnas.69.8.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]