Crizotinib is an anaplastic lymphoma kinase (ALK) inhibitor, which has brought marked clinical benefits to patients with adult non-small cell lung cancer with ALK rearrangement.1 The ALK gene is located on chromosome 2p23 and its rearrangements with variable fusion partners have been identified in various malignant diseases such as anaplastic large cell lymphoma, inflammatory myofibroblastic tumors and neuroblastoma.2 The potential efficacy of crizotinib for these diseases has been reported.2, 3, 4 Myeloid neoplasms with ALK rearrangement are uncommon but several reports indicate that crizotinib is effective for these diseases.5, 6 The efficacy of an ALK inhibitor on leukemia cells with ALK rearrangement has been shown in vitro.6 Furthermore, crizotinib reduced leukemia cells in acute myeloid leukemia (AML) with ALK rearrangement.5
Here we report a pediatric patient with relapsed and refractory AML with RAN-binding protein 2 (RANBP2)-ALK fusion with monosomy 7, who achieved complete remission (CR) after treatment with crizotinib and allogeneic hematopoietic cell transplantation (allo-HCT).
A previously healthy 2-year-old female developed fatigue and a respiratory disorder. Her blood tests showed marked leukocytosis and anemia, and she was referred to our department. Her laboratory results were as follows: hemoglobin level 3.5 g/dl, platelet count 10 × 103/μl and leukocyte count 159 × 103/μl with 39% blast cells. A bone marrow aspirate showed hypercellularity with leukemia cells. The patient was diagnosed as acute myelomonocytic leukemia. The karyotype determined by G-banding was 45,XX,inv(2)(p23q13), which indicates ALK rearrangement, in all 20 cells analyzed. Other gene abnormalities, including monosomy 7, were not detected.
The patient was treated in accordance with Japanese Pediatric Leukemia/Lymphoma Study Group AML-05 protocol.7 After induction therapy, hematological CR was achieved and further G-banding analysis revealed a normal karyotype. The first relapse occurred 6 months after diagnosis, during consolidation therapy. In addition to inv(2)(p23q13), monosomy 7 was identified by fluorescence in situ hybridization. The patient received IDA-FLAG (Idarubicin, 10 mg/m2, on days 1 and 2; fludarabine, 30 mg/m2, on days 1–5; and cytarabine, 2 g/m2, once daily on days 1–5. Granulocyte colony-stimulating factor, 300 mg/m2, was administered daily beginning 1 day before the commencement of chemotherapy and continued until a neutrophil count of >500/μl), and azacitidine as salvage therapy; however, these proved ineffective.
Because the patient's leukemia cells carried ALK rearrangement and were refractory to conventional salvage therapy, we decided to administer crizotinib based upon its potential efficacy for the disease.5 After the approval of an institutional review board and written informed consent from her guardians, the patient received crizotinib, 280 mg/m2, twice a day, without concomitant chemotherapy except for intrathecal therapy (12 mg methotrexate, 25 mg cytarabine and 10 mg hydrocortisone). The dose of crizotinib used was determined based on phase I clinical trials by the Children's Oncology Group.4
Complete cytogenetic remission (disappearance of monosomy 7 in fluorescence in situ hybridization of bone marrow aspirate) was confirmed 51 days after the initiation of crizotinib. Severe adverse reactions did not occur except for nausea and vomiting. Subsequently the patient underwent allo-HCT from the 5/8 HLA-matched mother. Crizotinib was administrated for 55 days in total and discontinued 6 days before the initiation of a conditioning regimen, with total body irradiation of 12 Gy and 90 mg/m2 melphalan for 2 days. HCT was well tolerated, and neutrophil engraftment was achieved on day 29. Complete donor chimerism and the absence of monosomy 7 in a bone marrow aspirate was confirmed on day 28. CR has remained for more than 1 year after the HCT.
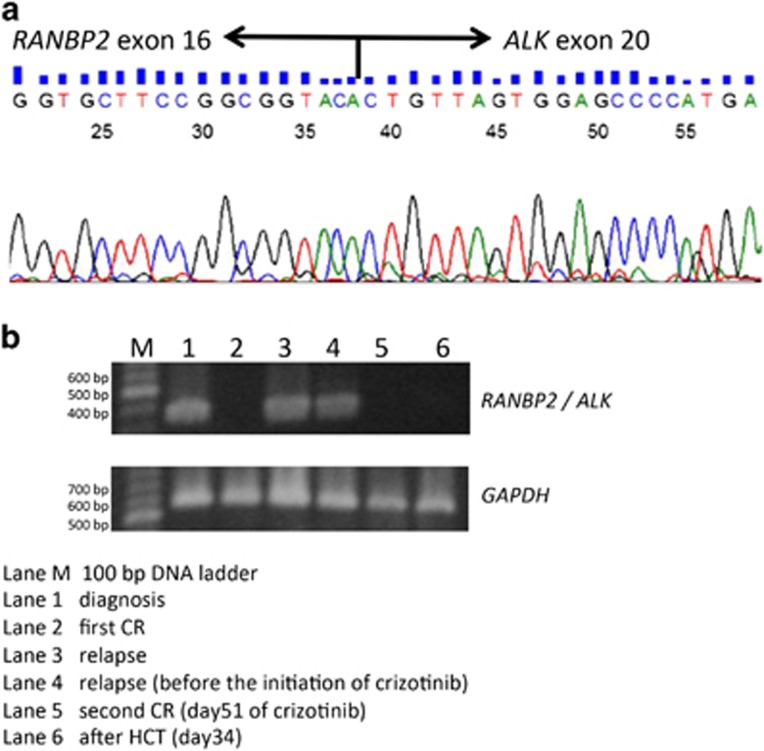
The presence of RANBP2-ALK fusion gene was confirmed by PCR with reverse transcription of a bone marrow sample at diagnosis and a relapse (Figure 1a).8 The RANBP2-ALK fusion gene disappeared after 51 days of administration of crizotinib and remained negative after HCT (Figure 1b).
Figure 1.
(a) Sanger sequencing of RANBP2-ALK fusion transcripts. The breakpoint lies between RANBP2 exon 16 and ALK exon 20. (b) RT-PCR for RANBP2-ALK fusion transcripts in bone marrow aspirate samples. Total cellular RNA was extracted from leukemia blast cells with an RNeasy Mini Kit (QIAGEN, Tokyo, Japan). For RT-PCR analysis, 500 ng of total RNA was reverse-transcribed by PrimeScriptTM RT Master Mix according to the manufacturer's instructions (TaKaRa Bio, Tokyo, Japan). PCR reactions contained cDNA template, TaKaRa Ex Taq (TaKaRa Bio), 10 × Ex Taq buffer (TaKaRa Bio), dNTPs (TaKaRa Bio), forward primer (5′-CATTCTACACCGTCTCCTACCAG-3′) and reverse primer (5′-CGAGGTGCGGAGCTTGCTCAGC-3′) in a 50 μl reaction.8 The cycling conditions were as follows: one cycle of 94 °C for 5 min; 30 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s; and one cycle of 72 °C for 7 min. Sequencing of the PCR product was performed by FASMAC Co., Ltd. (Kanagawa, Japan). CR, complete remission; HCT, hepatopoietic cell transplantation; RT-PCR, reverse transcription-PCR.
This is the first pediatric case suggesting the effectiveness of crizotinib and HCT for relapsed and refractory AML with ALK rearrangement.
The potential efficacy of crizotinib for hematological malignancies with ALK rearrangement has been reported previously. In a pediatric phase I trial, seven out of eight patients with ALK-positive anaplastic large cell lymphoma achieved a CR after treatment with crizotinib.4 Maesako et al.5 reported the efficacy of crizotinib in reducing leukemia cells in AML with ALK rearrangement. However, resistance to crizotinib due to a secondary ALK kinase domain mutation occurred.5, 9 The authors consequently suggested the necessity of consolidation therapy using cytotoxic agents to achieve long-term remission.5 Allogeneic HCT is also a tolerable and curative option after crizotinib therapy, as reported in a case of refractory anaplastic large cell lymphoma.3 Our case achieved CR by crizotinib administration only and then successfully underwent subsequent HCT.
A RANBP2-ALK fusion gene combined with monosomy 7 may be responsible for a certain type of hematologic disorder, such as acute myelomonocytic leukemia, and be related to a poor prognosis.10, 11 Clinical data for our patient and five other previously reported patients5, 10, 11 showing a myeloid neoplasm with ALK rearrangement are summarized in Table 1. The neoplasms of all patients, including our case, were accompanied by monosomy 7 and were resistant to multidrug cytotoxic chemotherapy. Maxson et al.6 identified an oncogenic ALK point mutation in adult AML and pediatric B-cell acute lymphoblastic leukemia, and suggested that ALK mutations likely require other cooperating mutations for progression to leukemia. Monosomy 7, a potentially unfavorable prognostic factor in AML and juvenile myelomonocytic leukemia, may, in our patient, have a role as the cooperating mutation, together with ALK rearrangement, which leads to resistance to standard cytotoxic chemotherapy.
Table 1. Characteristics of patients with myeloid malignancies and the RANBP2-ALK fusion gene.
| Characteristic | This case | Maesako et al.5 | Lim et al.10 | Rottgers et al.11 Patient 3 | Patient 4 | Patient 6 |
|---|---|---|---|---|---|---|
| Diagnosis | AML (M4) | AML | AML (M4) | MDS or JMML | AML (M4) | JMML |
| Karyotype | 46,XX,inv(2)(p23q13) [20] (at diagnosis) 45,XX,inv(2)(p23q13), -7 [5] (relapse) | 46, XX, inv(2)(p23q13) [1]/, 45, idem, -7 [8]/,46, idem, -7, +mar [1] | 45,XX,inv(2)(p23q13),-7 [20] | 45,XY,inv(2) (p23q13),-7 [5] | 46,XY,inv(2)(p23q13) [3]/, 45,idem,-7 [8] | 45,XY,t(2;2) (p23;q11~13),-7 |
| Age at diagnosis | 2.8 Years | 75 Years | 31.4 Years | 8 Years | 16.3 Years | 3.5 Years |
| Initial therapy regimen | VP-16, Ara-C, IDA | Ara-C, DNR, AZA (every 4 weeks) | Ara-C, DNR | Ara-C, DNR, VP-16 | Ara-C, DNR, VP-16 | Ara-C, DNR, VP-16 |
| Response to initial therapy | Relapse 6 months after diagnosis, during consolidation therapy | Relapse after eight cycles of AZA | Induction failure | Recurrence of blasts on day 26 | Early relapse after 6 months | Low persistent blast cells ranging between 2 and 6% during the next 4 months |
| Salvage therapy regimen | IDA+FLAG, AZA, Crizotinib | Crizotinib | Ara-C, MIT, VP-16 | — | liposomal DNR, FLU, Ara-C | — |
| Response to crizotinib therapy | Molecular CR after 51 days crizotinib | Blasts disappeared from peripheral blood after 71 days crizotinib: blasts reappeared on day 135 | — | — | — | — |
| HCT | HCT from HLA5/8 matched mother | — | MUD HCT | — | MFD HCT | MUD HCT |
| Clinical outcome | Alive 1 year after HCT | No data | Relapse 3 months after HCT; death 7 months after relapse | Early death due to infection | Alive 6 years after HCT | Alive 8 years after HCT |
Abbreviations: AML, acute myeloid leukemia; Ara-C, cytarabine; AZA, azacitidine; DNR, daunorubicin; FLAG, fluradabine, cytarabine and granulocyte colony-stimulating factor; FLU, fludarabine; HCT, hematopoietic cell transplantation; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndromes; MFD, matched family donor; MIT, mitoxantrone; MUD, matched unrelated donor; VP-16, etoposide.
The prognosis of AML with monosomy 7 is dismal; however, our patient successfully achieved molecular CR by crizotinib monotherapy treatment and maintained CR for more than a year after HCT. Crizotinib can be viewed as a promising treatment option for those high-risk patients with ALK rearrangement. Therefore, we suggest examining ALK rearrangement and using crizotinib in patients with a refractory or relapsed myeloid neoplasm and chromosome 2p23 aberration. Further studies of the use of crizotinib in AML with RANBP2-ALK fusion gene are required to enhance our understanding of the contribution of crizotinib to the successful treatment of this disease.
Acknowledgments
This study was funded by basic research expenditures of Yokohama City University.
The authors declare no conflict of interest.
References
- Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol 2013; 31: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013; 13: 685–700. [DOI] [PubMed] [Google Scholar]
- Cleary JM, Rodig S, Barr PM, Shinagare AB, Clark JW, Shapiro GI et al. Crizotinib as salvage and maintenance with allogeneic stem cell transplantation for refractory anaplastic large cell lymphoma. J Natl Compr Canc Netw 2014; 12: 323–326. [DOI] [PubMed] [Google Scholar]
- Mosse YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours on anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 2013; 14: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesako Y, Okumura A, Takeoka K, Kishimori C, Izumi K, Kamoda Y et al. Reduction of leukemia cell burden and restoration of normal hematopoiesis at 3 months of crizotinib treatment in RAN-binding protein (RANBP2)-anaplastic lymphoma kinase (ALK) acute myeloid leukemia. Leukemia 2014; 28: 1935–1937. [DOI] [PubMed] [Google Scholar]
- Maxson JE, Davare MA, Luty SB, Eide CA, Chang BH, Loriaux MM et al. Therapeutically targetable ALK mutation in leukemia. Cancer Res 2015; 75: 2146–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa D, Tawa A, Watanabe T, Saito AM, Kudo K, Taga T et al. Excess treatment reduction including anthracyclines results in higher incidence of relapse in core binding factor acute myeloid leukemia in children. Leukemia 2013; 27: 2413–2416. [DOI] [PubMed] [Google Scholar]
- Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 2003; 37: 98–105. [DOI] [PubMed] [Google Scholar]
- Takeoka K, Okumura A, Maesako Y, Akasaka T, Ohno H. Crizotinib resistance in acute myeloid leukemia with inv(2)(p23q13)/RAN binding protein 2 (RANBP2) anaplastic lymphoma kinase (ALK) fusion and monosomy 7. Cancer Genet 2015; 208: 85–90. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jang S, Park CJ, Cho YU, Lee JH, Lee KH et al. RANBP2-ALK fusion combined with monosomy 7 in acute myelomonocytic leukemia. Cancer Genet 2014; 207: 40–45. [DOI] [PubMed] [Google Scholar]
- Rottgers S, Gombert M, Teigler-Schlegel A, Busch K, Gamerdinger U, Slany R et al. ALK fusion genes in children with atypical myeloproliferative leukemia. Leukemia 2010; 24: 1197–1200. [DOI] [PubMed] [Google Scholar]