Abstract
Background
Postmortem studies of people who have successfully committed suicide and people with depression have implicated the serotonin-1A (5-HT1A) receptor system in the pathophysiology of depression. Several molecular imaging studies have investigated alterations in 5-HT1A receptors in patients with depression using positron emission tomography and have reported conflicting results.
Methods
We performed a meta-analysis of studies investigating the relationship between depression and 5-HT1A binding. We conducted a comprehensive search of Medline, Embase, ScienceDirect, Scopus and Springer databases for relevant studies published between January 1999 and October 2015. The meta-analysis was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines.
Results
Ten studies were included, comprising 218 patients with depression and 261 healthy controls. The results of these studies indicated a reduction in 5-HT1A receptors in mesiotemporal cortex, yielding a summary effect estimate of -0.8 (95 % CI −1.36, −0.24). Smaller reductions were reported in 5-HT1A receptor binding in the hippocampus, raphe nuclei, insular, anterior cingulate cortex and occipital cortex of people with depression. No clear effect of depression on 5-HT1A receptors was detected in the amygdala.
Conclusions
Reduced 5-HT1A receptor binding was associated with the pathology of depression and predicted altered serotonergic neurotransmission in various brain regions. These findings increase our understanding of the neurophysiological processes underlying depression.
Electronic supplementary material
The online version of this article (doi:10.1186/s12888-016-1025-0) contains supplementary material, which is available to authorized users.
Keywords: Meta-analysis, 5-HT1A, Molecular imaging, Depression
Background
Depression is a chronic mental illness characterized by depressed mood, anhedonia, irritability, concentration difficulties, and abnormalities in appetite and sleep. It has a lifetime prevalence of 10–15 % [21]. The classic biogenic amine hypothesis of depression suggests that the disorder is associated with a deficiency in several neurotransmitters, including serotonin (5-hydroxytryptamine, 5-HT), noradrenaline (NA), and acetylcholine (ACh) [3]. There is increasing evidence that alterations in the brain serotonergic system are involved in the pathophysiology of depression [13, 42]. It has been suggested that 5-HT receptor dysfunction might contribute significantly to the development of depression.
5-HT receptors are highly expressed in the human limbic system, including the amygdala, hippocampus, thalamus, putamen, anterior cingulate cortex and midbrain [37]. Among the 5-HT receptor types (5-HT1A, 5-HT1B, 5-HT2A, and 5-HT4), 5-HT1A has generated much research interest because of its involvement in recognition, learning memory, and hippocampal neurogenesis, as well as its response to antidepressant treatment [15, 36, 41]. Furthermore, 5-HT1A dysfunction often accompanies depression. Extensive rodent and human research suggests that 5-HT1A dysregulation is highly sensitive to stress, i.e., increased cortisol levels [9, 25].
A previous meta-analysis of molecular imaging studies of depression reported a relationship between the disorder and serotonin transporters, which indicated altered serotonergic availability in depression [14]. Recent functional neuroimaging has pointed to widespread abnormalities in 5-HT1A binding in depression. Since 1991, abnormalities in 5-HT1A binding in patients with depression have been investigated using positron emission tomography (PET) techniques under various scanning conditions [1]. Several such studies have reported decreased 5-HT1A binding in patients with depression compared with healthy controls [34, 38]. However, other studies have reported conflicting results or insufficient evidence to confirm any relationship [20, 22, 23, 31].
While converging lines of evidence indicate that 5-HT1A may contribute to the pathophysiology of depression, there is no consensus about the way 5-HT1A binding is altered in the condition. To address this contention, we performed a meta-analysis of studies investigating the relationship between depression and 5-HT1A binding. We hypothesized that at least some of the discrepancies between studies would be explained by our meta-analysis.
Methods
To ensure the quality of this meta-analysis, we followed the proposal for conducting and reporting described in ‘Meta-analysis of Observational Studies in Epidemiology (MOOSE)’ (Stroup DF 2000 [47]). The MOOSE checklist is included in the Additional file 1.
Search strategy
Two reviewers (LW and CjZ), one postgraduate student and one doctoral student, systematically searched Medline, Embase, ScienceDirect, Scopus, and Springer databases to identify relevant manuscripts published between January 1999 and October 2015. Databases were accessed via PubMed or directly via their website.
We used subject and free-form search terms as follows (the PubMed search string is provided as an example):
#1 depression [MeSH Terms] OR depress* OR bipolar disorder OR affective disorders, psychotic OR major depression;
#2 positron-Emission tomography [MeSH Terms] OR pet OR tomography, emission-computed, single-photon OR SPECT OR molecular imaging OR molecular diagno*;
#3 receptor, serotonin, 5-HT1A[MeSH Terms] OR serotonin 1A receptor;
#4 #1 AND #2 AND #3.
Study selection and data extraction
The inclusion criteria were: (1) original studies that indexed 5-HT1A receptors in patients with depression and healthy controls; (2) molecular imaging studies published in English. We excluded: (1) subjects with neurological, severe somatic, psychotic, chronic stress or affective disorders other than unipolar depression or bipolar depression; (2) reviews, comments, and case reports; (3) overlapping or duplicated samples; (4) subjects with depression accompanied by neuropsychiatric or physical diseases; and (5) studies that included relatives with significant symptoms. Where several contrasts or tasks were present in the same study, we chose one task to avoid including the sample twice.
The binding potential (BP) value of [11C]WAY-100635 was used as the primary outcome for the analysis. This radioligand binds specifically to 5-HT1A and is widely used in PET studies to measure 5-HT1A occupancy and density in psychiatric patients [12]. Secondary outcomes (listed by relevance to clinical and population characteristics) included: diagnosis, symptoms severity, diagnosis criteria for psychiatric diagnosis, rating tools, antidepressant treatment, study and patient characteristics, measurements performed, year of publication, population characteristics, and type of tracer. Two reviewers (LW and CjZ) extracted all data independently. Any disagreement between reviewers was resolved by discussion.
Statistical methods
Statistical analyses were performed using RevMan version 5.0.1 (The Cochrane Collaboration The Nordic Cochrane Centre, Copenhagen, Denmark). The BP value in given regions was weighted by standard mean differences (SMD) with a 95 % confidence interval (CI) for each individual study. We used Q-tests with a significance threshold of p < 0.05 (two-tailed) to evaluate the SMD.
We assessed heterogeneity using a chi-squared Q-statistic, and its magnitude was estimated using the inconsistency index I2. I2 indicates the percentage of effect size variance due to heterogeneity: I2 = 100 % × (Q-df)/Q [16]. If between-study variance clearly made the assessment of heterogeneity significant (p < 0.05), a random-effects model was used to estimate the effects of major depressive disorder (MDD) on 5-HT1A expression.
Publication bias is the tendency of small studies to report large effect sizes. We assessed this parameter using Begg’s funnel plots. The presence of bias was indicated by asymmetrical plots. In addition, we conducted a sensitivity analysis by excluding studies that might influence pooled SMD.
Results
Search results
We identified 408 potentially relevant studies, which were managed in Endnote X7 (Thomas Reuters Scientific, Philadelphia, PA, USA). After scanning each title and abstract, 355 studies were excluded. Of the 53 remaining articles, 43 were further excluded: seven articles were systematic reviews; four articles did not report 5-HT1A binding measures; 15 articles were not controlled clinical trials; ten articles included patients with depression accompanied by neuropsychiatric or physical diseases, or chronic stress or affective disorders other than unipolar depression or bipolar depression; two articles did not supply adequate data; and five articles were molecular imaging studies that likely overlapped with another included study. Finally, ten studies remained for inclusion in the meta-analysis ([4, 11, 18, 28, 32, 33, 35, 39, 43, 48]) (see Fig. 1). Three of the included studies (marked with a “Δ” in Table 1) did not provide quantitative data, and data were only available in a graphical format. For these studies, where we could not obtain primary data by contacting the author, we used GetData Graph Digitizer version 2.2 (GetData, Moscow, Russia) to analyze the graphical data.
Fig. 1.
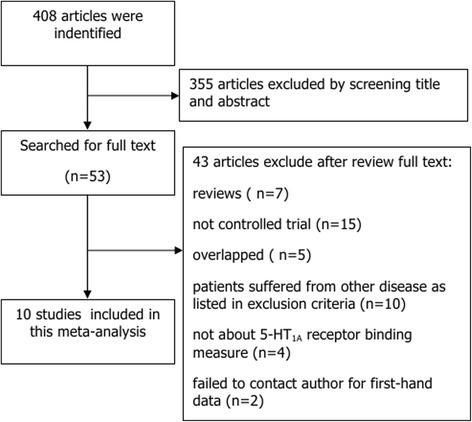
Flowchart of identification, screening and inclusion of eligible studies
Table 1.
Key characteristics of selected studies
| 1st author | Publication year | Method/Ligand | Reference region | Diagnosis | Severity ranting tools | Severity score | Controls (F) + age | Patients (F) + age | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Wayne C. Drevets | 1999 | PET [11C]WAY-100635 | Cerebellum | MDD, BPII | HAM-17 | 22 ± 6.4 | 8 (4) 35.3 ± 13.5 | 12 (7) 35.8 ± 9.7 | Drug free ≥2 week |
| Sargent P. Aa. | 2000 | PET [11C]WAY-100635 | Cerebellum | MDD, BPII | HAM-17 | 14.1 ± 194.9 | 18 (1) 36.4 ± 8.3 | 35 (3) 40 ± 34.5 | Drug free ≥12 |
| Z Bhagwagar | 2004 | PET [11C]WAY-100635 | Cerebellum | MDD | HAM-7 | 2.3 ± 1 | 18 (0) 43.2 ± 13 | 14 (0) 48 ± 14.9 | Drug free ≥25.7 week |
| Carolyn Cidis Meltzer | 2004 | PET [11C]WAY-100635 | Plasma | MDD | HAM-17 | 18.1 ± 2.7 | 17 (9) 70 ± 6.7 | 17 (13) 71.4 ± 5.9 | Drug free ≥2 week |
| Jussi Hirvonen△ | 2008 | PET [11C]WAY-100635 | Plasma | MDD | HAM-17 | 18.1 ± 2.9 | 15 (8) 32.6 ± 7.7 | 21 (8) 40.1 ± 9 | Drug free ≥17.1 week |
| Eydie L. Moses-kolko | 2008 | PET [11C]WAY-100635 | Cerebellum | Postpartum D. | HAM-17 | 21 ± 4.3 | 7 (7) 33 ± 3.9 | 9 (9) 26.9 ± 7.9 | 7 patients rdrug naïve |
| Ramin V. Parsey a△ | 2006b | PET [11C]WAY-100635 | Cerebellum | MDD | HAM-17 | 25.7 ± 7.08 | 43 (24) 38.2 ± 15 | 28 (7) 38.5 ± 29.3 | Drug free ≥2 week |
| Gregory M. Sullivan△ | 2009 | PET [11C]WAY-100635 | Cerebellar white matter | BPII | HAM-17 | 18 ± 4.9 | 47 (27) 38.1 ± 14.7 | 32 (19) 38.4 ± 9.7 | Drug free ≥2 week |
| Jeffrey Millera△ | 2012 | PET [11C]WAY-100635 | Cerebellum white matter | MDD | HAM-17 | 24.6 ± 5.3 | 51 (29) 37.3 ± 14.4 | 24 (17) 35 ± 13.3 | Drug free ≥3 |
| Allison C. Nugent | 2013a | PET [11C]WAY-100635 | Cerebellar white matte | BPII | MADRS | 23 ± 10 | 33 ± 9.4 | 26 (19) 33 ± 9.5 | Drug free ≥3 week |
MDD major depressive disorder, BP bipolar depression, PET positron emission tomography, HAMD Hamilton depression scale, MADRS Montgomery and Asberg depression rating scale
aData from subgroups were combined and averaged (severity score, age, BP values)
ΔBP values were acquired from figures by graphical software (GetData Graph Digitizer v2.2)
Table 1 shows data extracted from the ten included articles, comprising 218 patients with depression and 261 healthy controls. The most frequently studied brain regions in studies using PET to measure 5-HT1A binding were the limbic system (cingulate cortex, amygdala, hippocampus/parahippocampal), cortical regions (occipital cortex, temporal cortex, prefrontal cortex), raphe nuclei, and mesiotemporal cortex. In this meta-analysis, to ensure reliability of findings, regions were included if they were reported in a minimum of four studies. If sub-regions within a structure (e.g. left mesiotemporal cortex and right mesiotemporal cortex) were reported, the corresponding 5-HT1A BP values were combined and averaged, according to the Cochrane Handbook for Systematic Reviews of Interventions version 5.0 [17].
Meta-analysis of 5-HT1A binding in depression
This meta-analysis focused on differences in 5-HT1A binding between patients with depression and healthy controls in six reported regions: hippocampus (HIP), mesiotemporal cortex (MTC), anterior cingulate cortex (ACN), occipital cortex (OCC), raphe nucleus (RN), and insular cortex (INS).
Ten included studies investigated 5-HT1A binding in MTC. The analysis yielded significantly lower binding in people with depression, with an effect estimate of −0.8 (95 % CI −1.36, −0.24). Additionally, a moderate reduction of 5-HT1A binding in people with depression relative to healthy controls was detected in HIP: −0.29 (95 % CI −0.51, −0.07), CAN: −0.57 (95 % CI −1.24, −0.09), OCC: −0.35 (95 % CI −0.96, −0.04), RN: −0.60 (95 % CI −1.17, −0.04) and INS: −0.79 (95 % CI −0.54, −0.05) (Fig. 2).
Fig. 2.
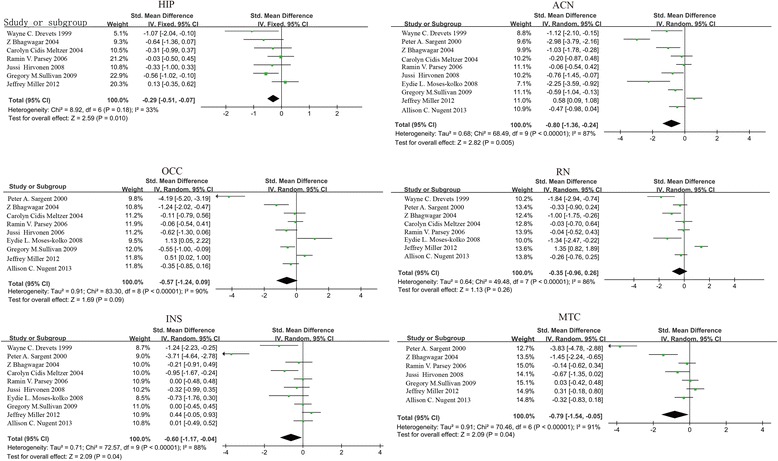
Forest plots showing the summary effect sizes of 5-HT1A binding in depression. HIP: hippocampus; MTC: mesiotemporal cortex; CAN: anterior cingulate cortex; OCC: occipital cortex; RN: raphe nucleus; INS: insular cortex
In some studies, differences in 5-HT1A binding were reported between patients with and without remission. Therefore, we assumed that the severity of depressive symptoms might influence 5-HT1A availability. However, no relationship was found between depressive symptom severity and 5-HT1A availability following subgroup analysis in the six regions mentioned above.
Sensitivity analysis
We hypothesized that depressive symptom severity, small sample size (each arm containing fewer than 15 patients), and the chosen reference tissue might influence 5-HT1A availability measures (Additional files 1, 2 and 3). To test this hypothesis, we performed several sensitivity analyses by deleting studies with each factor respectively to assess which factors influenced the results.
The results indicated that overall significance of the SMD was altered after exclusion of two studies with small sample sizes and one study with conflicting results [32, 33, 43]. The results following exclusion of these studies were as follows: MTC: −0.49 (95 % CI −0.71, −0.27), p = 0.0001 for Q test, I2 = 27; HIP: −0.40 (95 % CI −0.64, −0.19), p = 0.002 for Q test, I2 = 5; CAN: −0.41 (95 % CI −0.64, −0.19), p = 0.0004 for Q test, I2 = 36; OCC: −0.50 (95 % CI −1.02, −0.02), p = 0.01 for Q test, I2 = 68; RN: −0.21 (95 % CI −0.43, −0.02), p = 0.07 for Q test, I2 = 44; and INS: −0.43 (95 % CI −0.86, −0.00), p = 0.009 for Q test, I2 = 66. These changes suggest that the findings of the meta-analysis were strongly influenced by these three studies.
Publication bias
A funnel plot was generated to explore publication bias (Fig. 3). The results indicated that some publication bias was present in the analyses of MTC, CAN, INS, OCC, and RN. Publication bias may have been due to the limited number (n ≤ 10) of studies we included. There was no evidence for publication bias in HIP.
Fig. 3.
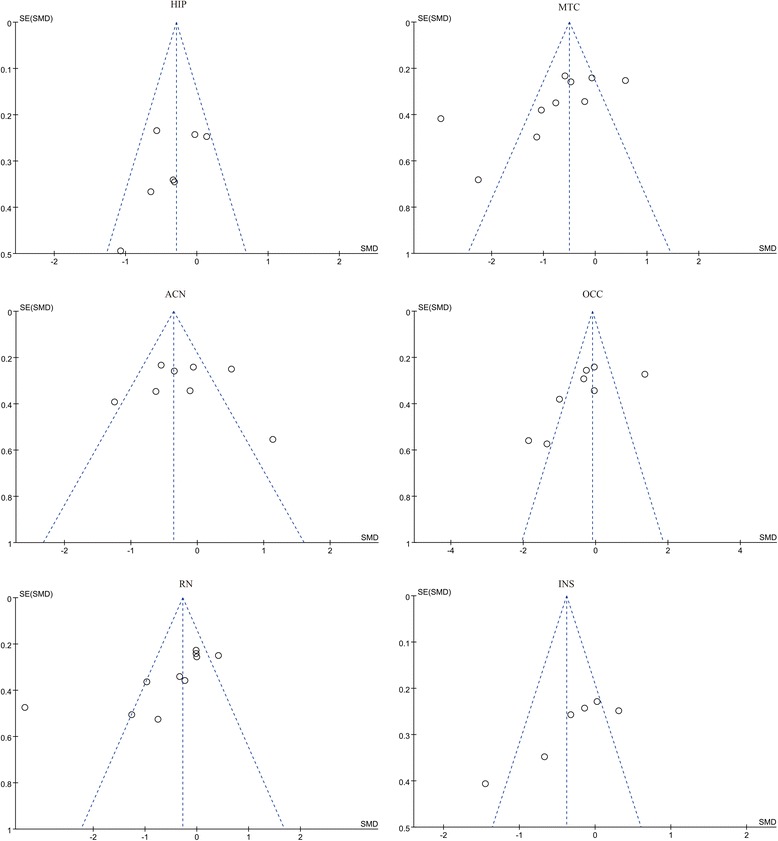
Funnel plot analyzing publication bias for the summary effect sizes of 5-HT1A binding in depression. HIP: hippocampus; MTC: mesiotemporal cortex; CAN: anterior cingulate cortex; OCC: occipital cortex; RN: raphe nucleus; INS: insular cortex
Discussion
5-HT1A changes in depression
Our literature search yielded ten published studies of 5-HT1A receptor binding in depression, comprising 218 patients with depression and 261 healthy controls. We found a mean group size of 22 patients and 26 controls per imaging study. Our meta-analysis indicated significantly decreased 5-HT1A density in MTC, and smaller reductions in 5-HT1A binding in RN, INS, HIP, CAN and OCC in patients with depression. The different PET imaging methods employed in each study to measure 5-HT1A binding in high-affinity regions (i.e., RN, INS, and OCC) may have contributed to high inter-study variability for these particular regions.
Some previous studies have reported that 5-HT1A alterations in depression are influenced by antidepressant medication, and remission and recovery status. In the 10 studies we included, patients were prescribed antidepressants prior to study enrollment, but details of drug doses and types were not reported. It was therefore difficult to investigate whether antidepressant medication affected 5-HT1A availability during treatment in our meta-analysis. We hypothesized that 5-HT1A binding would be associated with depressive symptom severity. However, no relationship was found between the severity of depressive symptoms and 5-HT1A availability in patients with depression.
Reduced 5-HT1A binding visualized with PET may reflect changes in receptor density or affinity. Alternatively, such reductions may reflect receptor down-regulation, inter-activation, or blockage by endogenous ligands (although [11C] WAY-100635 appears to be insensitive to endogenous levels of serotonin; [6]). In the current meta-analysis, we found significant reductions in 5-HT1A in MTC (including amygdala). This finding is consistent with previous postmortem studies indicating lower 5-HT1A binding and mRNA expression in MDD and bipolar disorder [24, 26, 27]. Postsynaptic 5-HT1A receptors exist in cortical inter-neurons and pyramidal dendrites. These receptors participate in feedback inhibition of 5-HT neuronal activity and the modulation of cortical circuits [37]. Decreased 5-HT1A binding may act as a compensating factor to improve postsynaptic serotonin reuptake during a depressive episode. We speculate that decreased binding in the MTC of people with depression may impair the integration of 5-HT signaling in cortico-mesiotemporal cortical circuits and disrupt limbic input back to the cortex [2, 40]. Thus, the reduction of 5-HT1A binding in depression found in our meta-analysis may represent mesiotemporal 5-HT1A-mediated dysregulation of cortical and limbic structures.
We detected smaller differences in 5-HT1A binding between depression and healthy controls in HIP, RN, CAN, and OCC. However, sensitivity analyses revealed stronger trends toward reduced 5-HT1A binding. The HIP has previously been shown to be structurally and functionally altered during depression [8, 29, 30]. However, the data regarding 5-HT1A binding in OCC and RN merit special attention for several reasons. First, there is evidence that the RN contains many serotonergic cell bodies that regulate 5-HT release and uptake. Collin et al. [10] found that the level of expression of 5-HT transporter mRNA was down-regulated in the rodent dorsal raphe nucleus in obese behaviorally depressed ob/ob mice. Decreased 5-HT1A autoreceptor binding was also found in the dorsal raphe nucleus of people with depression who successfully committed suicide [7]. Moreover, several studies have indicated that the OCC is involved in depression, and γ-aminobutyric acid (GABA) concentrations in this region have been shown to be functionally altered in people with depression [5, 44]. These data support the hypothesis that depression is associated with reduced presynaptic serotonergic activity, which leads to the down-regulation of postsynaptic 5-HT1A sites. The results of the current meta-analysis suggest that further imaging and animal studies are warranted to investigate the specific role of 5-HT1A and serotonergic dysfunction in the OCC and RN of patients with depression.
Study heterogeneity
Study heterogeneity occurs when multiple studies investigating a particular effect are actually measuring different effects. This may be caused by differences in samples, interventions, statistical analyses, or study designs, and may cause unreliability in meta-analyses. In the current study, several factors may have caused high heterogeneity, including the severity of depressive symptoms, small sample sizes, and the chosen reference tissue for normalization in PET. Differences in PET scanning protocols were also considered to be potentially important sources of variation between measurements [45]. Our results indicate that considerably more data are required for regions with high 5-HT1A abundance. Moreover, we found that the regions chosen for normalization of specific binding to the radioactivity concentration in a reference region differed between studies (plasma, cerebellum gray matter, cerebellum white matter, whole cerebellum). These limitations, in combination with the measurement error and substantial biological variability in 5-HT1A, may have caused instability in group differences, reflecting an inherently problematic aspect of data acquisition using PET. These findings highlight the ways that specific technical differences in data collection and analysis can produce conflicting or inconsistent results. The development of a gold standard for arterial blood with larger sample sizes in PET studies may provide a solution that enables definitive conclusions about 5-HT1A density in depression.
The association between the C(-1019)G polymorphism in the promoter region of the 5-HT1A gene and depression may be another relevant factor. There is evidence that higher expression of the G allele may increase the risk of developing depression [19, 46, 49]. Therefore, genetic variability may contribute to heterogeneity in studies of 5-HT1A receptors in depression. It is possible that the inter-study heterogeneity in SMD in the current results was caused by genetic variation in 5-HT1A receptors. However, we did not consider genetic variation in our meta-analysis.
Study limitations
Several limitations should be considered in the interpretation of our findings. First, the number of published studies included was too small to exclude small study bias (i.e., smaller studies contributing to larger effect sizes). This finding indicates that future clinical molecular imaging studies should include larger sample sizes. Second, some brain regions have been associated with altered 5-HT1A binding but were not included in the current analysis because of an insufficient number of studies reporting data for these regions. Third, we suspect that heterogeneity may result from genetic variation, as well as differences in gender, depression severity, and treatment. However, we did not analyze these factors, and did not obtain sufficiently detailed data to allow such an analysis. Finally, we detected publication bias in this meta-analysis, possibly resulting from the small number of studies included.
Conclusions
To our knowledge, this is the first meta-analysis of molecular imaging studies of 5-HT1A binding in depression. It has been widely assumed that depression is associated with changes in the 5-HT system. However, consistent evidence from molecular imaging studies is limited. To resolve this uncertainty, we performed a systematic review and meta-analysis, which yielded ten molecular imaging studies of depression. Our meta-analysis showed a decrease in 5-HT1A binding in MTC associated with depression. Smaller reductions in 5-HT1A binding in HIP, RN, CAN, OCC, and INS of patients with depression were also found.
We conclude that individual molecular imaging studies have lacked sufficient statistical power to detect serotonergic dysfunctions in depression. Furthermore, potentially relevant factors, such as sample size, scanning protocol, and genetic polymorphisms, should be considered in future PET studies to further elucidate the pathophysiological mechanisms underlying depression.
Acknowledgements
Non applicable.
Funding
This study was supported by the National Basic Research Program of China (973 Program, 2009CB918300).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary files.
Authors’ contributions
LW, CZ, DZ, and XW contributed equally to the study design and the protocol, analysis, drafting and revising the manuscript. LF and JZ performed the search strategies for the electronic Databases. QM, LS, XG, XJ and BL were responsible for reviewing articles for inclusion and carrying out the data extraction. PX was responsible for the study concept. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Non applicable.
Ethics approval and consent to participate
Non applicable.
Additional files
MOOSE Checklist. A list of factors that have been checked or done for this meta-analysis of observational studies. (DOC 77 kb)
Primary Data. Data extracted from the articles that met the inclusion criteria. (XLSX 25 kb)
Study Quality Assessment. The quality assessment for each article included. (XLSX 16 kb)
Contributor Information
Ling Wang, Email: 548937812@qq.com.
Chanjuan Zhou, Email: 602093929@qq.com.
Dan Zhu, Email: 75564090@qq.com.
Xinfa Wang, Email: 602335573@qq.com.
Liang Fang, Email: 495573042@qq.com.
Jiaju Zhong, Email: 452990318@qq.com.
Qiang Mao, Email: 297529551@qq.com.
Lu Sun, Email: 1197222862@qq.com.
Xue Gong, Email: 429315801@qq.com.
Jinjun Xia, Email: 1042893462@qq.com.
Bing Lian, Email: 1549920786@qq.com.
Peng Xie, Email: xiepeng@cqmu.edu.cn.
References
- 1.Agren H, Reibring L, Hartvig P, Tedroff J, Bjurling P, Hornfeldt K, Andersson Y, Lundqvist H, Langstrom B. Low brain uptake of L-[11C]5-hydroxytryptophan in major depression: a positron emission tomography study on patients and healthy volunteers. Acta Psychiatr Scand. 1991;83:449–55. doi: 10.1111/j.1600-0447.1991.tb05574.x. [DOI] [PubMed] [Google Scholar]
- 2.Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 3.Baldessarini RJ. The basis for amine hypotheses in affective disorders. A critical evaluation. Arch Gen Psychiatry. 1975;32:1087–93. doi: 10.1001/archpsyc.1975.01760270019001. [DOI] [PubMed] [Google Scholar]
- 4.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY–100635. Mol Psychiatry. 2004;9:386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 5.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex γ–aminobutyric acid concentrations in medication–free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–12. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Billard T, Le Bars D, Zimmer L. PET radiotracers for molecular imaging of serotonin 5–HT1A receptors. Curr Med Chem. 2014;21:70–81. doi: 10.2174/09298673113209990215. [DOI] [PubMed] [Google Scholar]
- 7.Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin–1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–42. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417. [PMC free article] [PubMed] [Google Scholar]
- 9.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 10.Collin M, Håkansson–Ovesjö ML, Misane I, Ogren SO, Meister B. Decreased 5–HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin–deficient ob/ob mouse. Mol Brain Res. 2000;81:51–61. doi: 10.1016/S0169-328X(00)00167-4. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87. doi: 10.1016/S0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 12.Farde L, Ginovart N, Ito H, Lundkvist C, Pike VW, McCarron JA, Halldin C. PET–characterization of [carbonyl–11C]WAY–100635 binding to 5–HT1A receptors in the primate brain. Psychopharmacology (Berl) 1997;133(2):196–202. doi: 10.1007/s002130050391. [DOI] [PubMed] [Google Scholar]
- 13.Graeff FG, Guimarães FS, De Andrade TG, Deakin JF. Role of 5–HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 14.Gryglewski G. Meta–analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab. 2014;34(7):1096–103. doi: 10.1038/jcbfm.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddjeri N, Blier P, de Montigny C. Long–term antidepressant treatments result in a tonic activation of forebrain 5–HT1A receptors. J Neurosci. 1998;18:10150–6. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistencyin meta–analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, VolumeVersion 5.1.0. 2011. Available from http://handbook.cochrane.org/.
- 18.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, Någren K, Salminen JK, Hietala J. Decreased brain serotonin 5–HT1A receptor availability in medication–naive patients with major depressive disorder: An in–vivo imaging study using PET and [carbonyl–11C]WAY–100635. Int J Neuropsychopharmacol. 2008;11:465–76. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 19.Hong C, Chen T, Yu YW, Tsa S. Response to fluoxetine and serotonin 1A receptor (C–1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2005;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson H, Hirvonen J, Salminen JK, Hietala J. No association between serotonin 5–HT 1A receptors and spirituality among patients with major depressive disorders or healthy volunteers. Mol Psychiatry. 2011;16:282–5. doi: 10.1038/mp.2009.126. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan MJ, Hesselgrave N, Ciarleglio A, Ogden RT, Sullivan GM, Mann JJ, Parsey RV. Higher pretreatment 5–HT1A receptor binding potential in bipolar disorder depression is associated with treatment remission: A naturalistic treatment pilot PET study. Synapse. 2013;67:773–8. doi: 10.1002/syn.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, Micskei Z, Stein P, Karanikas G, Wadsak W, Kasper S, Frey R. Global decrease of serotonin–1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry. 2013;18:93–100. doi: 10.1038/mp.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A. Impaired repression at a 5–hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesch KP, Söhnle K, Poten B, Schoellnhammer G, Rupprecht R, Schulte HM. Corticotropin and cortisol secretion after central 5–hydroxytryptamine–1A (5–HT1A) receptor activation: effects of 5–HT receptor and β–adrenoceptor antagonists. J Clin Endocrinol Metab. 1990;70:670–4. doi: 10.1210/jcem-70-3-670. [DOI] [PubMed] [Google Scholar]
- 26.Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. 5–HT1A Receptor binding sites in post–mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42:199–207. doi: 10.1016/S0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara S, Arora R, Meltzer HY. Serotonergic measures in suicide brain: 5–HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect. 1991;85:181–94. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- 28.Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsan BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late–life depression. Neuropsychopharmacology. 2004;29:2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 29.Mervaala E, Föhr J, Könönen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamäki H, Karjalainen AK. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–25. doi: 10.1017/S0033291799001567. [DOI] [PubMed] [Google Scholar]
- 30.Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus-from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–42. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–84. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JM, Hesselgrave N, Ogden RT, Zanderigo F, Oquendo MA, Mann JJ, Parsey RV. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol Psychiatry. 2013;74(10):760–7. doi: 10.1016/j.biopsych.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril. 2008;89:685–92. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nugent AC, Bain EE, Carlson PJ, Neumeister A, Bonne O, Carson RE, Eckelman W, Herscovitch P, Zarate CA, Jr, Charney DS. Reduced post–synaptic serotonin type 1A receptor binding in bipolar depression. Eur Neuropsychopharmacol. 2013;23:822–9. doi: 10.1016/j.euroneuro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugent AC, Carlson PJ, Bain EE, Eckelman W, Herscovitch P, Manji H, Zarate CA, Drevets WC. Mood stabilizer treatment increases serotonin type 1A receptor binding in bipolar depression. J Psychopharmacol. 2013;27:894–902. doi: 10.1177/0269881113499204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ögren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5–HT1A receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Palchaudhuri M, Flügge G. 5–HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005;321:159–72. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- 38.Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatr. 2006;163:52–8. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 39.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: A [carbonyl–C–11] WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Polter AM, Li X. 5–HT1A receptor–regulated signal transduction pathways in brain. Cell Signal. 2010;22:1406–12. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radley JJ, Jacobs BL. 5–HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–7. doi: 10.1016/S0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 42.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY–100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 44.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical γ–aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 45.Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, Innis RB. Serotonin–1A receptors in major depression quantified using PET: Controversies, confounds, and recommendations. NeuroImage. 2012;59:3243–51. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strobel A, Gutknecht L, Rothe C, Reif A, Mössner R, Zeng Y, Brocke B, Lesch KP. Allelic variation in 5–HT1A receptor expression is associated with anxiety–and depression–related personality traits. J Neural Transm. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- 47.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. JAMA. 2000;283(15):2008–12. Meta–analysis of observational studies in epidemiology: a proposal for reporting. Meta–analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan GM, Ogden RT, Oquendo MA, Kumar JSD, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron Emission Tomography Quantification of Serotonin–1A Receptor Binding in Medication–Free Bipolar Depression. Biol Psychiatry. 2009;66:223–30. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Xu Y, Sun Y, Wang YF, Li X, Lang XE, Wang WP, Zhang KR. Association between the serotonin 1A receptor C (–1019) G polymorphism and major depressive disorder in the northern Han ethnic group in China. Chin Med J. 2008;121:874. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary files.


