Abstract
Red blood cell (RBC) alloimmunization is a significant clinical complication of sickle cell disease (SCD). It can lead to difficulty with cross-matching for future transfusions and may sometimes trigger life-threatening delayed hemolytic transfusion reactions. We conducted a retrospective study to explore the association of clinical complications and age of RBC with alloimmunization in patients with SCD followed at a single institution from 2005 to 2012. One hundred and sixty six patients with a total of 488 RBC transfusions were evaluated. Nineteen patients (11%) developed new alloantibodies following blood transfusions during the period of review. The median age of RBC units was 20 days (interquartile range: 14–27 days). RBC antibody formation was significantly associated with the age of RBC units (P = 0.002), with a hazard ratio of 3.5 (95% CI: 1.71–7.11) for a RBC unit that was 7 days old and 9.8 (95% CI: 2.66–35.97) for a unit that was 35 days old, 28 days after the blood transfusion. No association was observed between RBC alloimmunization and acute vaso-occlusive complications. Although increased echocardiography-derived tricuspid regurgitant jet velocity (TRV) was associated with the presence of RBC alloantibodies (P = 0.02), TRV was not significantly associated with alloimmunization when adjusted for patient age and number of transfused RBC units. Our study suggests that RBC antibody formation is significantly associated with older age of RBCs at the time of transfusion. Prospective studies in patients with SCD are required to confirm this finding.
Introduction
Red blood cell (RBC) transfusion therapy is an important management strategy in sickle cell disease (SCD) for several acute and chronic indications [1–5]. Data from the Cooperative Study of Sickle Cell Disease show that most patients over the age of 20-years have received a RBC transfusion [6]. Despite its benefits, RBC transfusion has multiple complications [3,5–8]. Alloimmunization, the development of a clinically significant antibody to a RBC antigen, is an important complication following RBC transfusion therapy. Alloimmunization leads to both acute and delayed hemolytic transfusion reactions and limits the availability of compatible blood for future transfusions. The rates of RBC alloimmunization in the US population are reported to range from 18 to 36% [6,9]. In the Jamaican population where blood is racially matched, the rate of alloimmunization is only 2.6% [8], whereas in the United Kingdom where most transfusions are racially mismatched, the rate of alloimmunization has been reported to be as high as 76% [8]. The increased frequency of RBC alloimmunization in SCD patients has been hypothesized to be due to incompatibility of minor RBC antigens due to a donor pool of racially mismatched blood [10]. More recently, it has been reported that specific Rh variants are more frequent in individuals of African descent, and SCD patients may develop alloantibodies despite testing positive for these antigens on serologic studies [11]. Additional risk factors associated with alloimmunization include increased number of transfusions, older age of patient at the time of first transfusion, and female gender [12–14].
A murine model of alloimmunization to RBC antigens exposed to poly(I:C), a synthetic double-stranded RNA molecule that induces viral-like inflammation, demonstrated a significant enhancement of humoral immunization both in frequency and magnitude to transfused alloantigens [13,15]. This suggests that, in addition to having the “responder” phenotype, other host factors such as inflammation may contribute to the pathophysiology of alloimmunization. Patients with SCD exhibit marked elevation of inflammatory markers at baseline, which are further increased during acute vaso-occlusive episodes [16–21]. In addition, stored RBCs have been reported to produce higher rates of immunogenicity than fresh blood in a murine model for alloimmunization [22].
With the evidence that inflammation and RBC age play complex regulatory roles in RBC alloimmunization in murine models, we conducted a retrospective study to evaluate the association of clinical complications and age of RBCs with alloimmunization in patients with SCD.
Methods
We conducted a retrospective chart review to explore the association of clinical complications and age of RBC with alloimmunization in patients with SCD followed at the University of North Carolina at Chapel Hill from 2005 to 2012. As is the standard of care at our center, all patients were transfused with RBC units that were at a minimum, matched for Rh C, c, D, E and Kell antigens. A list of patients was generated using the following ICD-9 codes for SCD: 282. 60 Sickle cell anemia NOS, 282.61 HbSS without Crisis, 282.62 HbSS with Crisis, 282.63 Sickle cell/HbC disease without crisis, 282.64 Sickle cell/HbC disease with crisis, 282.41 Sickle Cell Thalassemia without crisis, 282.42 Sickle Cell Thalassemia with crisis, 282.68 Other sickle-cell disease without crisis, 282.69 Other SCD with crisis, 289.52 Splenic sequestration, and 517.3 Acute chest syndrome. This query was combined with blood bank query for the following codes: Transfuse Packed Red Cells, Packed Red Cells, Transfuse Packed Cell – PRBC’s. The data mining was supplemented by a physical review of medical history and blood bank records.
Patients were included in the study if they had a confirmed diagnosis of SCD and a type and screen following RBC transfusion. If a patient was transfused with RBCs, a new type and screen was usually obtained prior to the next transfusion beyond 72 hours. Patients were excluded if they were on chronic exchange transfusion, did not have a type and screen following transfusion or had a history of bone marrow transplantation. Transfusions were categorized into 2 groups: transfusions for acute vaso-occlusive complications (acute chest syndrome and vaso-occlusive crisis) versus other indications for transfusion (subsequently referred to as non-acute indications). All clinical complications (acute pain crises, active infection, acute chest syndrome, stroke, retinal artery occlusion, surgery, or leg ulcer) and use of hydroxyurea at the time of transfusion were noted. Follow up type and screens were evaluated for the formation of new antibodies.
Statistical Methods
Descriptive statistics are provided for all study variables, and comparisons between groups were made using Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables. If multiple RBC units were transfused in a single transfusion, the average age of the RBC units was used. A multivariable logistic regression model was used to evaluate the combined effect of patient characteristics on the development of antibodies, using all data available from the last type and screen for each patient. Cox proportional hazard models were used to account for the time dependent nature of the data in describing the relationship with development of new antibodies. At each of the failure times (the time from baseline until the next type and screen detected a new antibody), we used data from all transfusions that occurred in the 60 day window prior to the failure time. We chose a 60 day window since a transfusion will not contribute to risk indefinitely and we assumed that the risk will be minimal after 2 months. All times were calculated from baseline, defined as the first type and screen, for each patient in the study period. A log transformation was used for the age of the RBCs to account for the skewed distribution. An additional sensitivity analysis was conducted, excluding patients who had received frozen RBC units. Variables were considered significantly associated with development of antibodies if the likelihood ratio p-value for the model was significant at the 0.05 level. All analyses were conducted using SAS statistical software v9.3 (Cary, NC).
Results
One hundred and sixty six patients (HbSS = 121; HbSC = 25; HbSβ0 = 6; HbSβ+ = 9; other genotypes = 5), 94 of them females (57%) were evaluated during the study period. The mean patient age at the time of first transfusion during the period of study was 26 years (range: 5 months – 72.4 years). The baseline demographics of the study patients at the start of the follow-up period are shown in Table I. There were a total of 488 transfusions and 652 types and screens in the study patients between September 2005 and July 2012. Most patients (82%) had received at least one RBC transfusion prior to the study period. The median time in the study (defined as time from first to last type and screen) was 1.04 years (interquartile range [IQR]: 69 days – 2.82 years). The median number of transfusions was 2 (IQR: 1–4) and the median number of types and screens was 3 (IQR: 2–4). The median number of RBC units per transfusion was 2 (IQR: 1 – 3), and the median age of transfused RBCs was 20 days (IQR: 14–27). Nine patients were transfused with frozen RBC units, for a total of 16 transfusions (age of RBC units [range]: 78 – 3640 days). Patients received only one unit of RBC in 260 of the 495 transfusion events (53%). For those patients who received more than one unit of RBC, we averaged the age of the RBC units for our analysis. The difference in the age of RBC was less than 10 days in 74% of transfusion events involving more than 1 unit of RBC. Follow-up types and screens were obtained for all patients with a median time of 42 days (IQR: 9–198 days). Fifty seven percent of the follow-up types and screens were obtained within 60 days of the RBC transfusion.
TABLE I.
Baseline Patient Demographics (n = 168)
| Variable | Frequency or median (IQRa) |
|---|---|
| Age | 22.6 years (12–39) |
| Genotype | |
| SS | 121 (73%) |
| Sβ0 | 6 (4%) |
| SC | 25 (15%) |
| Sβ+ | 9 (5%) |
| Other | 5 (3%) |
| Female | 94 (57%) |
| African American | 161 (97%) |
| Tricuspid regurgitant Jet Velocity≥2.5 (m/s) | 15 (9%) |
| Avascular necrosis | 38 (23%) |
| History of stroke | 9 (5%) |
| Retinopathy | 23 (14%) |
| Number of patients with prior antibodies | 27 (16%) |
| Number of transfusions/patient | 2 (1–4; range: 1–38 Transfusions) |
| Number of units/transfusion | 2 (1–3; range: 1–15 Units) |
| Age of RBC units | 20 days (14–27; range: 2–42 days) |
IQR: interquartile range.
Transfusions occurred for varied reasons, including symptomatic anemia associated with acute painful episodes (59%), acute chest syndrome (33%), acute stroke (1.6%), leg ulcers (10%), acute retinal artery occlusion (1%) and surgery (26%). In 19% of transfusions, patients had laboratory confirmed infections. Patients were taking hydroxyurea on the day of transfusion in 27% of transfusions. Patients were transfused for acute vaso-occlusive complications (acute painful episodes or acute chest syndrome) in 61% of cases. Overall, 40% of patients received transfusions for only acute vaso-occlusive indications, 34% received only non-acute transfusions, and 26% received transfusions for nonacute as well as for acute vaso-occlusive indications.
Twenty-seven patients (16%) were known to have RBC antibodies at the start of the study period. Four of these patients (15%) had an additional antibody detected during a subsequent type and screen (median of 33.5 days after first transfusion during the study period). Of the 139 (84%) patients who did not have any antibodies at the start of the study period, 15 (10.6%) had an antibody identified during a subsequent type and screen. Overall, 19 patients (11%) developed a total of 25 new alloantibodies during the study period. Two of the 19 patients who developed new alloantibodies did so following multiple RBC transfusions within the 72-hr type and screen window. One patient with a new antibody was excluded from the analysis as she was transfused at an outside facility and the details of the transfusion were unknown. Following the last type and screen during the study period, 42 patients (25%) had at least one antibody detected (15 new patients, in addition to the 27 who had at least one antibody at the start of the study). The alloantibodies detected included Anti-C,-Cob, -Cw, -E, -Fya -Jka, -Jkb, -Jsa, -K, -Lea, -M, -S, and -V. Of the identified antibodies, 11 were targeted against Rh antigens.
Associations with ever having an antibody detected were evaluated based on patient characteristics at the last type and screen during the study period (Table II). Patients with tricuspid regurgitant jet velocities (TRV) ≥2.5 m/s were significantly more likely to have antibodies than patients with TRV < 2.5 m/s (50% vs. 22%, P = 0.02). There were no associations between genotype, gender, use of hydroxyurea, or other evaluated clinical complications and RBC antibody formation (P > 0.05). There was no difference in antibody formation when patients who had been transfused for acute vaso-occlusive indications during the study period were compared with patients only transfused for nonacute indications (P = 0.9). Patients who developed antibodies were older (median age: 31 years [IQR: 22–49] vs. 22 years [IQR: 12–39], P = 0.0006) and had received more RBC transfusions (median number of transfusions: 2.5 [IQR: 1–4] vs. 2 [IQR: 1–3], P = 0.023) than patients who did not develop antibodies. A multivariable model including TRV, age, and number of transfusions revealed that TRV was no longer associated with the development of antibodies after controlling for patient age and the number of transfusions (P = 0.5). However, both increasing age and the number of RBC transfusions retained their significant associations with higher risk of developing antibodies (P = 0.01 and 0.04, respectively).
TABLE II.
Association Between Clinical Variables at End of Study and RBC Alloimmunizationa
| Variable | Frequency (%) | P-value | |
|---|---|---|---|
| Genotype | SS | 33/121 (27%) | 0.6 |
| SC | 6/25 (24%) | ||
| Other | 3/19 (16%) | ||
| Gender | Male | 16/72 (22%) | 0.5 |
| Female | 26/94 (28%) | ||
| Indication for Transfusion | Acute Vaso-occlusive Indication | 26/100 (26%) | 0.9 |
| Nonacute Indication | 16/66 (24%) | ||
| TRV≥2.5 (m/s) | Yes | 9/18 (50%) | 0.02 |
| No | 33/148 (22%) | ||
| History of leg ulcers | Yes | 7/16 (43%) | 0.1 |
| No | 35/150 (23%) | ||
| History of AVN | Yes | 14/39 (36%) | 0.09 |
| No | 28/127 (22%) | ||
| History of ACS | Yes | 36/127 (28%) | 0.1 |
| No | 6/39 (15%) | ||
| History of Stroke | Yes | 2/10 (20%) | 0.9 |
| No | 40/156 (25%) | ||
| History of Retinopathy | Yes | 10/25 (40%) | 0.08 |
| No | 32/141 (23%) | ||
| Use of Hydroxyurea | Yes | 20/60 (33.3%) | 0.09 |
| No | 84/106 (79.2%) |
Using summary data at the time of the last type and screen on study.
TRV: tricuspid regurgitant jet velocity.
As relatively few patients developed new antibodies, Cox proportional hazards models were fit using one to two variables at a time. Time since transfusion was considered, in addition to mean age of the blood, number of RBC units, and transfusion for acute vaso-occlusive indications in three separate models. Neither the number of units (P = 0.16) nor having any transfusions for acute vaso-occlusive indications (P = 0.64) was significantly associated with the development of new antibodies. However, there was a significant association between the mean age of RBCs and the development of antibody (P = 0.002; Fig. 1). The estimated hazard ratio for a 7 day old RBC unit is 1.5 (95% CI: 1.15 – 2.02) on day 7 and 3.5 (95% CI: 1.71 – 7.11) on day 28, while the estimated hazard ratio for a 35 day old RBC unit is 2.2 (95% CI: 1.29 – 3.60) on day 7 and 9.8 (95% CI: 2.66 – 35.97) on day 28 (Table III). This means, for example, that if a patient was transfused on day one with 7-day-old RBC units, his/her hazard of developing antibodies at day 28 was 3.5 times that of a person who did not receive a transfusion on day one.
Figure 1.
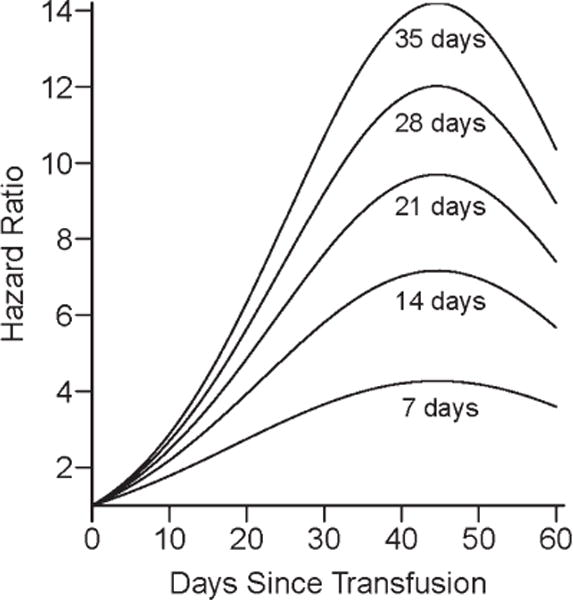
The model evaluating mean age of red blood cells shows that the hazard of developing new antibodies increases and then decreases over the 60 days following the transfusion, with a peak at 47 days. Each separate line shows the hazard of developing antibodies for a transfusion compared to a patient not transfused, for different ages of blood.
TABLE III.
Hazard Ratio Estimates of New Antibody Formation for RBC Units of Different Ages
| Age of RBCs (days) | Hazard Ratio and 95% Confidence Intervals
|
||||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 28 | Day 42 | Day 60 | |
| 7 | 1.5 (1.15, 2.02) | 2.2 (1.32, 3.53) | 3.5 (1.71, 7.11) | 4.2 (2.05, 8.72) | 3.6 (1.53, 8.28) |
| 14 | 1.8 (1.21, 2.59) | 2.8 (1.45, 5.52) | 5.4 (2.07, 14.29) | 7.1 (2.65, 18.86) | 5.6 (1.78, 17.58) |
| 21 | 1.9 (1.24, 3) | 3.3 (1.54, 7.18) | 7.1 (2.31, 21.5) | 9.5 (3.08, 29.62) | 7.3 (1.94, 27.31) |
| 28 | 2.1 (1.27, 3.33) | 3.7 (1.6, 8.65) | 8.5 (2.5, 28.73) | 11.8 (3.42, 40.8) | 8.8 (2.07, 37.33) |
| 35 | 2.2 (1.29, 3.6) | 4.1 (1.65, 10) | 9.8 (2.66, 35.97) | 13.9 (3.72, 52.31) | 10.2 (2.17, 47.56) |
| Patients who did not receive frozen RBC units | |||||
| 7 | 1.5 (1, 2.32) | 2.1 (1.03, 4.45) | 3.4 (1.17, 9.58) | 3.8 (1.32, 11.07) | 2.9 (0.78, 10.39) |
| 14 | 1.8 (1, 3.13) | 2.8 (1.04, 7.58) | 5.2 (1.24, 21.44) | 6.2 (1.46, 26.06) | 4.1 (0.72, 23.92) |
| 21 | 1.9 (1, 3.72) | 3.3 (1.05, 10.35) | 6.6 (1.29, 34.33) | 8.2 (1.55, 43.01) | 5.2 (0.68, 38.96) |
| 28 | 2.1 (1, 4.22) | 3.7 (1.06, 12.9) | 7.9 (1.32, 47.95) | 10 (1.62, 61.36) | 6 (0.66, 55.07) |
| 35 | 2.2 (1, 4.64) | 4 (1.06, 15.32) | 9.1 (1.34, 62.14) | 11.6 (1.67, 80.84) | 6.8 (0.64, 72.03) |
As frozen RBC units are processed and may age differently compared with nonfrozen RBC units, we ran similar analyses using only patients who were not transfused with any frozen RBC units; 9 patients who ever received frozen units were excluded. The association between the mean age of RBCs and the development of antibody remained, with similar estimated hazard ratios (Table III), but was no longer statistically significant (P = 0.053), likely due to the smaller number of events.
Discussion
In this study, we observed the relationship between the age of RBCs and alloimmunization. RBCs stored for up to 42 days prior to transfusion meet licensure requirements of allowing 75% of the cells to remain in the circulation 24 hr after infusion [23]. However, older RBCs approaching the 42 day expiration mark have numerous attributes that may increase inflammation in the recipient and develop what is described as a storage lesion [24–28]. Older RBCs have higher intracellular heme, which may overwhelm the heme oxygenase system that normally neutralizes the oxidative and inflammatory effects of heme [24]; increased plasma non-transferrin-bound iron, which may lead to increased risk of sepsis and possibly mortality [25]; higher RBC membrane loss, which may result in phospholipid changes, and the release of microparticles (which are considered procoagulant and proinflammatory) [26]; and lower levels of nitric oxide, due in large part to ongoing hemolysis and generation of reactive oxygen species [27,28]. Increased RBC age is associated with increased mortality, as well as higher risk of renal failure, pneumonia, deep venous thrombosis, and postoperative infections in critically ill patients, cardiovascular surgery patients, and in patients with trauma [29–31]. Considering the important role of RBC transfusion in the prevention and treatment of many SCD-related complications, our observation of an association between RBC age and alloimmunization may have important implications for clinical practice.
We did not observe any association between transfusion for acute complications, such as ongoing painful episodes or acute chest syndrome, and new RBC antibody formation. Although it has been reported that the baseline increased inflammatory state in SCD increases even further during acute painful episodes and acute chest syndrome [32–35], no inflammatory markers were measured in our retrospective study. As such, prospective studies in which inflammatory markers are assessed are required to definitively exclude a role for inflammation in alloimmunization in SCD. The increased alloimmunization in patients with increased TRV on univariate analysis was unexpected. However, TRV was not significantly associated with alloimmunization when adjusted for patient age and the number of RBC transfusions.
The study has limitations due to its retrospective design and small number of patients with new antibody formation. Follow up types and screens were obtained in patients on average 42 days after a RBC transfusion, with occasional delays greater than sixty days in obtaining a type and screen following a RBC transfusion. As such, we may not have captured all formed alloantibodies. In addition, a type and screen was not always performed immediately before each transfusion, usually due to multiple transfusions within a 72 hr period. In such cases, we cannot directly attribute the development of antibodies to a particular transfusion. As a result, a repeated measures analysis at the transfusion level could not be performed. However, the rate of alloimmunization in our study is comparable to those obtained in studies that have examined antibody formation in SCD patients transfused with RBC units matched for Rh C, D, E, and Kell antigens [29]. Patients on chronic RBC exchange transfusion were excluded due to the anticipated difficulty in ascertaining the contribution of RBC age to antibody formation in patients on such treatment. In situations where patients were transfused with multiple RBC units in close proximity, we cannot directly attribute the development of antibodies to a particular transfusion. However, our analysis takes this into account by using the information from all transfusions within 60 days prior to each event time in the Cox proportional hazards model. Finally, as frozen RBC units are processed and age differently from nonfrozen RBC units, we conducted a sensitivity analysis excluding patients transfused with frozen RBC units. The results were similar, although not statistically significant, likely due to the smaller number of events when frozen units were excluded. As only four patients transfused with frozen RBC units developed new antibodies, we did not evaluate the association of these units with alloimmunization.
In summary, transfusion for acute vaso-occlusive complications was not associated with increased alloimmunization in our retrospective study. However, RBC antibody formation was significantly associated with increased patient age and the number of transfused RBC units. In addition, following adjustment for the number of RBC units and transfusion for acute vaso-occlusive indications, older age of RBCs at the time of transfusion was significantly associated with RBC antibody formation. This finding is consistent with the reports indicating that older age of RBCs leads to higher levels of oxidative stress, and possibly subsequent increase in morbidity and mortality. Our findings warrant further investigation in a prospective study evaluating the role of RBC age in alloimmunization in patients with SCD.
Acknowledgments
Contract grant sponsor: NIH (to K.I.A.); Contract grant numbers: U01HL117659, R01HL111659, and UL1TR001111.
Contract grant sponsors: North Carolina State Sickle Cell Program and The North Carolina Translational and Clinical Sciences (NC TraCS) Institute (University of North Carolina at Chapel Hill).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Author Contributions
P.C. Desai designed research, interpreted data, and wrote the manuscript; A.M. Deal performed statistical analysis, interpreted data and wrote the manuscript; E. R. Pfaff collected data and critically reviewed the manuscript; B. Qaqish performed statistical analysis, interpreted the data and critically reviewed the manuscript; L.M. Hebden collected data and critically reviewed the manuscript; Y Park interpreted data and critically reviewed the manuscript; K.I. Ataga designed research, interpreted data, and wrote the manuscript.
References
- 1.Danielson CFM. The role of red blood cell exchange transfusion in the treatment and prevention of complications of sickle cell disease. Ther Apher. 2002;6:24–31. doi: 10.1046/j.1526-0968.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Davies SC, Luce PJ, Win AA, et al. Acute chest syndrome in sickle-cell disease. Lancet. 1984;1:36–38. doi: 10.1016/s0140-6736(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe C, Adams R, Vichinsky E. Transfusion therapy in sickle cell disease. Renaissance Sickle Cell Dis Res Genome Era. 2007;117 [Google Scholar]
- 4.Morrison JC, Schneider JM, Whybrew WD, et al. Prophylactic transfusions in pregnant patients with sickle hemoglobinopathies: Benefit versus risk. Obstet Gynecol. 1980;56:274. [PubMed] [Google Scholar]
- 5.Piomelli S. Chronic transfusions in patients with sickle cell disease. Indications and problems. Am J Pediatr Hematol Oncol. 1985;7:51. [PubMed] [Google Scholar]
- 6.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The cooperative study of sickle cell disease. Blood. 1990;76:1431. [PubMed] [Google Scholar]
- 7.Orlina AR, Unger PJ, Koshy M. Post transfusion alloimmunization in patients with sickle cell disease. Am J Hematol. 1978;5:101–106. doi: 10.1002/ajh.2830050204. [DOI] [PubMed] [Google Scholar]
- 8.Olujohungbe A, Hambleton I, Stephens L, et al. Red cell antibodies in patients with homozygous sickle cell disease: A comparison of patients in Jamaica and the United Kingdom. Br J Haematol. 2001;113:661–665. doi: 10.1046/j.1365-2141.2001.02819.x. [DOI] [PubMed] [Google Scholar]
- 9.Vichinsky E. Consensus document for transfusion-related iron overload. Semin Hematol. 2001;38:2–4. doi: 10.1016/s0037-1963(01)90054-x. [DOI] [PubMed] [Google Scholar]
- 10.Vichinsky EP, Earles A, Johnson RA, et al. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 11.Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 12.Bauer MP, Wiersum Osselton J, Schipperus M, et al. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007;47:2066–2071. doi: 10.1111/j.1537-2995.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 14.Schonewille H, Van De Watering LMG, Loomans DSE, et al. Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006;46:250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 15.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 16.Bourantas KL, Dalekos GN, Makis A, et al. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998;61:49–54. doi: 10.1111/j.1600-0609.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 17.Hibbert JM, Hsu LL, Bhathena SJ, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med (Maywood) 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jison ML, Munson PJ, Barb JJ, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathare A, Al Kindi S, Alnaqdy AA, et al. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77:323–328. doi: 10.1002/ajh.20196. [DOI] [PubMed] [Google Scholar]
- 20.Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: Pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrickson JE, Hod EA, Spitalnik SL, et al. Immunohematology: Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 50:642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao W, Zhong H, Li X, et al. Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and beta-thalassemia major. Am J Hematol. 2011;86:1001–1006. doi: 10.1002/ajh.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: Pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong Z, Cavaretta J, Qu L, et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 51:610–621. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and Cell-free hemoglobin as a mechanism for the red cell storage lesion/clinical perspective. Circulation. 124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli F, Kabbara N, Ruggeri A, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–1078. doi: 10.1182/blood-2013-03-489112. [DOI] [PubMed] [Google Scholar]
- 30.Aubron C, Nichol A, Cooper DJ, et al. Age of red blood cells and transfusion in critically ill patients. Ann Intensive Care. 2013;3:2. doi: 10.1186/2110-5820-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettila V, Westbrook A, Nichol A, et al. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15:R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94:1555–1560. [PubMed] [Google Scholar]
- 33.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 34.Bean CJ, Boulet SL, Ellingsen D, et al. Heme oxygenase-1 gene promoter polymorphism is associated with reduced incidence of acute chest syndrome among children with sickle cell disease. Blood. 2012;120:3822–3828. doi: 10.1182/blood-2011-06-361642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88:2311–2320. [PubMed] [Google Scholar]


