Abstract
Aim:
CCL19 and its receptor CCR7 are essential molecules for facilitating the trafficking of mature dendritic cells (DCs) and helping to establish a microenvironment in lymphoid tissues to initiate primary immune responses, whereas CCL17 is required in the CCR7-CCL19-dependent migration of DCs. In this study we examined whether co-administration of CCL17 and CCL19 could enhance the immunogenicity of an anti-caries DNA vaccine, pCIA-P, in rodents.
Methods:
Plasmids encoding CCL17 (pCCL17/VAX) and CCL19 (pCCL19/VAX) were constructed. BALB/c mice were intranasally administered pCCL17/VAX, pCCL19/VAX, or pCCL17/VAX plus pCCL19/VAX, the migration of DCs to the spleen and draining lymph nodes (DLNs) was assessed with flow cytometry. The mice were co-administered pCIA-P; and the anti-PAc antibodies in the serum and saliva were detected with ELISA. Wistar rats were orally challenged with Streptococcus mutans and then administered pCIA-P in combination with pCCL17/VAX, pCCL19/VAX, or pCCL17/VAX plus pCCL19/VAX. The amount of S mutans sustained on rat molar surfaces was assessed using a colony forming assay. Caries activity was scored with the Keyes method.
Results:
Co-administration of the CCL17 and CCL19 genes in mice caused a greater increase in the number of mature DCs in the spleen and DLNs compared with administration of CCL17 or CCL19 genes alone. CCL17 and CCL19 double-adjuvant plus pCIA-P induced significantly higher levels of anti-PAc salivary IgA and anti-PAc serum IgG antibody in mice, and strengthened the ability of pCIA-P in inhibiting the colonization of S mutans on rat tooth surfaces. The caries activity of the combined adjuvant group was significantly lower than that of the pCCL17/VAX or the pCCL19/VAX group.
Conclusion:
A nasal adjuvant consisting of a combination of CCL17 and CCL19 attracts more mature DCs to secondary lymphoid tissues, inducing enhanced antibody responses against the anti-caries DNA vaccine pCIA-P and reducing S mutans infection in rodents.
Keywords: chemokine, CCL17, CCL19, DNA vaccination, pCIA-P, dendritic cells, dental caries
Introduction
DNA vaccination offers attractive new approaches for a series of infectious diseases that remain without proper prophylactic and therapeutic treatment throughout the world. We have previously reported that an anti-caries DNA vaccine, pCIA-P, which encodes the A-P region of the pac gene of Streptococcus mutans (S mutans), the principal etiologic agent of human dental caries, could induce protective immune responses in rodents1. Within the past decade, a few DNA plasmid products have been licensed for animal use2,3,4,5. However, despite promising results in small animal models and improved efficacy in large animal models, the clinical use of DNA vaccines remains unproven. More effort should be made toward finding new approaches to enhance the immunogenicity of DNA vaccines.
After plasmid DNA immunization, somatic cells, primarily myocytes or keratinocytes, as well as a small number of dendritic cells (DCs) and monocytes at the inoculation site, can be transfected6,7,8. Upon entering the nucleus of the transfected cells, the plasmid-encoded genes are expressed, and foreign antigens are generated and processed into peptide strings by the host cell machinery. These peptides can then be associated with the MHC class I or II molecules of DCs, which migrate from the peripheral tissues to the secondary lymphoid organs, where the DCs can present antigens to T cells and prime antigen-specific immunity9. Upon encountering the antigens and during migration to the secondary lymphoid organs, DCs undergo radical changes from immature phagocytic precursor cells to potent antigen-presenting cells (APCs) with the upregulation of chemokine receptors, such as C-C chemokine receptor type 7 (CCR7)10,11. The magnitude of the immune responses to antigens is strongly correlated with the T cell-DC interactions. Improved DC migration to secondary lymphoid organs results in an increased frequency of encounters with naive T cells, thereby promoting immunity. Therefore, an efficacious approach to improve the performance of DNA vaccines is to attract more DCs to secondary lymphoid organs, such as the draining lymph nodes (DLNs).
The migration of DCs to secondary lymphoid organs is regulated by multiple chemokine-chemokine receptor pairs. The chemokine CCL19, mainly expressed by stromal cells in the T-cell area of secondary lymphoid organs, can bind to CCR7, which is upregulated on mature DCs12,13. CCL19 and its receptor CCR7 are generally considered essential molecules for facilitating the trafficking of mature DCs and helping to establish a microenvironment in lymphoid tissues to initiate primary immune responses14,15. CCL19 gene-deleted mice have defects in DC localization16. Several studies showed that enhanced humoral and cellular immunity were obtained by the codelivery of plasmids encoding CCL19 along with DNA vaccines17,18,19. Our previous study demonstrated that the codelivery of the CCL19 gene with the anti-caries DNA vaccine pCIA-P significantly augmented systemic immune responses. Moreover, an increased number of mature DCs were detected in the DLNs of mice following the co-administration of CCL19 DNA20. These results indicate that the elevated immune responses in CCL19-coadministered mice occurred as a result of increased DC-T cell encounters in the secondary lymphoid tissues. Studies showed that the expression of CCR7 on the DC surface alone was not sufficient to guarantee its full function in guiding DC migration. CCL17-deficient DCs, which express normal levels of CCR7, fail to undergo efficient directional migration to the CCR7 ligand CCL1921. These results indicate that CCL17 is required in the CCR7-CCL19-dependent migration of DCs. To explore whether CCL17 can enhance DC migration after codelivery with the CCL19 gene and the anti-caries DNA vaccine pCIA-P, we constructed the plasmid pCCL17/VAX, which encodes the CCL17 gene, and investigated the in vivo chemotactic activity of CCL17 together with CCL19 on mature DCs in the present study. Moreover, we evaluated whether the intranasal delivery of pCCL17/VAX along with pCCL19/VAX could enhance the systemic and mucosal immunity in response to the anti-caries DNA vaccine pCIA-P and provide more protection against dental caries in rodents. Here, we aimed to offer a new approach for enhancing the immunogenicity of the anti-caries DNA vaccine by strengthening the interactions between T cells and DCs.
Materials and methods
Plasmid construction
The murine CCL19 and CCL17 genes were amplified from mouse spleen by RT-PCR and cloned into the NheI and KpnI sites of the pVAX1 vector (Clontech Laboratories, Inc, Mountain View, CA, USA) to obtain plasmids encoding CCL19 and CCL17, which were designated pCCL19/pVAX and pCCL17/pVAX, respectively. The plasmids were isolated and purified with the EndoFree Plasmid Giga kit (Qiagen, Valencia, CA, USA) and stored at -20 °C for further use.
Plasmid in vitro expression analysis
COS-7 cells (Chinese Center for Type Culture Collection, CCTCC, Wuhan, China) were transfected with pCCL19/VAX, pCCL17/VAX or pVAX1 using Sofast transfection reagent (Sunma Biotechnology Co, Ltd, Xiamen, China). The supernatants were collected 48 h later and kept at -80 °C. The proteins in the supernatant were analyzed using a mouse CCL19 and CCL17 ELISA kit (R&D Systems, Minneapolis, MN, USA).
In vivo DC migration assay
The chemokine plasmids pCCL17/VAX and pCCL19/VAX were prepared in a DNA-chitosan-liposome complex packed with a drug delivery system22 before inoculation. Six-week-old female BALB/c mice (Hubei Medical Laboratory Animal Center, Wuhan, China) were intranasally administered pCCL17/VAX (100 μg), pCCL19/VAX (100 μg) or pCCL17/VAX (50 μg) along with pCCL19/VAX (50 μg). Specifically, 100 μL of complex solution containing 100 μg DNA was deposited into both nostrils of the mice with the aid of a micropipette. All animal experiments in this study were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All animal protocols were reviewed and approved by the review board of Hubei Medical Laboratory Animal Center. The DLNs and spleens were collected 3 d after administration and digested with 0.25% collagenase IV (Gibco, USA) at 37 °C for 1 h with agitation. The cells were then filtered through a nylon sieve. For flow cytometric analysis, the cells were resuspended, labeled with conjugated FITC-anti-mouse CD11c and PE-anti-mouse MHCII (I-Ab α chain) mAbs (Becton Dickinson, Mountain View, CA, USA) for 30 min on ice, and analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA).
Immunization of mice
The DNA vaccine pCIA-P, encoding the A-P region of the pac gene of S mutans, was used as an antigenic plasmid. Six-week-old female BALB/c mice, 5 per group, were intranasally administered 50 μg of pCCL17/VAX plus 50 μg of pVAX1, 50 μg of pCCL19/VAX plus 50 μg of pVAX1, 50 μg of pCCL17/VAX plus 50 μg of pCCL19/VAX, or 100 μg of pVAX1. Twenty four hours later, 100 μg of pCIA-P was delivered into the intranasal cavity of all groups. All mice were boosted at weeks 2 and 4. As mentioned above, all the DNA plasmids were prepared in a DNA-chitosan-liposome complex before immunization and then delivered into the nostrils of the mice using a micropipette. Blood and saliva samples were collected before the first immunization and at 4, 6, 8, 10, 12, and 14 weeks after the first immunization. Peripheral blood was collected from the retro-orbital plexus and maintained at 4 °C overnight. Serum was obtained by centrifugation the next day. Saliva samples were collected after the stimulation of salivary flow by an intraperitoneal injection of 5 μg of pilocarpine (Sigma, St Louis, MO, USA). All samples were kept at -80 °C until use.
Antibody analysis
The levels of specific antibodies against PAc in the serum and saliva of rodents were determined by an enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Corning Costar, Cambridge, MA, USA) were coated with recombinant PAc (10 μg/mL in carbonate buffer, pH 9.6) overnight at 4 °C and incubated with serially diluted serum or salivary samples in blocking buffer (phosphate-buffered saline containing 3% bovine serum albumin and 0.1% Tween 20) followed by incubation with peroxidase-conjugated goat anti-mouse IgG (1:10 000, Pierce Biotechnology, Rockford, IL, USA), peroxidase-conjugated goat anti-mouse IgA (1:500, Pierce Biotechnology), peroxidase-conjugated goat anti-rat IgG (1:10 000, Bethyl Laboratories, Montgomery, TX, USA) or peroxidase-conjugated goat anti-rat IgA (1:500, Bethyl Laboratories). The plates were then incubated with o-phenylenediamine substrate with H2O2 and then stopped with 2 mol/L H2SO4. The optical density at 490 nm (OD490) was recorded. For calculating the serum PAc-specific IgG antibody values, a standard curve was established for each plate, at which point the wells were coated with purified unconjugated goat anti-mouse IgG (Sigma-Aldrich, St Louis, USA) or unconjugated goat anti-rat IgG (Pierce Biotechnology), and mouse reference serum (Bethyl Laboratories) or rat reference serum (Bethyl Laboratories) was added in serial dilutions. The sample antibody concentration was calculated by a computer program based on multi-parameter logistic algorithms and then interpolated on standard curves. For calculating the salivary PAc-specific IgA antibody values, purified unconjugated goat anti-mouse IgA (Sigma-Aldrich, St Louis, USA) or unconjugated goat anti-rat IgA (Bethyl Laboratories) was used to coat the plates to establish the standard curve. Total IgA in the saliva was detected, and the levels of IgA antibody activity in saliva were expressed as the ratio of specific IgA to total IgA levels to normalize for variation in the total Ig content in the samples.
Experimental rat caries model
Four groups of female Wistar rats, 6 per group, were weaned at 18 d of age and bred with a Keyes 2000 cariogenic diet23. Antibiotics (ampicillin, chloramphenicol, and carbenicillin, 1.0 g/kg food) were added to the food from d 20 to d 22 to temporarily suppress the oral flora to facilitate bacterial infection. On d 23, the rats were immunized intranasally with pCCL17/VAX plus pCIA-P, pCCL19/VAX plus pCIA-P, pVAX1 plus pCIA-P, or pCCL17/VAX and pCCL19/VAX plus pCIA-P. All animals were boosted twice at 14-d intervals. From d 25 to d 28, all rats were orally challenged with 2×109 CFU of S mutans Ingbritt using a swab that was pre-soaked with the bacterial solution. On d 79, bacterial samples from molar surfaces were collected with sterile swabs and transferred into 0.5 mL of sterile saline. The samples were then ultrasonically dispersed. The bacterial suspension was diluted in saline at 1:100, and then a 100-μL aliquot was plated on MSB solid medium to examine the S mutans infection. The plates were incubated anaerobically at 37 °C. The numbers of CFUs were counted after 48 h. On d 80, serum and saliva samples were collected for specific anti-PAc IgG and IgA antibody analysis. On d 120, all rats were sacrificed, and the mandibles were removed, defleshed, cleaned and stained with murexide. The teeth were then sectioned, and caries activity was scored by the Keyes method24.
Statistics
SPSS 10.0 software (SPSS, Inc, Chicago, IL, USA) was used to perform the statistical analyses. The differences in the antibody levels, S mutans colonization, and caries scores among the groups were determined by one-way analysis of variance (ANOVA) followed by multiple-mean comparisons using the Student-Newman-Keuls test. A value of P<0.05 was considered significant.
Results
Expression of recombinant proteins in vitro
The recombinant CCL19 and CCL17 proteins in the cultured supernatants of COS-7 cells were detected by ELISA 48 h after transfection. The concentrations of the CCL19 protein and CCL17 proteins in the supernatants collected from pCCL19/VAX- and pCCL17/VAX-transfected cells were 71 500 pg/mL and 69 100 pg/mL, respectively. The CCL19 and CCL17 concentrations in the pVAX1-transfected cell culture supernatants were not detectable.
Enhanced migration of DCs to secondary lymphoid tissues mediated by CCL17 and CCL19
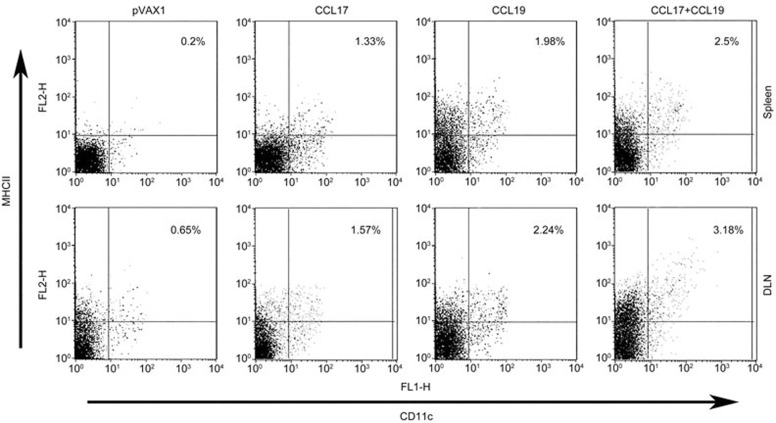
To examine the role of CCL17 and CCL19 in DC migration to secondary lymphoid tissues, the number of mature DCs (CD11c+ major histocompatibility complex class IIhigh) was measured in the spleen and DLNs. All three groups (CCL17, CCL19, and CCL17 plus CCL19) showed increased total cell numbers in the DLNs and spleens compared with the group treated with the control vector (Figure 1). The co-administration of CCL17 and CCL19 had a greater effect in increasing the number of mature DCs in secondary lymphoid tissues than did CCL17 or CCL19 administered alone.
Figure 1.
CCL17 and CCL19 increase the number of mature DCs in secondary lymphoid tissues. Splenocytes and DLN cells were collected from mice treated with pCCL17/VAX, pCCL19/VAX, pCCL17/VAX plus pCCL19/VAX, or pVAX1, digested with collagenase IV 3 days after immunization, stained with a DC marker (CD11c) and a class II marker (I-Ab α chain), and analyzed by flow cytometry. This result is representative of three experiments.
Combined adjuvants enhanced specific anti-PAc antibody responses in mice
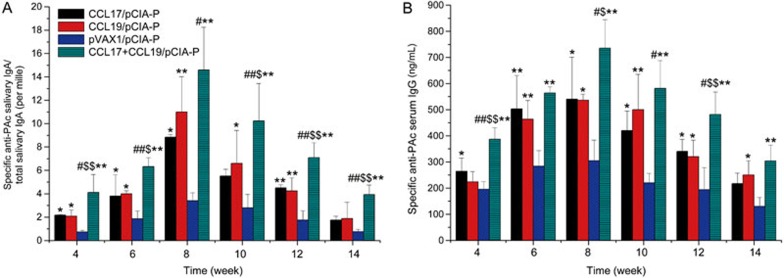
The immune-enhancing effects of CCL17 combined with CCL19 were examined by detecting salivary PAc-specific IgA levels and serum PAc-specific IgG levels after the intranasal delivery of pCIA-P plus pCCL17/VAX, pCIA-P plus pCCL19/VAX, pCIA-P plus pCCL17/VAX and pCCL19/VAX, or pCIA-P plus pVAX1. For salivary PAc-specific IgA levels, the peaks appeared at 8 weeks after the first immunization in all groups (Figure 2A). At each time point, including weeks 4, 6, 8, 10, 12, and 14 after the first immunization, the three experimental groups showed higher levels of salivary anti-PAc IgA antibodies than the pVAX1 control group. The salivary IgA anti-PAc antibody levels in the pCCL17/VAX group were significantly higher than that of the control group at weeks 4, 6, 8, and 12 (P<0.01 or P<0.05). Similar enhanced responses were observed in the pCCL19/VAX group compared with the control group in the entire experimental period except at week 14 (P<0.01 or P<0.05). The pCCL17/VAX and pCCL19/VAX-co-administered group showed a significant difference from the control group at all time points (P<0.01). Comparing the co-administered group with the pCCL17/VAX group, the salivary PAc-specific IgA antibody responses were significantly enhanced at all time points (P<0.01 or P<0.05). When compared with the pCCL19/pVAX group, the co-administered group showed significantly enhanced responses at weeks 4, 6, 10, 12, and 14 (P<0.01 or P<0.05).
Figure 2.
Salivary PAc-specific IgA and serum PAc-specific IgG antibody levels in mice immunized intranasally with pCIA-P in combination with pCCL17/VAX, pCCL19/VAX, pCCL17/VAX plus pCCL19/VAX, or pVAX1. Saliva and serum samples were collected at 4, 6, 8, 10, 12, 14, and 16 weeks after the first immunization. The specific anti-PAc salivary IgA (A) and specific anti-PAc serum IgG (B) concentrations were determined by ELISA. The data are expressed as the mean±SD. n=5. *P<0.05, **P<0.01 vs the pVAX1 group. ##P<0.01 vs the pCCL17/VAX1 group. $P<0.05, $$P<0.01 vs the pCCL19/VAX1 group.
The peak serum PAc-specific IgG levels appeared in week 8 in all groups (Figure 2B). At each time point, the three experimental groups showed higher antibody levels than the control group. In the pCCL17/pVAX group, the serum IgG anti-PAc antibody responses were significantly higher than that of the control group at all time points except week 14 (P<0.01 or P<0.05), while the pCCL19/pVAX group showed a significant difference from the control group at all time points except week 4 (P<0.01 or P<0.05). The pCCL17/VAX and pCCL19/VAX co-administered group showed a significant difference from the control group throughout the entire experimental period (P<0.01). When comparing the co-administered group with the pCCL17/pVAX group, the serum PAc-specific IgG antibody levels were significantly enhanced at weeks 4, 8, 10, and 12 (P<0.01 or P<0.05). When compared with the pCCL19/VAX group, the responses were significantly enhanced at weeks 4, 8, and 12 (P<0.01 or P<0.05).
These data demonstrated that the combination of CCL17 and CCL19 as a nasal adjuvant with pCIA-P markedly enhanced PAc-specific antibody responses in mice.
Anti-caries efficacy in rats
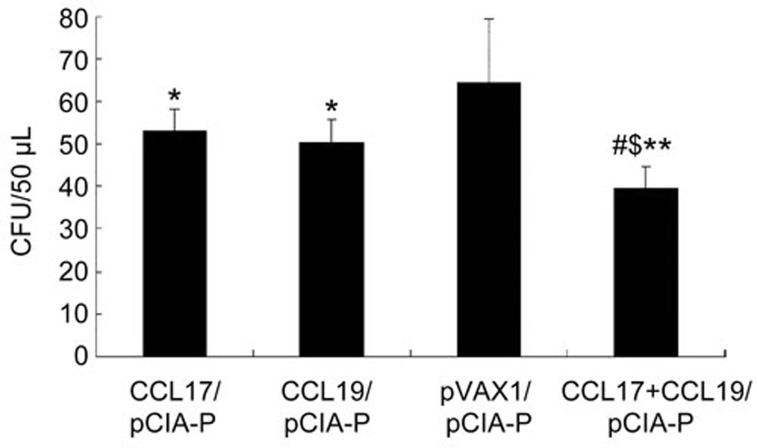
Inhibition of S mutans colonization
We detected the amount of S mutans sustained on the molar surfaces after immunization using a colony forming assay (Figure 3). The colony-forming unit (CFU) counts of rats immunized with pCIA-P together with pCCL17/VAX, pCCL19/VAX, or pCCL17/VAX plus pCCL19/VAX were significantly lower than those of rats immunized with pCIA-P without adjuvant (P<0.05 or P<0.01). The CFU counts were significantly lower in the pCCL17/VAX and pCCL19/VAX co-administered group than in the pCCL17/pVAX group or the pCCL19/pVAX group (P<0.05). These data showed that the combined use of CCL17 and CCL19 strengthened the ability of pCIA-P to inhibit the colonization of S mutans on rat tooth surfaces.
Figure 3.
CFU counts of S mutans detected on the molar surfaces of immunized rats. The data are expressed as the mean±SD. n=5. **P<0.01 vs the pVAX1 group. #P<0.05 vs the pCCL17/VAX1 group. $P<0.05 vs the pCCL19/VAX1 group.
Rat antibody responses
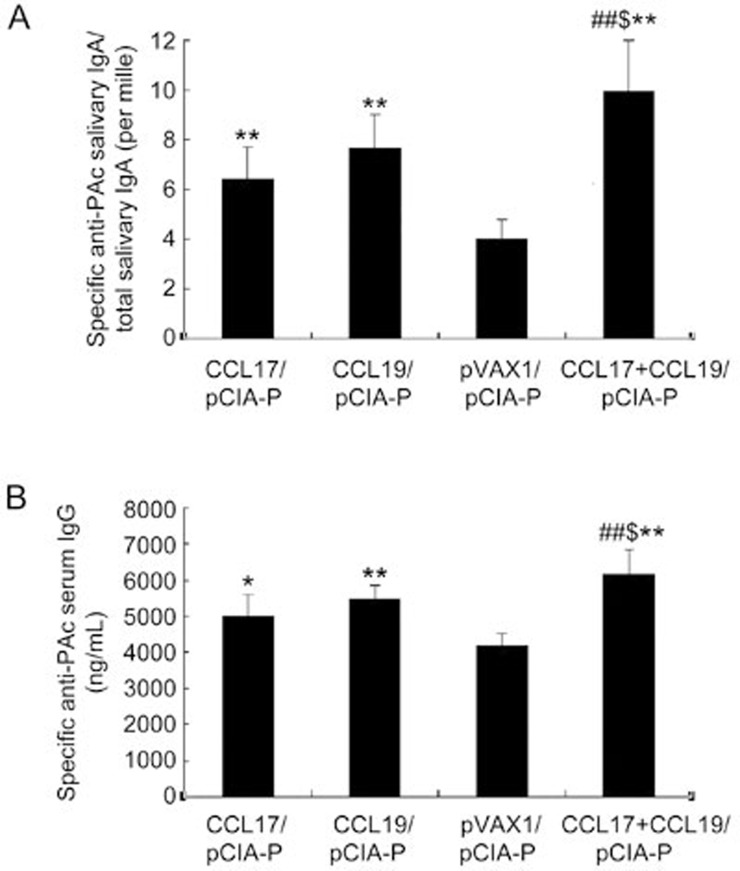
Salivary-specific anti-PAc IgA titers were significantly higher in rats immunized with pCIA-P along with pCCL17/VAX, pCCL19/VAX, or pCCL17/VAX plus pCCL19/VAX compared with those immunized with pCIA-P without adjuvant (P<0.01) (Figure 4A). The salivary-specific anti-PAc IgA titers in the pCCL17/VAX and pCCL19/VAX co-administered group were significantly higher than those of the pCCL17/VAX group or the pCCL19/VAX group (P<0.01 and P<0.05, respectively). The serum-specific anti-PAc IgG titers in the co-administered group were significantly higher than those of the pCCL17/VAX group or the pCCL19/VAX group (P<0.01 and P<0.05, respectively) (Figure 4B). These data demonstrated that the combined use of CCL17 and CCL19 as nasal adjuvants with pCIA-P markedly enhanced PAc-specific antibody responses in rats.
Figure 4.
Salivary PAc-specific IgA and serum PAc-specific IgG antibody levels in rats immunized intranasally with pCIA-P in combination with pCCL17/VAX, pCCL19/VAX, pCCL17/VAX plus pCCL19/VAX, or pVAX1. Saliva and serum samples were collected 52 days after the first immunization. The specific anti-PAc salivary IgA (A) and specific anti-PAc serum IgG (B) concentrations were determined by ELISA. The data are expressed as the mean±SD. n=5. *P<0.05, **P<0.01 vs the pVAX1 group. ##P<0.01 vs the pCCL17/VAX1 group. $P<0.05 vs the pCCL19/VAX1 group.
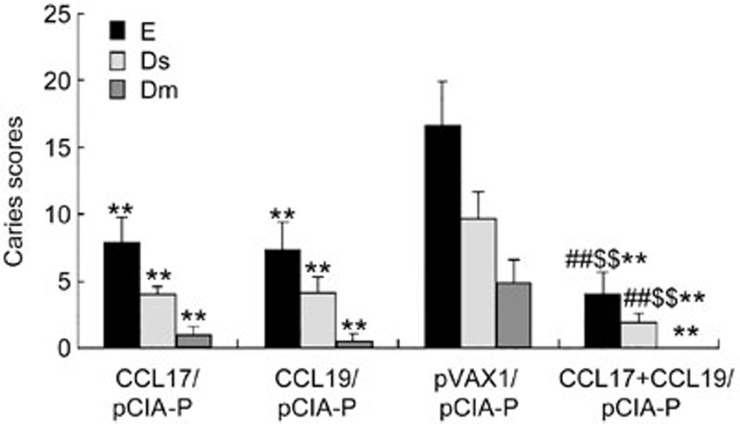
Caries protection against S mutans infection
The degree of caries was scored as enamel lesions (E), slight dentinal lesions (Ds) and moderate dentinal lesions (Dm) (Figure 5). Significantly fewer E, Ds and Dm caries lesions were observed in the pCCL17/VAX, pCCL19/VAX, and pCCL17/VAX plus pCCL19/VAX groups compared with the pVAX1 group (P<0.01). The E and Ds scores of the combined adjuvant group were significantly lower than those of the pCCL17/VAX or pCCL19/VAX groups (P<0.01). No significant difference in Dm scores was observed among the pCCL17/VAX, pCCL19/VAX, and combined adjuvant groups (P>0.05).
Figure 5.
Caries scores of rats immunized intranasally with pCIA-P in combination with pCCL17/VAX, pCCL19/VAX, pCCL17/VAX plus pCCL19/VAX, or pVAX1. **P<0.01 vs the pVAX1 group. ##P<0.01 vs the pCCL17/VAX1 group. $$P<0.01 vs the pCCL19/VAX1 group.
Discussion
Several studies have demonstrated that mature DCs failed to migrate into the DLNs in the absence of CCR7 in mice14,25,26. The data derived from experiments on CCR7-deficient mice showed severely delayed kinetics of the antibody responses against a T-dependent antigen and a lack of contact sensitivity and delayed-type hypersensitivity reactions14. CCL19, which is secreted by stromal cells in the T-cell area of DLNs, is a high-affinity functional ligand for CCR7. Our previous findings showed that CCL19 is a potent chemoattractant for in vitro-expanded bone marrow-derived DCs, and the specificity was confirmed by CCR7 blockade of mature DCs20. In this study, we showed that exogenous overexpression of CCL19 enhanced the recruitment of DCs to DLNs. Taken together, these results indicate that CCL19 is required for the CCR7-dependent migration of DCs.
Recent studies showed that the expression of CCR7 on the DC surface alone was not sufficient to guarantee its full function of guiding DC migration21. CCL17-deficient DCs, which express normal levels of CCR7, failed to undergo efficient directional migration in response to the CCR7 ligand CCL19. Moreover, CCL19-induced Ca2+ flux was strongly reduced in CCL17-deficient DCs. These results indicate that CCL17 deficiency does not affect the surface expression of CCR7, but rather, alters its functionality. Here, our findings showed that the exogenous overexpression of CCL17 alone could enhance the recruitment of DCs to DLNs, which demonstrates in vivo that CCL17 is involved in the process of DC migration. CCL17 was identified as a specific functional ligand for CCR4, a receptor that is selectively expressed on certain immune cells27,28. However, CCR4 knockout mice showed no defect in cutaneous DC migration and no amelioration of atopic dermatitis pathology, suggesting that CCL17 must bind to another receptor in addition to CCR421. Although the molecular mechanisms by which CCL17 acts on CCR7 remain enigmatic, our results indicated that the co-delivery of CCL17 and CCL19 led to enhanced DC migration to DLNs compared with CCL17 or CCL19 delivered alone.
Regimens using chemokines as adjuvants for DNA vaccination have been shown to act through the recruitment of DCs and other immune cells to immune inductive sites29,30,31. Our previous studies showed that a CCL19 DNA plasmid entered the secondary lymphoid tissues and expressed the chemokine there following intramuscular administration. Using a CCL19 plasmid in combination with the anti-caries DNA vaccine pCIA-P, an increased serum PAc-specific IgG level was observed20. To date, by virtue of its paramount importance in diverse effects on DC and lymphocyte migration and immune response formation, it has been postulated that CCL19 is an interesting candidate vaccine adjuvant for a number of infectious diseases19,32,33. However, few studies have investigated the adjuvant role of CCL17. Based on the role of CCL17 in the CCR7-dependent recruitment of DCs, we speculated that CCL17 had an immunomodulatory effect on the DNA vaccine pCIA-P. In accordance with this idea, pCIA-P and the CCL17 plasmid were intranasally administered to mice. We observed significantly increased serum PAc-specific IgG levels and salivary PAc-specific IgA levels compared with the vector DNA-treated control group. Moreover, the co-delivery of CCL17 and CCL19 with pCIA-P led to enhanced antibody responses compared with CCL17 or CCL19 delivered alone at most time points after immunization. Consistent with the findings of the augmented salivary PAc-specific IgA antibody levels, S mutans colonization on the tooth surfaces and caries scores were reduced in CCL17 and CCL19 co-administered rats compared with CCL17- or CCL19-administered rats. These results confirmed the immunopotentiator effect of CCL17 when co-administered with CCL19 and the anti-caries DNA vaccine. To our knowledge, this report is the first study using CCL17 as an adjuvant to enhance the immunogenicity of DNA vaccines.
The immunomodulatory mechanism by which CCL17 acts as a vaccine adjuvant remains unknown. This mechanism is not likely due to the interaction through its receptor, CCR4, because CCR4 KO mice developed allergic skin inflammation similar to WT mice in an atopic dermatitis mouse model21. Another possibility for enhancing Th1- and Th2-type cytokine production by CD4+ Th cells can be excluded because neither IFN-γ nor IL-4 cytokine levels were significantly higher in the splenocytes of mice that received the CCL17 plasmid and pCIA-P compared with the vector- and pCIA-P-immunized groups (data not shown here).
In conclusion, our present study clearly showed that the combination of CCL17 and CCL19 as a nasal adjuvant attracted more mature DCs to secondary lymphoid tissues to induce enhanced PAc-specific antibody responses against the anti-caries DNA vaccine pCIA-P and reduced S mutans infection in rodents. The mechanisms by which CCL17 acts as an immunopotentiator remain to be further elucidated.
Author contribution
Qing-an XU designed the study and revised the manuscript; Yan-hong YAN, Li-hua CAO, Zhou ZHANG, and Fei YU performed the study; and Fei YU and Chang ZENG wrote the manuscript.
Acknowledgments
This study was financially supported by grants from the National Natural Science Foundation of China (No 81570968 and 81271129).
References
- Fan MW, Bian Z, Peng ZX, Zhong Y, Chen Z, Peng B, et al. A DNA vaccine encoding a cell-surface protein antigen of Streptococcus mutans protects gnotobiotic rats from caries. J Dent Res 2002; 81: 784–7. [DOI] [PubMed] [Google Scholar]
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol 2001; 75: 4040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver KA, LaPatra SE, Kurath G. Efficacy of an infectious hematopoietic necrosis (IHN) virus DNA vaccine in Chinook Oncorhynchus tshawytscha and sockeye O. nerka salmon. Dis Aquat Organ 2005; 64: 13–22. [DOI] [PubMed] [Google Scholar]
- Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 2006; 24: 4582–5. [DOI] [PubMed] [Google Scholar]
- Draghia-Akli R, Ellis KM, Hill LA, Malone PB, Fiorotto ML. High-efficiency growth hormone-releasing hormone plasmid vector administration into skeletal muscle mediated by electroporation in pigs. FASEB J 2003; 17: 526–8. [DOI] [PubMed] [Google Scholar]
- Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD Jr. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med 1996; 2: 1122–8. [DOI] [PubMed] [Google Scholar]
- Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol 2000; 165: 2850–8. [DOI] [PubMed] [Google Scholar]
- Kichaev G, Mendoza JM, Amante D, Smith TR, McCoy JR, Sardesai NY, et al. Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Hum Vaccin Immunother 2013; 9: 2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet 2008; 9: 776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med 1999; 189: 611–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 2004; 21: 279–88. [DOI] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8: 362–71. [DOI] [PubMed] [Google Scholar]
- Liu C, Lin J, Zhao L, Yang Y, Gao F, Li B, et al. Gamma-ray irradiation impairs dendritic cell migration to CCL19 by down-regulation of CCR7 and induction of cell apoptosis. Int J Biol Sci 2011; 7: 168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev 2000; 177: 134–40. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999; 189: 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo SK, Lee S, Kumaraguru U, Rouse BT. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine 2001; 19: 4685–93. [DOI] [PubMed] [Google Scholar]
- Westermann J, Nguyen-Hoai T, Baldenhofer G, Höpken UE, Lipp M, Dörken B, et al. CCL19 (ELC) as an adjuvant for DNA vaccination: induction of a TH1-type T-cell response and enhancement of antitumor immunity. Cancer Gene Ther 2007; 14: 523–32. [DOI] [PubMed] [Google Scholar]
- Hartoonian C, Sepehrizadeh Z, Tabatabai Yazdi M, Jang YS, Langroudi L, Amir Kalvanagh P, et al. Enhancement of immune responses by co-delivery of CCL19/MIP-3beta chemokine plasmid with HCV core DNA/protein immunization. Hepat Mon 2014; 14: e14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YH, Qi SC, Su LK, Xu QA, Fan MW. Co-delivery of ccl19 gene enhances anti-caries DNA vaccine pCIA-P immunogenicity in mice by increasing dendritic cell migration to secondary lymphoid tissues. Acta Pharmacol Sin 2013; 34: 432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutte S, Quast T, Gerbitzki N, Savinko T, Novak N, Reifenberger J, et al. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc Natl Acad Sci U S A 2010; 107: 8736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhu J, Li Y, Lu J, Gao L, Xu H, et al. Enhanced nasal mucosal delivery and immunogenicity of anti-caries DNA vaccine through incorporation of anionic liposomes in chitosan/DNA complexes. PLoS One 2013; 8: e71953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia JM. Animal models in dental research. Tuscaloosa: University of Alabama Press; 1977. p 280.
- Keyes PH. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res 1958; 37: 1088–99. [DOI] [PubMed] [Google Scholar]
- Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, et al. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol 2006; 177: 7346–54. [DOI] [PubMed] [Google Scholar]
- Junt T, Scandella E, Förster R, Krebs P, Krautwald S, Lipp M, et al. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol 2004; 173: 6684–93. [DOI] [PubMed] [Google Scholar]
- Bonner K, Pease JE, Corrigan CJ, Clark P, Kay AB. CCL17/thymus and activation-regulated chemokine induces calcitonin gene-related peptide in human airway epithelial cells through CCR4. J Allergy Clin Immunol 2013; 132: 942–50. [DOI] [PubMed] [Google Scholar]
- Vijayanand P, Durkin K, Hartmann G, Morjaria J, Seumois G, Staples KJ, et al. Chemokine receptor 4 plays a key role in T cell recruitment into the airways of asthmatic patients. J Immunol 2010; 184: 4568–74. [DOI] [PubMed] [Google Scholar]
- Luo K, Zhang H, Zavala F, Biragyn A, Espinosa DA, Markham RB. Fusion of antigen to a dendritic cell targeting chemokine combined with adjuvant yields a malaria DNA vaccine with enhanced protective capabilities. PLoS One 2014; 9: e90413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartoonian C, Sepehrizadeh Z, Mahdavi M, Arashkia A, Jang YS, Ebtekar M, et al. Modulation of hepatitis C virus core DNA vaccine immune responses by co-immunization with CC-chemokine ligand 20 (CCL20) gene as immunoadjuvant. Mol Biol Rep 2014; 41: 5943–52. [DOI] [PubMed] [Google Scholar]
- Nguyen-Hoai T, Hohn O, Vu MD, Baldenhofer G, Sayed Ahmed MS, Dörken, B, et al. CCL19 as an adjuvant for intradermal gene gun immunization in a Her2/neu mouse tumor model: improved vaccine efficacy and a role for B cells as APC. Cancer Gene Ther 2012; 19: 880–7. [DOI] [PubMed] [Google Scholar]
- Toka FN, Gierynska M, Rouse BT. Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J Virol 2003; 77: 12742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez V, Pasolli HA, Hellwig A, Garbi N, Arregui AC. Virus-like particles harboring CCL19, IL-2 and HPV16 E7 elicit protective T cell responses in HLA-A2 transgenic mice. Open Virol J 2012; 6: 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]