Abstract
Aim:
Major depressive disorder (MDD) is a debilitating mental disorder associated with dysfunction of the neurotransmitter-neuroendocrine system and neuroinflammatory responses. Salvianolic acid B (SalB) has shown a variety of pharmacological activities, including anti-inflammatory, antioxidant and neuroprotective effects. In this study, we examined whether SalB produced antidepressant-like actions in a chronic mild stress (CMS) mouse model, and explored the mechanisms underlying the antidepressant-like actions of SalB.
Methods:
Mice were subjected to a CMS paradigm for 6 weeks. In the last 3 weeks the mice were daily administered SalB (20 mg·kg−1·d−1, ip) or a positive control drug imipramine (20 mg·kg−1·d−1, ip). The depressant-like behaviors were evaluated using the sucrose preference test, the forced swimming test (FST), and the tail suspension test (TST). The gene expression of cytokines in the hippocampus and cortex was analyzed with RT-PCR. Plasma corticosterone (CORT) and cerebral cytokines levels were assayed with an ELISA kit. Neural apoptosis and microglial activation in brain tissues were detected using immunofluorescence staining.
Results:
Administration of SalB or imipramine reversed the reduced sucrose preference ratio of CMS-treated mice, and significantly decreased their immobility time in the FST and TST. Administration of SalB significantly decreased the expression of pro-inflammatory cytokines IL-1β and TNF-α, and markedly increased the expression of anti-inflammatory cytokines IL-10 and TGF-β in the hippocampus and cortex of CMS-treated mice, and normalized their elevated plasma CORT levels, whereas administration of imipramine did not significantly affect the imbalance between pro- and anti-inflammatory cytokines in the hippocampus and cortex of CMS-treated mice. Finally, administration of SalB significantly decreased CMS-induced apoptosis and microglia activation in the hippocampus and cortex, whereas administration of imipramine had no significant effect on CMS-induced apoptosis and microglia activation in the hippocampus and cortex.
Conclusion:
SalB exerts potent antidepressant-like effects in CMS-induced mouse model of depression, which is associated with the inhibiting microglia-related apoptosis in the hippocampus and the cortex.
Keywords: major depressive disorder, chronic mild stress, antidepressant, salvianolic acid B, imipramine, cytokines, apoptosis, microglia activation
Introduction
Major depressive disorder (MDD) is a debilitating mental disorder that is characterized by depressed mood, or anhedonia, in combination with psychomotor retardation and fatigue. MDD is a common and serious health problem, with a high morbidity and mortality1, which affects approximately 350 million individuals worldwide2. Over the past several decades, the monoamine hypothesis has grown to be the most extensively accepted etiology of depression3. Several drugs that are based on monoamine neurotransmitters have been used to reduce depressive symptoms4. However, meta-analyses suggest that these agents are effective for only one-third to one-half of patients who suffer from depression5. In addition to having a 3 to 6 weeks delay before the patient experiences the antidepressant effect, current antidepressant drugs often produce side effects that limit their usefulness6. Therefore, the development of new antidepressants is urgently needed for the treatment of depression.
Accumulating evidence suggests that neuroinflammation is accompanied by the activation of microglial cells and the release of pro-inflammatory cytokines, which is associated with the pathophysiology of MDD7. Treatment with IL-2 or IFN-α in patients with cancer was found to result in high depression rates8. Furthermore, depression is considered a high comorbidity with immune activation-related diseases9. Human postmortem and animal studies have revealed hippocampal atrophy in patients with depression10. Inflammatory cytokines activate cellular cascades that cause excitotoxicity, thereby resulting in apoptosis in the hippocampus and cortex, which is associated with depressive-like behavior11. Conversely, down-regulation of chronic inflammatory responses can subserve neuroprotective and anti-inflammatory functions12. The antidepressant activity of anti-inflammatory therapy has also been observed in patients with autoimmune and inflammatory disorders13. Therefore, inhibition of neuroinflammation may be a potential therapeutic target for MDD14.
Recently, research has confirmed the effectiveness of herbal medicine therapies on depression15. A growing number of studies have demonstrated that herbs and their components act as promising candidates for depression therapy in rodent16. Salvianolic acid B (SalB) is a major bioactive compound that is extracted from the traditional Chinese medicinal herb Salvia miltiorrhiza, which has a variety of pharmacological actions, including anti-inflammatory, antioxidant and neuroprotective effects in vivo and in vitro17,18. There is also evidence suggesting that SalB is capable of maintaining self-renewal and proliferation of neural stem/progenitor cells, thereby protecting neurons from apoptosis and alleviating the learning and memory impairment that is induced by transient cerebral ischemia19,20. In addition, SalB can traverse the blood-brain barrier21 and act directly on the central nervous system.
The present study sought to investigate the antidepressant-like effects of SalB in the chronic mild stress (CMS) mouse model, which exhibited a series of behavioral, neurochemical, neuroendocrine, and neuroimmune alterations that are similar to some of the dysfunctions observed in patients with depression22. The antidepressant-like effects of SalB were evaluated by sucrose preference, the forced swimming test (FST), and the tail suspension test (TST). In addition, we assessed cytokines expression in the hippocampus and cortex, plasma corticosterone (CORT) levels, cell apoptosis and microglial activation in order to explore the association of neuroimmunomodulation with antidepressant-like effects of SalB.
Materials and methods
Animals
Male C57BL/6 mice (18–22 g) were purchased from the Laboratory Animals Center of Sichuan Academy of Medical Sciences, Chengdu, China. The mice were housed individually under standard condition (23±2 °C, 50%–70% humidity, on a 12–12 h light-dark cycle with lights on at 07:00, with access to food and water ad libitum) and were allowed to habituate to laboratory conditions for 1 week prior to the experiments. The behavioral tests were conducted between 13:00 and 19:00 h. All experimental procedures were approved by the Ethics Committee of the University of Electronic Science and Technology of China and carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2010).
CMS procedure
The CMS procedure was performed according to the method described previously23, with minor modification. Briefly, the mice were subjected to various stressors according to a semi-random schedule for 6 consecutive weeks (Figure 1A). The stress regime for each week consisted of forced swimming at 6 °C for 10 min, tail suspension for 10 min, food deprivation for 24 h, water deprivation for 24 h, cage tilting (45°) for 24 h, damp bedding for 24 h, stroboscopic illumination for 24 h, and behavior restriction for 2 h. The mice received one of these stressors on one day of the week, with no same stressor applied for two consecutive days. The control mice were housed in a separate room under identical conditions and had no contact with these stressed animals.
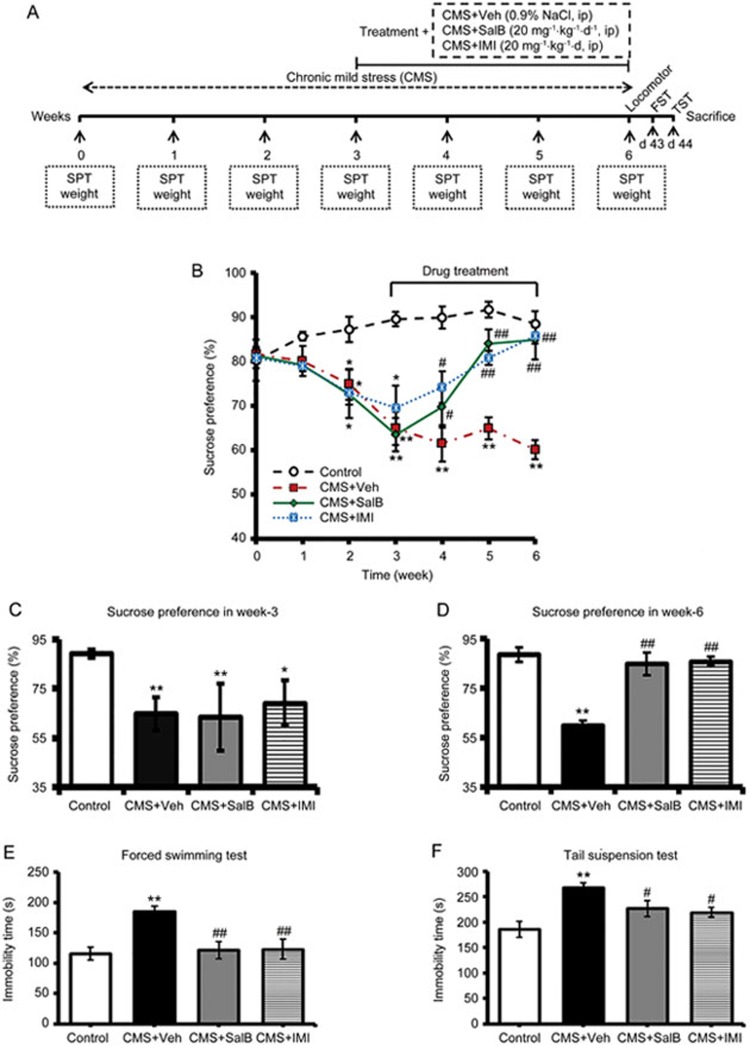
Figure 1.
Effects of SalB and IMI treatment on depressive-like behaviors in CMS-treated C57BL/6 mice. (A) The experimental design. (B) Changes in sucrose consumption over 6 weeks in the control, CMS+Veh, CMS+SalB, and CMS+IMI groups. (C and D) The sucrose preference ratio of C57BL/6 mice at the 3rd and 6th weeks, respectively. (E and F) The duration of immobility of mice in the FST and in TST at the 6th week, respectively. Data are shown as the mean±SEM. n=8. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the CMS+Veh group.
Drug administration
After the third week of the CMS paradigm, when depressive-like behaviors occurred, drugs were administered once per day for three weeks. All drugs and vehicle (0.9% normal saline) were injected intraperitoneally (ip) between 17:00 and 19:00 h in a total volume of 10 mL/kg. The drugs SalB and imipramine (IMI) hydrochloride (Sigma-Aldrich, St Louis, USA) were dissolved in normal saline to reach appropriate concentrations (2 mg/mL for both SalB and IMI). SalB was isolated from S miltorrhiza powder, to a purity of 95% to meet the experimental requirement, according to the method described in our previous work24. The non-CMS control mice were given the same volume of vehicle. Within 14 h of the last injection of drugs and vehicle, behavioral tests were performed.
All mice were randomly divided into four groups: one control and three experimental groups [CMS and Vehicle (CMS+Veh), CMS and SalB (CMS+SalB), CMS and IMI (CMS+IMI)]. In the control group, animals did not receive the CMS procedure and received only saline, the CMS+Veh group was exposed to the CMS procedure and received freshly prepared vehicle (normal saline), the CMS+SalB as exposed to the CMS procedure and received SalB (20 mg/kg, concentration determined based on data from our previous trial)24, and the CMS+IMI group as exposed to the CMS procedure and received IMI (20 mg/kg).
Sucrose preference test (SPT) and body weight measurement
The SPT was performed as described previously25, with minor modification. Briefly, 72 h before the test, mice were habituated to drink 1% sucrose solution, followed by deprivation of water and food for 12 h. Then, mice were free to access either of two bottles containing 1% sucrose solution or water. The positions of the two bottles were switched and kept for another 24 h. The mice were housed in individual cages. The volumes of consumed sucrose solution and water were recorded for six weeks through the whole experiment. The sucrose preference ratio (SPR) was calculated according to the following equation: SPR=sucrose intake (g)/sucrose intake (g)+water intake (g).
Body weight was measured between 15:00 and 17:00 h on Monday every week to calculate the mean body weight gain during the entirety of the experiment.
Forced swimming test (FST)
The FST was conducted using a method adapted from Porsolt's26, with minor modification. Mice were individually placed in a glass cylindrical container (total volume of approximately 1000 mL, 21 cm in height and 12 cm in diameter) that was filled with water (22±1 °C) to a depth of 12 cm. The FST started 24 h after the last drug administration. Each mouse was exposed to a test session for 6 min, and judged to be immobile when it remained floating passively in the water without struggling. The duration of immobility was accurately scored by a blinded observer during the last 4 min of the total swimming time.
Tail suspension test (TST)
The TST was carried out based on a previously described procedure27. Briefly, 48 h after the last drug administration, acoustically and visually isolated mice were suspended by their tail from a ledge with adhesive tape (5 cm in width), 10 cm above the tabletop, for 6 min. The tape was placed approximately 1 cm from the tip of the tail. Immobility was defined as the absence of movement, with the time of immobility recorded by an observer that was blinded to the drug treatment.
Measurement of locomotor activity
In order to exclude false positive results of SalB as a psychostimulant drug that reduces the immobility time in depression-related behavior in the FST and TST, the locomotor activity test was conducted before the FST and TST. Thirty minutes after the treatment on d-42, the spontaneous activity level was examined. Locomotor activity was measured with a 36-point infrared ray passive sensor system (model No ZZ-6, Taimeng Tech Ltd Chengdu, China). The mice were placed in six, separate chambers with an autonomous movement instrument. The total locomotor activity (standing and movement activity) of mice was automatically recorded during the 10-min test. All mice were allowed to adjust to the environment for 1 min before the test.
Plasma CORT level
The mice were anesthetized by sodium pentobarbital (60 mg/kg, ip), and the blood samples were collected in tubes with heparin for plasma preparation. The samples were centrifuged for 20 min at 1000×g, and the supernatants of each sample were collected and stored at −20 °C for assays. Plasma CORT levels were assayed with an enzyme-linked immunosorbent assay (ELISA) kit (50R- E.1444M, Biovalue Co Ltd, China) according to the manufacturer's instruction. The lowest assay sensitivity of the kit is <0.1 ng/mL.
RNA extraction and real-time PCR (RT-PCR)
After the blood samples were collected, all the brains of mice were removed using aseptic techniques. The hippocampus was quickly dissected out, placed in sterile tubes, and frozen on dry ice. Meanwhile, the whole cortex was collected and also placed in sterile tubes on dry ice. Total RNA was extracted according to the manufacturer's protocol with Trizol Reagent (Invitrogen Life Technologies, USA). The final RNA samples were resuspended in 30 μL of nuclease-free water. The cDNA was synthesized from 1 μL of the RNA sample with the First Strand cDNA Synthesis Kit (Invitrogen Life Technologies, USA), according to the manufacturer's instructions. The cDNA was subsequently stored at −20 °C.
Pro- and anti-inflammatory cytokine expression was analyzed by RT-PCR. For all cytokines, the oligonucleotide primers are listed in Table 1. The reaction mixture for RT-PCR consisted of 1 μL of template cDNA, 2 μL of primer and 5 μL of SsoFast™ EvaGreen Supermix (BIO-RAD, USA) in a total reaction volume of 10 μL. Duplicate samples were used for analysis in a CFX96 RT-PCR System (BIO-RAD, USA). The conditions of RT-PCR used for amplification were as follows: initial 95 °C for 3 min followed by 40 cycles at 95 °C for 10 s and annealing/extension at 72 °C for 10 s. The lengths of mRNA products of IL-1β, TNF-α, IL-10, TGF-β, and β-actin are 191, 81, 264, 236, and 73 bp, respectively. The collected data were analyzed, and the fold-expression changes were normalized against the housekeeping gene β-actin. Final data were expressed in relative quantification units using the equation of relative cytokines expression: Ratio (reference/target)=2Ct(reference) - Ct(target).
Table 1. Primers of RT-PCR.
| Gene | Forward (5′ – 3′) | Reverse (5′ – 3′) |
|---|---|---|
| IL-1β | CCAGCAGGTTATCATCATCATCC | CTCGCAGCAGCACATCAAC |
| TNF-α | CAGCCGATGGGTTGTACCTT | TGTGGGTGAGGAGCACGTAGT |
| IL-10 | TGGCCCAGAAATCAAGGAGC | CAGCAGACTCAATACACACT |
| TGF-β | GACCGCAACAACGCCATCTA | GGCGTATCAGTGGGGGTCAG |
| β-Actin | CCGTGAAAAGATGACCCAGATC | CACAGCCTGGATGGCTACGT |
Enzyme-linked immunosorbent assay (ELISA)
Mice were sacrificed at 24 h after the last drug administration and the brain was removed using aseptic technique. The hippocampal and cortex tissues were quickly dissected out, and then homogenized and centrifuged at 1000×g for 30 min. Supernatants were used to examine the concentration of TNF-α and IL-10. The protein levels of TNF-α and IL-10 were quantified using ELISA kits (QuantiCyto, China), according to the manufacturer's protocol. The detection limit for TNF-α and IL-10 were 8 pg/mL. All groups (n=6−8) were used in the ELISA test.
Immunohistochemistry and image analysis
Cell apoptosis and microglial activation were examined with immunofluorescence staining for the control (n=6), the CMS group (n=6) and the SalB-treated CMS group (n=6). The mice were deeply anesthetized with 10% pentobarbital and perfused with pH 7.2 phosphate-buffered saline (PBS) for 4 min and then with 4% paraformaldehyde (PFA) in pH 7.2 PBS for 6 min. Brains were excised and post-fixed in 4% PFA for 3 d, then dehydrated in 30% sucrose for 48 h. The brains were stored at −80 °C before coronal cryostat sections (35 μm thick) were obtained using a sliding vibratome (CM1900; Leica Microsystems, Wetzlar, Germany). Six sequential slices were collected into each well of a 12-well plate containing PBS with 0.02% sodium azide and stored at 4 °C. To quantify apoptotic cells, every sixth section containing the hippocampus was selected and permeabilized with 0.5% Triton X-100 in PBS for 15 min. The sections were blocked in 10% donkey serum for 1.5 h, incubated with primary antibodies (mouse anti-cleaved caspase-3 (1:100, Cell Signaling Technology, Inc) or goat anti-Iba1 (1:400, Abcam) overnight at 4 °C and with fluorescent-dye-conjugated secondary antibodies (DyLight 488-conjugate donkey anti-mouse (1:300; Jackson ImmunoResearch); DyLight 549-conjugate donkey anti-goat (1:300; Jackson ImmunoResearch) for 2 h at room temperature. Sections were imaged using fluorescence microscopy (Olympus BX51).
Image analysis was used to quantify the apoptotic cells and activated microglia. The images of every sixth section were imported to Image J software (version 1.45 J) and used to determine a threshold for positive staining while excluding background staining. The amount and average percent area of the positive threshold for all representative pictures are reported. The volumes of the hippocampus were estimated based on the Cavalieri principle. Every sixth section that contained the hippocampus was selected and stained with DAPI antibody. The volume measurements were performed using Image J software (version 1.45 J). The volume of the hippocampus was estimated by the sum of the measured areas of the hippocampus and multiplied by the cutting thickness (35 μm) and the number of series.
Statistical analysis
The statistical analyses were performed using SPSS statistical software package for Windows® v.17 (SPSS Inc, Chicago, USA). Potential differences between the mean values were analyzed using one-way analysis of variance (ANOVA) followed by the least significant difference test for post hoc comparisons. The correlative analyses were performed using GraphPad Prism 5.01. The level of confidence was set at 95% (P<0.05). All data are shown as the mean±SEM.
Results
SalB ameliorated CMS-induced depressive-like behavior
As a principal evaluation index for anhedonia in an animal model of depression, SPR was measured before initiating the CMS regimen and then weekly until the end of the 6-week CMS procedure (Figure 1A). All the groups were not significantly different in their baseline SPR score (week 0: F3,28=0.048, P>0.05, Figure 1B). After three weeks of CMS, SPR showed a significant decrease (week 3: F3,28=5.609, P<0.05) compared with the control group (Figure 1B and 1C). However, there were no significant differences in SPR among the CMS+Veh, CMS+SalB and CMS+IMI groups (Week 3: F3,28=0.031, P>0.05; Figure 1B and 1C). After three weeks of treatment, SPR gradually increased in mice that were treated with SalB or IMI compared with CMS+Veh mice (Week 6: F3,28=6.061, P<0.01, P<0.01, respectively, Figure 1B and 1D). In the FST, compared with the control group, the mice of CMS+Veh exhibited a significantly longer duration of immobility time (F3,28=6.351; P<0.01, Figure 1E). Conversely, the CMS+SalB and CMS+IMI groups showed a shorter duration of immobility in the FST (P<0.01 and P<0.01, respectively, Figure 1E). In the TST, immobility time was significantly shorter both in the CMS+SalB and CMS+IMI groups than in the CMS+Veh group at the end of week 6 (F3,28=6.671, P<0.05, P<0.05, respectively, Figure 1F). Meanwhile, there were no significant differences in immobility time between the CMS+SalB and CMS+IMI groups (P>0.05, Figure 1F).
SalB alleviated the depression-related physiological indices
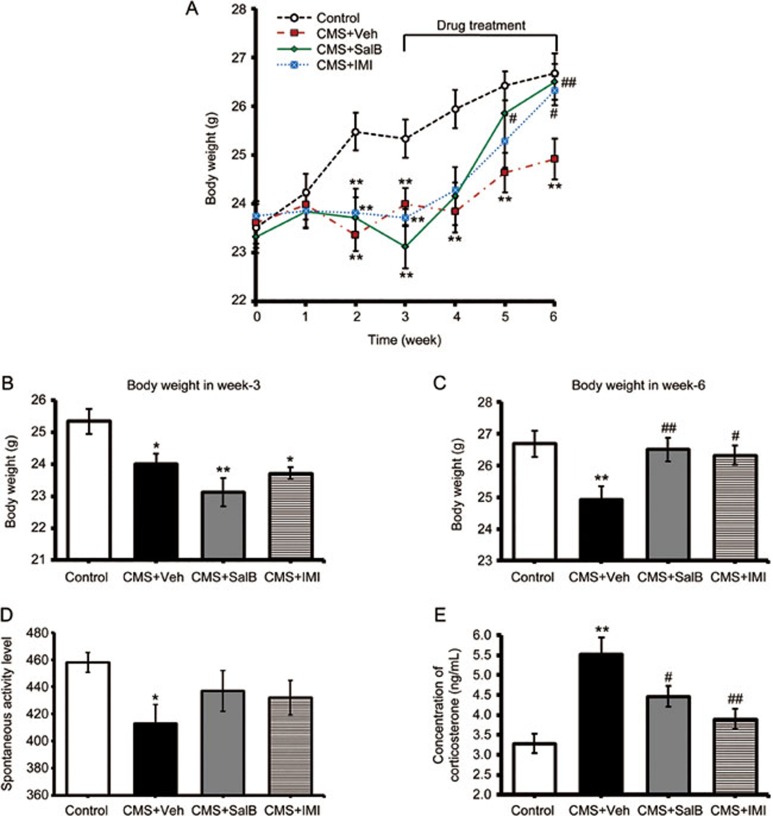
Depression is often accompanied by weight loss, spontaneous activity decreases and increased plasma CORT levels. These physiological indices were examined in all groups. As shown in Figure 2A, there were no differences in baseline body weight among the groups (week 0: F3,28=0.236, P>0.05, Figure 2A). After three weeks of stress, body weight of mice in all the three CMS groups was significantly diminished (week 3: F3,28=4.586, P<0.05; Figure 2B). With a continuous 3-week treatment of SalB (20 mg/kg) or IMI (20 mg/kg) in CMS mice, the weight loss was significantly reversed compared with the CMS-vehicle mice (P<0.01 and P<0.05, respectively, Figure 2C). The CMS+Veh mice showed a significantly decreased spontaneous movement level (F3,28=2.151, P<0.05, Figure 2D). However, SalB and IMI had no significant effect on spontaneous movement level (P>0.05). Chronic stress induced a significant increase of plasma CORT levels in depression-model mice (F3,20=10.069, P<0.01, Figure 2E). Both SalB and IMI significantly reduced stress-induced increases in plasma CORT levels (P<0.05 and P<0.01, respectively, Figure 2E), but their levels were still higher than those of the control mice.
Figure 2.
Effects of SalB and IMI treatment on depression-related physiological indices. (A) Changes in body weight of mice over 6 weeks. (B and C) The body weights of control, CMS+Veh, CMS+SalB, and CMS+IMI mice at the 3rd and 6th weeks, respectively. (D) The locomotor activities of mice at the 6th week. (E) The plasma CORT levels in control and experimental groups. Data are shown as the mean±SEM. n=8. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the CMS+Veh group.
SalB suppressed CMS-induced inflammatory cytokines expression in hippocampus and cortex
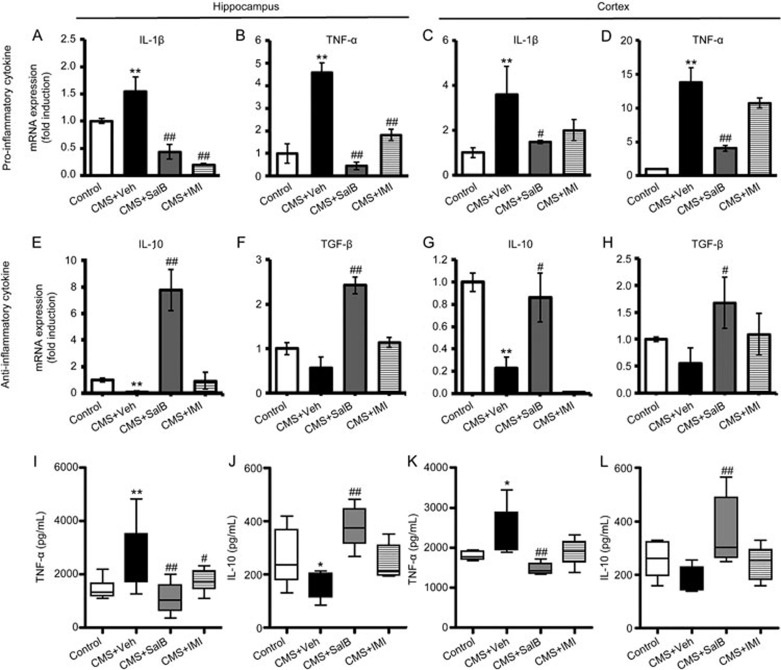
This study also assessed the mRNA or protein expression of pro-inflammatory and anti-inflammatory cytokines in the hippocampus and cortex using RT-PCR or ELISA, respectively. Expression levels of IL-1β were significantly higher in the hippocampus (F3,24=22.964, P<0.01; Figure 3A) and cortex (F3,24=4.189, P<0.01; Figure 3C) in the CMS+Veh mice than in control mice. Both SalB and IMI effectively restrained IL-1β expression in the hippocampus (P<0.01 and P<0.01, respectively, Figure 3A), while only SalB significantly inhibited the CMS-induced increase of IL-1β in the cortex (P<0.05; Figure 3C). In agreement with IL-1β expression, mRNA expression of TNF-α was significantly increased in the hippocampus (F3,28=27.149, P<0.01; Figure 3B) and cortex (F3,27=19.080, P<0.01; Figure 3D) of CMS+Veh mice. After three weeks of treatment, TNF-α mRNA expression was suppressed by SalB both in the hippocampus (P<0.01; Figure 3D) and cortex (P<0.01; Figure 3D) of CMS mice. IMI decreased TNF-α expression in the hippocampus (P<0.01; Figure 3D) but not in the cortex (P>0.05; Figure 3D). Nevertheless, the expression of anti-inflammatory cytokine IL-10 was significantly decreased in the hippocampus (F3,24=13.118, P<0.01; Figure 3E) and cortex (F3,24=11.818, P<0.01; Figure 3G) of CMS-exposed mice, while TGF-β showed no significant changes in the hippocampus (F3,28=3.371, P>0.05; Figure 3F) or cortex (F3,28=1.741, P>0.05; Figure 3H). With a 3-week treatment of SalB, the expression of IL-10 and TGF-β was significantly increased not only in the hippocampus (P<0.01 and P<0.01, respectively) but also in the cortex (P<0.05 and P<0.05, respectively) compared with the CMS+Veh group (Figure 3E–3H). Treatment with IMI had no effects on the expression of anti-inflammatory cytokines IL-10 and TGF-β in the hippocampus and the cortex except for decreasing IL-10 expression in the cortex (Figure 3E–3H).
Figure 3.
Effects of SalB and IMI treatment on pro- and anti-inflammatory cytokines expression in the hippocampus and cortex. mRNA expression of the pro-inflammatory cytokines IL-1β (A, C) and TNF-α (B, D) and anti-inflammatory cytokines IL-10 (E, G) and TGF-β (F, H) in the hippocampus and the cortex were assayed by RT-PCR. Protein levels of the pro-inflammatory cytokines TNF-α (I, K) and anti-inflammatory cytokines IL-10 (J, L) in the hippocampus and the cortex were assayed using ELISA. Scale bars represent the mean values, and the error bars represent the mean±SEM. n=6−8. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the CMS+Veh group.
Consistent with mRNA expression, the TNF-α protein level was enhanced in the hippocampus (F3,28=6.356, P<0.01; Figure 3I) and cortex (F3,28=6.471, P<0.05; Figure 3K) in a CMS mouse model of depression. Both SalB and IMI effectively restrained the concentration of TNF-α in the hippocampus (P<0.01 and P<0.05, respectively, Figure 3I), while only SalB significantly inhibited the CMS-induced increase in TNF-α in the cortex (P<0.01; Figure 3K). The protein level of IL-10 was downgraded in the hippocampus (F3,24=3.612, P<0.05; Figure 3J) but not in the cortex (F3,32=4.048, P>0.05; Figure 3L) of CMS-treated mice. SalB treatment significantly increased IL-10 levels in the hippocampus (P<0.01; Figure 3J) and cortex (P<0.01; Figure 3K), while IMI treatment showed no significant effects on IL-10 levels in the hippocampus (P>0.05; Figure 3J) or cortex (P>0.05; Figure 3L).
SalB decrease CMS-induced apoptosis in hippocampus and cortex
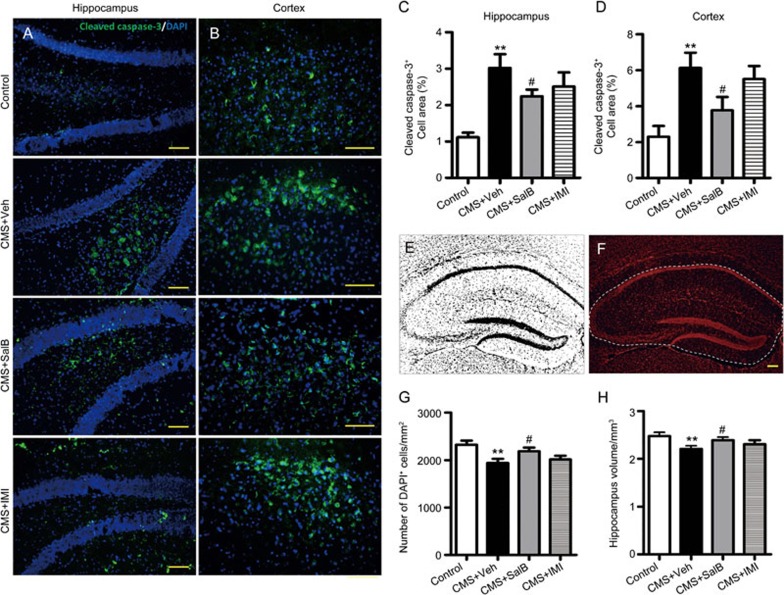
We examined the effects of SalB on CMS-induced apoptosis using cleaved caspase 3, a cell-specific marker that detects apoptotic cells. In doing so, we showed that CMS induced an increase in the number of apoptotic cells in the hippocampus (Figure 4A) and cortex (Figure 4B). The cleaved caspase-3+ area was analyzed by Image J software, and the results showed that the percentage of cleaved caspase-3+ area was significantly increased in the hippocampus (F3,24=7.501, P<0.01; Figure 4C) and cortex (F3,24=5.343, P<0.01; Figure 4D) of CMS+Veh mice. After 3 weeks of treatment using SalB, the percentage of cleaved caspase-3+ area was significantly decreased not only in the hippocampus (P<0.05, Figure 4C) but also in the cortex (P<0.05, Figure 4D) compared with the CMS+Veh group, while IMI treatment showed no significant decrease in the percentage of cleaved caspase-3+ area in the hippocampus (P>0.05, Figure 4C) and cortex (P>0.05, Figure 4D). In addition, we also assessed the density of DAPI+ (nucleus) and hippocampal volume. The results indicated that CMS procedures significantly decreased the cell density in the hippocampus of C57BL/6 mice (F3,24=4.590, P<0.01; Figure 4G). The hippocampal cell density was restored by treatment with SalB (P<0.05, Figure 4G) but not with IMI (P>0.05, Figure 4G). Similarly, chronic stress resulted in the reduction of hippocampal volume in mice (F3,24=2.543, P<0.01; Figure 4H). Stress-induced decreases in volumes of the hippocampus were significantly reversed after treatment with SalB (P<0.05, Figure 4H), while the IMI treatment showed no significant effect on the volumes of the hippocampus (P>0.05, Figure 4H).
Figure 4.
Effects of SalB treatment on CMS-induced apoptosis in the hippocampus and cortex. (A) Representative images of apoptotic cells (cleaved caspase 3+) scattering in the hippocampus. (C) The bar graph indicates cleaved caspase 3+ cells (percentage of area) in the hippocampus. (B) Representative images of apoptotic cells scattering in the cortex. (D) The bar graph indicates cleaved caspase 3+ cells (percentage of area) in the cortex. (E) Representative image of the threshold set for DAPI+ staining with no background was used to measure cell density. (F) Representative image of the volume of the hippocampus. The bar graph indicates cell density (G) and the volumes of the hippocampus from various animals (H). Data are shown as the mean±SEM. n=6. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the CMS+Veh group. The scale bar represents 100 μm.
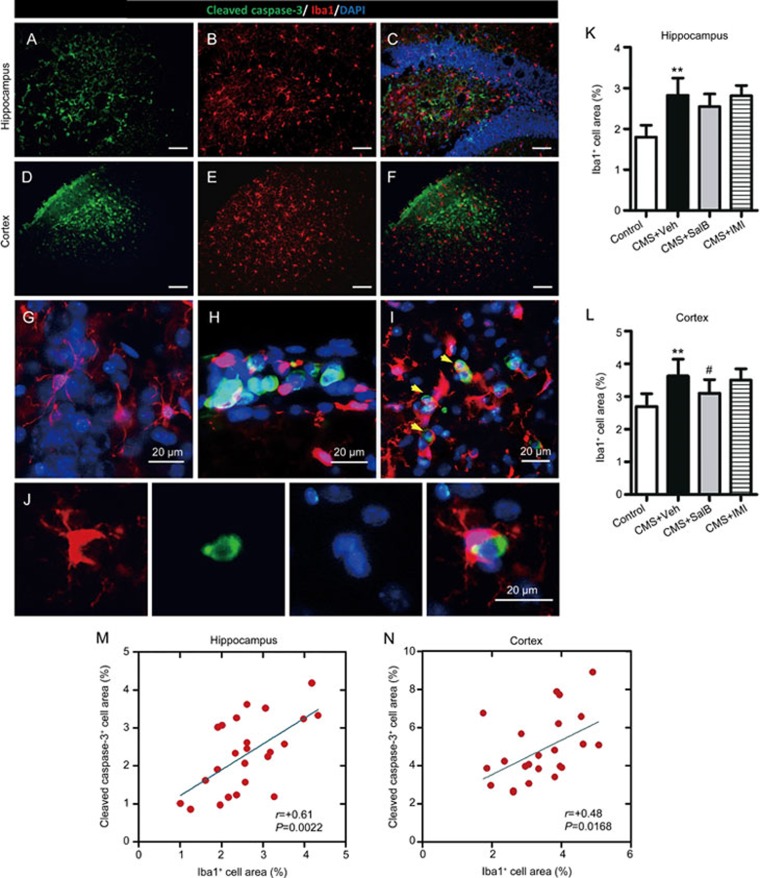
Apoptosis associated with microglial activation
Immunofluorescence double labeling was performed using a cell-specific marker for apoptotic cell (cleaved caspase 3+) and microglia (Iba1+) in the hippocampus (Figure 5A–5C) and cortex (Figure 5D–5F). The percentage of Iba1+ area was significantly increased in the hippocampus (F3,24=2.187, P<0.01; Figure 5G) and cortex (F3,24=3.928, P<0.01; Figure 5H) of CMS+Veh mice. SalB decreased the percentage of Iba1+ area in the cortex (P<0.05, Figure 5H) but not in the hippocampus (P>0.05, Figure 5G) of CMS-exposed animals. Meanwhile the IMI treatment showed no significant effect on the percentage of Iba1+ area both in the hippocampus (P>0.05, Figure 5K) and cortex (P>0.05, Figure 5L). Much more interestingly, amoeba-like Iba1+ cells (activated microglia) were recruited into the regions of apoptosis both in the hippocampus (Figure 5C and 5H) and cortex (Figure 5F). Removal of cleaved caspase-3+ cell was performed by phagocytosis by amoeba-like Iba1+ cell (Figure 5I and 5J). Moreover, we found a significant correlation between the percentage of Iba1+ area and the percentage of cleaved caspase-3+ area both in the hippocampus (r=+0.61, P= 0.0022, Figure 5M) and cortex (r=+0.48, P= 0.0168, Figure 5N).
Figure 5.
Effects of SalB treatment on microglial activation in the hippocampus and cortex. (A and D) Representative staining with the apoptotic cell marker (cleaved caspase 3) in the hippocampus and cortex, respectively. (B and E) Representative labeling with the microglial activation marker (Iba1) in the hippocampus and cortex, respectively. (C and F) Merged images, which indicate that cleaved caspase 3+ cells are associated with Iba1+ cells in the hippocampus and cortex. (G) Representative resting-state microglia images in the absence of apoptotic cells. (H) Representative activated microglia images in the regions of apoptosis. (I) Representative amoeba-like microglia images, which show their phagocytic activity in targeting apoptotic cells. (J) Representative high-resolution images of the phagocytosis of microglia. The bar graph indicates the percentage of Iba1+ area in the hippocampus (K) and cortex (L). Correlation between the percentage of cleaved caspase 3+ area and the percentage of Iba1+ area in the hippocampus (M) and cortex (N), with each circle representing individual mice (n=24). All data without (M and N) are shown as the mean±SEM. n=6. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the CMS+Veh group. The scale bar represents 100 μm.
Discussion
The present study evaluated the potential antidepressant-like effects of SalB. Our results showed that SalB significantly reversed the decrease of SPR and markedly reduced the immobility in the FST and TST in CMS mice. In addition, we also observed that SalB decreased the levels of CORT in plasma and regulated the imbalance of pro- and anti-inflammatory cytokines in the hippocampus and the cortex of depressive-like C57BL/6 mice.
Our previous data from the acute screening paradigm of the FST and TST showed that SalB attenuated depressive-like behaviors24. In the present study, we further determined the potent antidepressant-like activity and investigated the active mechanism of SalB in the CMS model of depression. After exposure to a series of unpredictable stressors, C57BL/6 mice exhibited depressive-like behaviors. Sucrose preference and body weight were significantly reduced, and the immobility time of the FST and TST were increased. The model provides a natural induction of a chronic depressive-like state that develops gradually over time in response to stress and results in persistent changes in behavior, neurochemistry, and neuroimmune and neuroendocrinological variables resembling those that are observed in depressed patients28. This model would therefore be the most valid animal model of depression29. C57BL/6 mice respond well to the CMS treatment and are commonly used in antidepressant drug research30. The CMS protocol provides a robust, easily replicable rodent animal model of depression for investigating the antidepressant-like consequences of SalB.
Depression is usually manifested in diverse debilitating symptoms, including hopelessness and anhedonia1. Anhedonia, which is reflected by sucrose preference, is a core symptom of MDD31. Immobility in the FST and TST paradigms is interpreted as a passive stress-coping strategy or depressive-like behavior32. Such depressive-like behaviors were ameliorated by SalB treatment. The dose of SalB at 20 mg/kg was chosen based on our previous experiments24. After SalB administration for 21 d, we observed that SalB not only enhanced sucrose preference but also decreased the immobility time of CMS mice in the FST and TST. Weight change is one of several additional symptoms in patients with MDD33. CMS mice weight loss was also reversed after three weeks of SalB treatment. There was no significant difference between the effects of SalB and those of IMI in the SPT, FST, and TST tests. These findings indicate that SalB, similar to IMI, could improve depressive-like behaviors in mice subjected to a model of unpredictable CMS procedures. The locomotor activity was reduced in CMS mice. Similar to IMI, SalB increased locomotor activities in CMS mice, but there was no significant difference in overall locomotor activity compared with the saline group. Therefore, the effect of SalB on reduction of immobility time in the FST and TST seemed unlikely to be due to the activation of locomotion.
Recent research has suggested that inhibition of neuroinflammation might represent a novel mechanism of action of antidepressant treatment34. SalB has been shown to provide anti-inflammatory, antioxidant, and neuroprotective activity by regulating microglial activation17,35. According to recent studies, besides functioning as a monoaminergic neurotransmitter, neuroinflammation may also have a key role in the pathogenesis of MDD13,36. Growing evidence suggests that there is a causative link between depressive-like behaviors and life events, inflammation and apoptosis in the brain37. Our previous research indicated that the dysregulation of pro- and anti-inflammatory cytokines have a crucial role in the pathophysiology of depression38. Pro-inflammatory cytokines (TNF-α, IL-1β) or lipopolysaccharide-induced depressive-like behavior and neuroinflammatory reaction can be reversed by anti-inflammatory cytokines, such as TGF-β and IL-1039,40. Conversely, anti-inflammatory agents have therapeutic benefits for mood disorders41,42. The present study demonstrated that CMS induced the imbalance of pro- and anti-inflammatory cytokines in the hippocampus and cortex. SalB suppressed the expression of the pro-inflammatory cytokines IL-1β and TNF-α in the hippocampus and cortex. SalB also enhanced the expression of the anti-inflammatory cytokines IL-10 and TGF-β in the hippocampus and cortex of CMS-treated mice. Chen et al35 reported that SalB treatment had similar effects on IL-1β, TNF-α, IL-10, and TGF-β in the brain tissue of mice, thereby suggesting that the neuroprotective effects of SalB were associated with its anti-inflammatory activities. These findings indicate that SalB is involved in the regulation of cytokines expression in the brain and the subsequent depressive-like behaviors that are induced by CMS. Conversely, an abnormal hypothalamic-pituitary adrenal (HPA) axis is a key endophenotype in MDD patients and animal models43. Plasma CORT, an indicator of HPA axis activity in rodents, is over-secreted in animal models of depression and can be normalized by antidepressants44. In our experiment, SalB treatments significantly reduced the CMS-induced enhancement in plasma CORT, thereby suggesting that SalB has the function of regulating CORT levels and normalizing HPA axis hyperactivity, which may be involved in neuroimmunomodulation45.
The loss of neurons in hippocampal and cortical regions contributes to the etiology of MDD46. Recent studies have suggested that neuroinflammation is accompanied by the activation of microglial cells and the release of inflammatory mediators, which activates cellular cascades and results in neural apoptosis in the brain47,48. Our results indicate that SalB blocks the CMS-induced increases in apoptosis in both the hippocampus and cortex and prevents the decrease of hippocampal volume and cell density. Interestingly, activated microglia were recruited into the regions of apoptosis and a significant correlation between apoptosis and microglial activation was observed both in the hippocampus and cortex, thereby indicating that CMS-induced apoptosis is associated with a microglia-mediated pro-inflammatory response49. Moreover, we observed that apoptotic cells were phagocytized by activated microglia, which suggests that microglia plays a dual role in the phagocytosis of apoptotic cells and the regulation of neuroinflammation. Overall, we observed that SalB suppressed CMS-induced pro-inflammatory activation in the brain, reversed apoptosis and hippocampal atrophy, and alleviated depression-like behaviors in mice. These findings support the observation that the antidepressant-like effects of SalB involved the regulation of microglia activation, inflammatory cytokines release, and inflammatory-mediated neural apoptosis (Figure 6).
Figure 6.
Schematic representation of the antidepressant-like effects of SalB, which targets the neuroinflammatory pathway. Chronic stress can lead to inflammatory cytokine release and subsequent apoptosis, which results in depressive-like behaviors in mice. SalB treatment can regulate the balance of pro- and anti-inflammatory cytokines, and inhibit inflammatory-mediated apoptosis in the brain, which have a role in antidepressant activity.
In summary, the present study demonstrates the potential antidepressant-like effects of SalB in a CMS-induced mice model of depression, which is associated with the regulation of microglia-related apoptosis in the hippocampus and the cortex.
Author contribution
Zi-li YOU and Cheng PENG conceived and designed the experiments; Jin-qiang ZHANG, Xiao-hui WU, Yi FENG, and Yong-hua FAN performed research; Xiao-fang XIE and Shuo YAN isolated natural product; Qiu-ying ZHAO conducted the data analysis; Jin-qiang ZHANG, Xiao-hui WU wrote the first-draft manuscript and Zi-li YOU revised the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81571174), the Open Research Fund of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources (No 2015003), and Key Technologies R&D Program of Sichuan Province (No 2013SZ0011, 2015SZ0058-5). We are grateful to Ms Xiong WAN for her help in copyediting.
References
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psycho 2007; 3: 137–58. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2012) Media Centre Fact Sheet No 369. http://www.who.int/mediacentre/factsheets/fs369/en/.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002; 34: 13–25. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 2006; 7: 137–51. [DOI] [PubMed] [Google Scholar]
- Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiat 2001; 62: 869–77. [DOI] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 2005; 143: 415–26. [DOI] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research Review: The role of cytokines in depression in adolescents: a systematic review. J Child Psychol Psyc 2013; 54: 816–35. [DOI] [PubMed] [Google Scholar]
- Piser TM. Linking the cytokine and neurocircuitry hypotheses of depression: A translational framework for discovery and development of novel anti-depressants. Brain Behav Immun 2010; 24: 515–24. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: Comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 2008; 85: 1–74. [DOI] [PubMed] [Google Scholar]
- Huang YS, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatr 2013; 74: 62–8. [DOI] [PubMed] [Google Scholar]
- Makhija K, Karunakaran S. The role of inflammatory cytokines on the aetiopathogenesis of depression. Aust Nz J Psychiat 2013; 47: 828–39. [DOI] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun 2010; 24: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Kim HW, Jackson SH, Bosetti F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem 2012; 120: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YJ, Cheng IC, Yeh LL, Cho YM, Feng J. Utilization of traditional Chinese medicine in patients treated for depression: a population-based study in Taiwan. Complement Ther Med 2013; 21: 215–23. [DOI] [PubMed] [Google Scholar]
- Xue WD, Zhou X, Yi N, Jiang LH, Tao WW, Wu RJ, et al. Yueju Pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brain. Evid-Based Compl Alt 2013. [DOI] [PMC free article] [PubMed]
- Wang SX, Hu LM, Gao XM, Guo H, Fan GW. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res 2010; 35: 1029–37. [DOI] [PubMed] [Google Scholar]
- Liu CS, Cheng Y, Hu JF, Zhang W, Chen NH, Zhang JT. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761). Acta Pharmacol Sin 2006; 27: 1137–45. [DOI] [PubMed] [Google Scholar]
- Du GH, Qiu Y, Zhang JT. Salvianolic acid B protects the memory functions against transient cerebral ischemia in mice. J Asian Nat Prod Res 2000; 2: 145–52. [DOI] [PubMed] [Google Scholar]
- Zhang N, Kang TG, Xia Y, Wen QP, Zhang XD, Li HY, et al. Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur J Pharmacol 2012; 697: 32–9. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Wu L, Zhang QL, Li J, Yin FX, Yuan Y. Pharmacokinetics of phenolic compounds of Danshen extract in rat blood and brain by microdialysis sampling. J Ethnopharmacol 2011; 136: 129–36. [DOI] [PubMed] [Google Scholar]
- McArthur R, Borsini F. Animal models of depression in drug discovery: A historical perspective. Pharmacol Biochem Be 2006; 84: 436–52. [DOI] [PubMed] [Google Scholar]
- Micale V, Kucerova J, Sulcova A. Leading compounds for the validation of animal models of psychopathology. Cell Tissue Res 2013; 354: 309–30. [DOI] [PubMed] [Google Scholar]
- Feng Y, You ZL, Yan S, He G, Chen YB, Gou XJ, et al. Antidepressant-like effects of Salvianolic acid B in the mouse forced swim and tail suspension tests. Life Sci 2012; 90: 1010–14. [DOI] [PubMed] [Google Scholar]
- Zhao QY, Peng C, Wu XH, Chen YB, Wang C, You ZL. Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiol Dis 2014; 68: 57–65. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Lepichon M, Lalfre M. Behavioral despair revisited–a citation-classic commentary on depression — a new animal-model sensitive to antidepressant treatments. Life Sci 1993; (20): 9. [Google Scholar]
- Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice. Prog Neuro-psychopharmacol Biol Psychiatr 2006; 30: 1419–25. [DOI] [PubMed] [Google Scholar]
- Seok JH, Kim LS, Hong N, Hong HJ, Kim SJ, Kang HJ, et al. Psychological and neuroendocrinological characteristics associated with depressive symptoms in breast cancer patients at the initial cancer diagnosis. Gen Hosp Psychiat 2010; 32: 503–8. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KGC, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav R 2012; 36: 2085–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer MC, Henniger MS, Sillaber I. Chronic mild stress (CMS) in mice: of anhedonia, 'anomalous anxiolysis' and activity. PLoS One 2009; 4: e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith P. Anhedonia — a neglected symptom of psychopathology. Psychol Med 1993; 23: 957–66. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav R 2005; 29: 547–69. [DOI] [PubMed] [Google Scholar]
- Trief PM, Cibula D, Delahanty LM, Weinstock RS. Depression, stress, and weight loss in individuals with metabolic syndrome in SHINE, a DPP Translation Study. Obesity 2014; 22: 2532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Du GH, Zhang JT. Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol Sin 2000; 21: 463–6. [PubMed] [Google Scholar]
- Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun 2011; 25: 221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatr 2009; 66: 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZL, Luo CM, Zhang WZ, Chen YB, He JJ, Zhao QY, et al. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res 2011; 225: 135–41. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim YK. Transforming growth factor-beta 1 and major depressive disorder with and without attempted suicide: Preliminary study. Psychiatr Res 2010; 178: 92–6. [DOI] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo XK, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One 2013; 8: e58488. [DOI] [PMC free article] [PubMed]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog neuro-psychopharmacol Biol Psychiatr 2014; 53: 23–34. [DOI] [PubMed] [Google Scholar]
- Powell TR, Tansey KE, Breen G, Farmer AE, Craig IW, Uher R, et al. ATP-binding cassette sub-family F member 1 (ABCF1) is identified as a putative therapeutic target of escitalopram in the inflammatory cytokine pathway. J Psychopharmacol 2013; 27: 609–15. [DOI] [PubMed] [Google Scholar]
- Wainwright SR, Galea LAM. The neural plasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast 2013; 2013: 805497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience 2007; 146: 1734–42. [DOI] [PubMed] [Google Scholar]
- Wu JF, Du JA, Xu CQ, Le JJ, Xu YZ, Liu BJ, et al. Icariin attenuates social defeat-induced down-regulation of glucocorticoid receptor in mice. Pharmacol Biochem Be 2011; 98: 273–8. [DOI] [PubMed] [Google Scholar]
- Chen C, Wang Y, Zhang J, Ma L, Gu J, Ho G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Disease Models Mechanism 2014; 7: 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol 2007; 179: 3231–41. [DOI] [PubMed] [Google Scholar]
- Yan N, Liu Y, Liu S, Cao S, Wang F, Wang Z, et al. Fluoride-induced neuron apoptosis and expressions of inflammatory factors by activating microglia in rat brain. Mol Neurobiol 2016; 53: 4449–60. [DOI] [PubMed] [Google Scholar]
- Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro 2013; 5: e00126. [DOI] [PMC free article] [PubMed] [Google Scholar]