Abstract
Congestive heart failure (CHF) is associated with an increase of leukocyte infiltration, pro-inflammatory cytokines and fibrosis in the heart and lung. Regulatory T cells (Tregs, CD4+CD25+FoxP3+) suppress inflammatory responses in various clinical conditions. We postulated that expansion of Tregs attenuates CHF progression by reducing cardiac and lung inflammation. We investigated the effects of Interleukin-2 (IL-2) plus IL-2 monoclonal antibody clone JES6-1 complexes (IL2/JES6-1) on induction of Tregs, transverse aortic constriction (TAC)-induced cardiac and lung inflammation and CHF progression in mice. We demonstrated that end-stage CHF caused a massive increase of lung macrophages and T cells, as well as relatively mild LV leukocyte infiltration. Administration of IL2/JES6-1 caused a ~6-fold increase of Tregs within CD4+ T cells in the spleen, lung and heart of mice. IL2/JES6-1 treatment of mice with existing TAC-induced left ventricular (LV) failure markedly reduced lung and right ventricular (RV) weight, and improved LV ejection fraction and LV end-diastolic pressure. Mechanistically, IL2/JES6-1 treatment significantly increased Tregs, suppressed CD4+ T-cell accumulation, dramatically attenuated leukocyte infiltration including decreasing CD45+ cells, macrophages, CD8+ T cells and effector memory CD8+, and reduced pro-inflammatory cytokine expressions and fibrosis in the lung of mice. Furthermore, IL2/JES6-1 administered before TAC attenuated the development of LV hypertrophy and dysfunction in mice. Our data indicate that increasing Tregs through administration of IL2/JES6-1 effectively attenuates pulmonary inflammation, RV hypertrophy and further LV dysfunction in mice with existing LV failure, suggesting strategies to properly expand Tregs may be useful in reducing CHF progression.
Keywords: heart failure, regulatory T cell, heart, lung, inflammation, fibrosis
Introduction
In clinic, patients with congestive heart failure (CHF) usually do not seek treatment until the symptoms of left ventricular (LV) dysfunction occur. Additionally, in many cases, CHF inevitably transits from LV failure to right ventricular (RV) failure and turns into the end-stage even with a proper therapy to improve the heart function. Therefore, in addition to improving the LV function, more effective therapies to attenuate the progression of existing LV failure is desired.
End-stage CHF exhibits increased pulmonary venous pressure, increased lung weight and lung leukocyte infiltration, pulmonary arterial hypertension, and subsequent RV hypertrophy and failure.1, 2 Inflammation plays an important role in CHF development.3 The expression of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β are up-regulated in cardiac tissues and blood of patients and experimental animals with CHF.4 T cells and CD4+ T cells accumulate in the LV tissues of mice with CHF and contribute to the pathogenesis of CHF.5, 6 We recently demonstrated that end-stage CHF is associated with a profound lung leukocyte infiltration in mice,7 suggesting that inflammation plays an important role in LV failure-induced lung remodeling and the transition from LV failure to RV failure.
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are a subpopulation of T cells that modulate immune tolerance and suppress inflammatory responses in various clinical diseases.8 Interleukin-2 (IL-2) is a critical cytokine for the survival and functional competence of Tregs. Administration of IL-2 alone results in a robust expansion of Tregs in vitro, but only a mild induction of Tregs in vivo due to its rapid enzymatic degradation.9, 10 A particular IL-2 monoclonal antibody clone JES6-1 binds to a site on IL-2 that could reduce IL-2 degradation in vivo and selectively lead to vigorous stimulation of CD25+ cells.11, 12 Administration of IL-2 plus IL-2 monoclonal antibody clone JES6-1 complexes (IL2/JES6-1) could result in a robust increase of endogenous Tregs in experimental animals.9, 10
We demonstrated that end-stage CHF in mice is associated with a dramatic accumulation of lung immune cells including macrophages and T cells. Administration of IL2/JES6-1 can selectively expand Tregs ~6 fold and attenuate the progression of transverse aortic constriction (TAC)-induced CHF by suppressing cardiac and lung and/or systemic inflammation in mice. Our findings suggest that cytokine therapy to selectively expand Tregs or cell therapy by transplantation of Tregs may be an attractive new therapeutic approach in treating CHF. It has been developed an intense interest in using Tregs for immunotherapy and has already shown efficacy in suppressing immune inflammation-related diseases.13 Therefore, the translational potential of treating CHF with Tregs is high.
In this study, we treated the mice when they already had LV failure induced by TAC. Our experimental strategy is to examine the effect of IL2/JES6-1 complexes on the LV and lung in mice with existing LV failure, which is more clinically relevant.
Methods
Detailed methods are available in the online-only Data Supplement.
Animals and experiment design
IL2/JES6-1 complexes (1 µg mouse IL-2 plus 5 µg anti-IL-2 antibodies clone JES6-1, Biolegend) were administrated (i.p.) to mice on 3 consecutive daily doses every 6 days during the study. PBS was used for vehicle control.
To determine the effect of IL2/JES6-1 on mice with existing CHF, male Balb/c mice (4–5 weeks of age) from the Jackson Laboratory were used because Balb/c mice develop LV dysfunction rapidly in response to TAC. Briefly, Male Balb/c mice were subjected to a TAC procedure created with a 27G needle,14 a model that mimics clinical systemic hypertension or aortic stenosis. IL2/JES6-1 was commenced when LV ejection fraction (EF) of Balb/c mice reached to ~55% 10 days after TAC.7 Samples were collected 4 weeks after TAC, at a time when the PBS treated mice had average LV ejection fraction of 32%.
To evaluate the effect of IL2/JES6-1 on the development of TAC-induced LV hypertrophy and cardiac dysfunction, male C57/B6 mice (4–5 weeks of age) from Jackson Laboratory were subjected to a TAC procedure created with a 26G needle. IL2/JES6-1 treatment was commenced 3 days before TAC. Samples were collected 12 weeks after TAC, at a time when the PBS treated mice had average LV ejection fraction of 63%.
Statistics
A normality test (Shapiro-Wilk) provided by SigmaPlot was used to determine whether data were normally distributed. If data were normally distributed, the data were presented as mean ± SEM. A Student’s t-test was used to test for differences between 2 groups. A two-way ANOVA followed by a Bonferroni correction post-hoc test was used to test for differences among more than 2 groups. If mouse physiological data were not normally distributed or the sample size in one of the experimental groups was less than 10, a non-parametric test (Mann-Whitney or Kruskal-Wallis) followed by a Bonferroni post hoc correction was performed. All pairwise p-values are two-sided. The null hypothesis was rejected at P < 0.05.
Results
End-Stage CHF Exhibits Dramatic Leukocyte Infiltration in Lungs and Mild Leukocyte Infiltration in Hearts
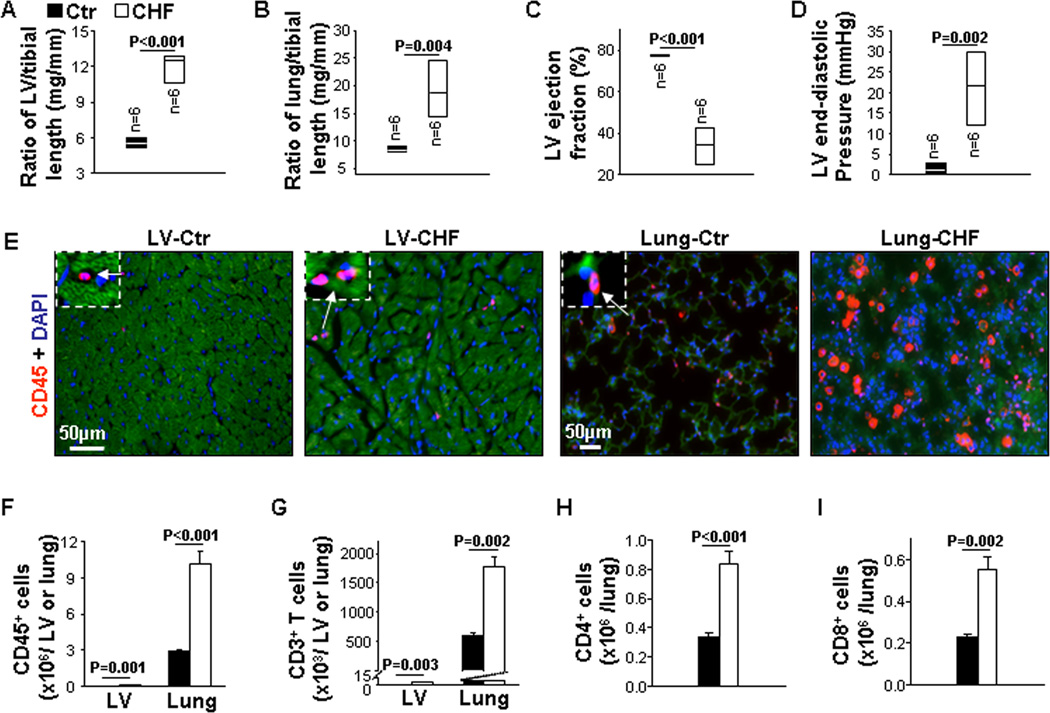
We examined the relative inflammatory status in the lungs and hearts of mice with end-stage CHF produced by TAC. As shown in Figure 1A and 1B, ratios of LV and lung weight to tibial length were significantly increased 2.2-fold and 2.3-fold in CHF mice, respectively. Echocardiography and hemodynamic measurement showed that mice with CHF exhibited a significant decrease of LV EF (34% in CHF vs 77.6% in control group) (Figure 1C) and a dramatic increase (12.3-fold) of LV end-diastolic pressure (LVEDP) (Figure 1D), indicating that TAC caused end-stage CHF in these mice.
Figure 1. End-stage congestive heart failure (CHF) exhibits robust leukocyte infiltration in lungs and moderate leukocyte infiltration in hearts.
Data were collected from mice under basal (Ctr) and CHF conditions. A and B, The ratio of left ventricle (LV) and lung weight to tibial length of mice. C, Echocardiographic measurements of LV ejection fraction from hearts. D, Hemodynamics of LV end-diastolic pressure from hearts. E and F, Representative images of immunostaining and flow cytometry quantitative data of CD45+ cells in the LV and lung. G, Flow cytometry quantitative data represent the number of CD3+ T cells in the LV and lung. H and I, Flow cytometry quantitative data represent the number of CD4+ and CD8+ T cells in the lung. n=5 per group.
LV and lung leukocyte infiltration was significantly increased in CHF mice, as revealed by immunostaining and flow cytometry analysis of CD45+ cells. And notably, CD45+ cell infiltration was 0.15 × 106 cells per LV and 10.1 × 106 cells per lung in CHF mice (Figure 1E and 1F). In addition, flow cytometry analysis showed that CD3+ T cells were significantly increased in both the LV and the lung of CHF mice; while CD3+ T cell infiltration was 13.8 × 103 cells per LV and 1780 × 103 cells per lung in CHF mice (Figure 1G). Both C4+ and CD8+ T cells were 2.2-fold and 2.7-fold increased in the lung of CHF mice (Figure 1H and 1I). Flow cytometry gating strategies are presented in Figure S1 and S2. These data indicate that end-stage CHF is associated with a dramatic lung inflammation and relatively mild LV inflammation. Similar results were observed in both C57/B6 and Balb/c background mice.
IL2/JES6-1 Treatment was Effective in Increasing Endogenous Tregs in Mice
Flow cytometry gating strategies are presented in Figure S2 and S3. IL2/JES6-1 treatment resulted in a ~6-fold increase of Tregs in the spleen and lung after 5 days, while total numbers of CD4+ and CD8+ T cells were not altered (Figure S4A–S4F). Foxp3 immunostaining and quantitative data also demonstrated a significant increase of Tregs in the LV and lung in mice treated with IL2/JES6-1, while Tregs were rarely found in the LV compared to those in the lung (Figure S4G and S4H). At the end point of the treatment for 12 weeks, Tregs still maintained a ~6-fold increase in the spleen with IL2/JES6-1 treatment without altering the total number of CD4+ or CD8+ T cells (Figure S4I–S4K). These data demonstrate that IL2/JES6-1 treatment can efficiently expand Tregs in mouse lungs and hearts.
IL2/JES6-1 Treatment Effectively Attenuated CHF Progression in Mice with Existing LV Failure
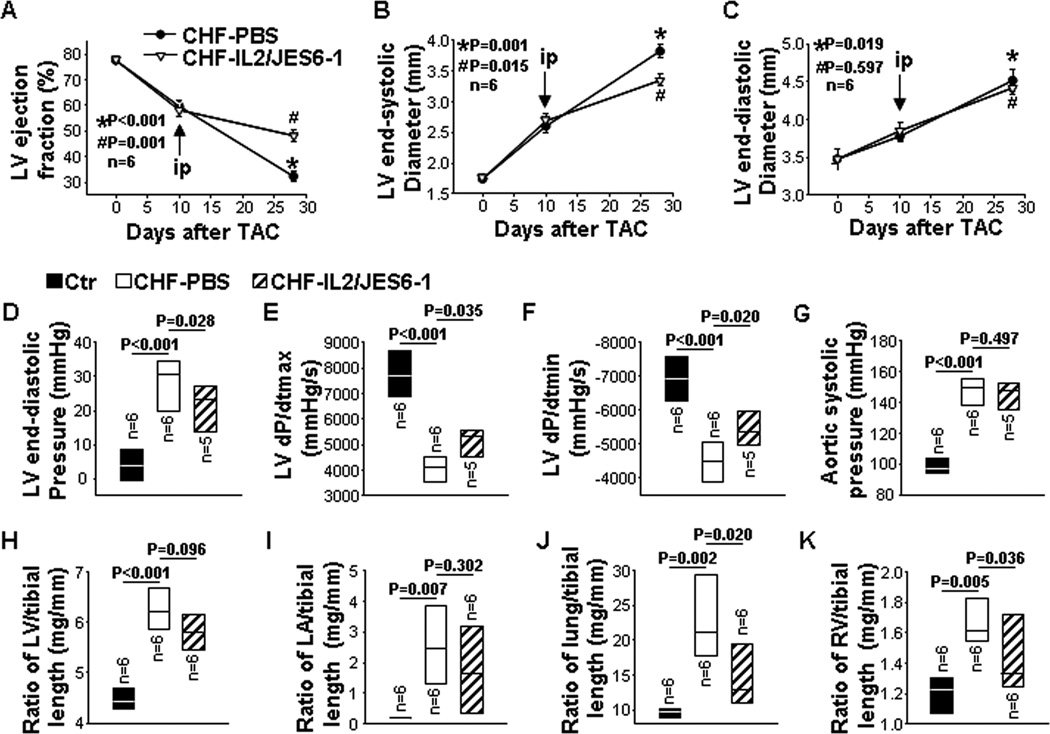
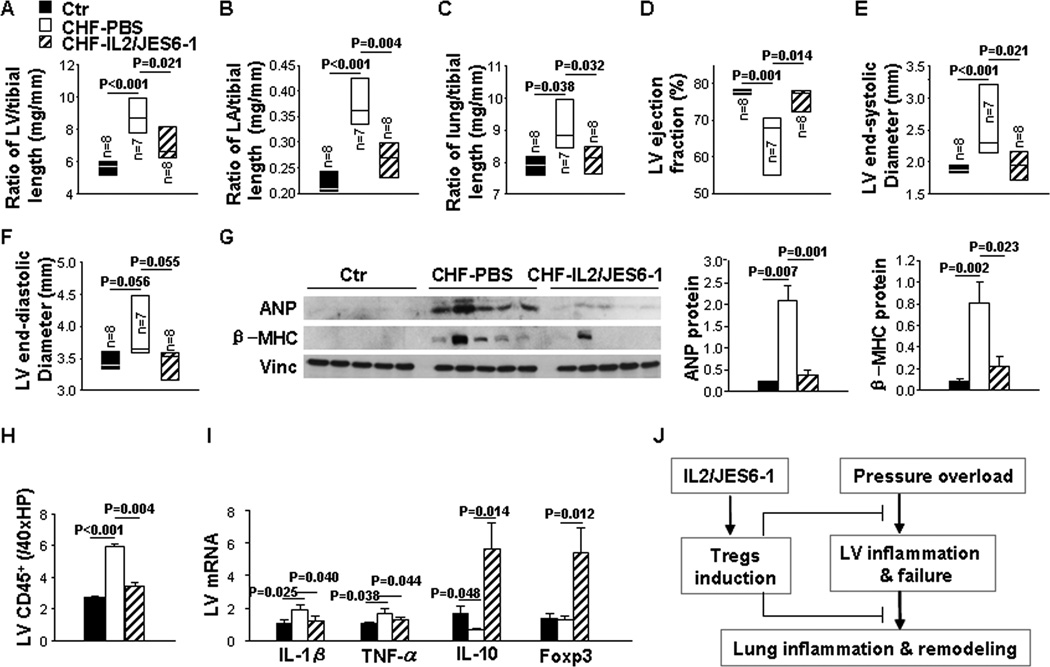
To examine whether IL2/JES6-1 treatment affected the progression of CHF in Balb/c mice with exhibiting LV dysfunction, we used Balb/c mice because they usually develop LV dysfunction rapidly in response to TAC. Briefly, IL2/JES6-1 treatment was commenced to mice when LV EF reduced to 58% 10 days after TAC. Final studies were performed 4 weeks after TAC (Figure 2A).
Figure 2. IL2/JES6-1 treatment attenuates progression of congestive heart failure (CHF) in mice with existing left ventricle (LV) failure.
The treatment was commenced when LV ejection fraction (EF) reached around 55% due to TAC. Data were collected from mice under basal conditions (Ctr), or treated with IL2/JES6-1 or PBS under TAC conditions (CHF-IL2/JES6-1 or CHF-PBS). A to C, Echocardiographic measurements of LV EF, LV end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD) from hearts. D to G, Hemodynamics of LV end-diastolic pressure, LV maximum rate of rise of pressure (dP/dtmax), LV maximum rate of decline of pressure (dP/dtmin) and aortic systolic pressure from hearts. H to K, The ratio of LV, LA, lung and right ventricle weight to tibial length of mice. *p as compared with control before TAC. #p as compared with CHF-PBS.
Echocardiographic measurements showed that mice exhibited a significant decrease of LV EF (32% in CHF vs 78% before TAC) and a 2.1-fold increase in LV end-systolic diameter (LVESD) 4 weeks after TAC. IL2/JES6-1 treatment significantly attenuated the further loss of LV function, as indicated by significantly less reduction of LV EF and less increase of LVESD (Figure 2A and 2B). LV end-diastolic diameter (LVEDD) was also increased 29% 4 weeks after TAC, while it was comparable between PBS and IL-2/JES6-1-treated mice (Figure 2C). Moreover, hemodynamic measurements showed that LVEDP was significantly (6.3-fold) increased by TAC, and IL2/JES6-1 treatment significantly reduced this increase (Figure 2D). IL2/JES6-1 treatment also significantly attenuated TAC-induced reductions of LV maximum rate of rise of pressure (dP/dtmax) and LV maximum rate of decline of pressure (dP/dtmin) with similar aortic systolic pressures (Figure 2E–2G). Furthermore, ratios of LV, LA, lung and RV weight to tibial length were respectively increased 1.4-fold, 14.5-fold, 2.2-fold and 1.3-fold 4 weeks after TAC, suggesting severe LV hypertrophy and dysfunction, and intense pulmonary congestion due to TAC (Figure 2H–2K and Table S1). Interestingly, although ratios of LV weight to tibial length were not significantly different (Figure 2H and Table S1), ratios of lung and RV weight to tibial length were remarkably reduced in IL2/JES6-1-treated mice as compared to PBS-treated mice (Figure 2J and 2K and Table S1), suggesting that IL2/JES6-1 treatment might have exerted a prominent role in reducing lung remodeling and RV hypertrophy in these mice.
IL2/JES6-1 Treatment Significantly Reduced Lung Inflammatory Cytokines and Mildly Affected LV Inflammatory Cytokines in Mice with Existing LV Failure
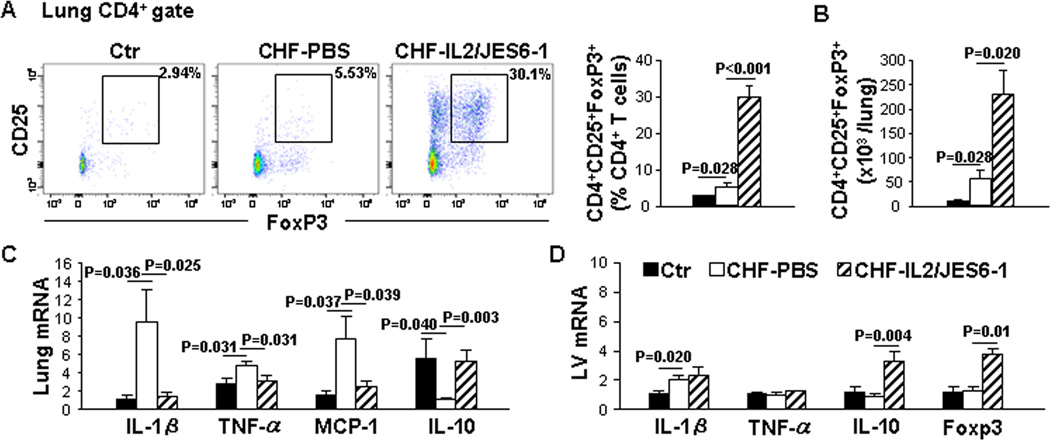
To determine whether IL2/JES6-1 treatment plays a role in pulmonary or myocardial inflammation with existing LV failure, Lung and LV mRNA levels of inflammatory cytokines were measured by quantitative RT-PCR. The percentage of Tregs and the total number of Tregs in the lung were 1.9-fold and 5.1-fold increased in CHF mice as compared to those in control mice. As expected, IL2/JES6-1 treatment resulted in a robust increase of Tregs in CHF mice (Figure 3A and 3B). As shown in Figure 3C, mRNA levels of IL-1β, TNF-α and monocyte chemoattractant protein-1 (MCP-1) were respectively up-regulated 8.4-fold, 1.7-fold and 4.8-fold in the lung of CHF mice, while IL-10 was reduced 81% as compared to that in control mice. IL2/JES6-1 treatment extensively suppressed IL-1β, TNF-α and MCP-1 by 85%, 33% and 68%, respectively, and significantly (5.1-fold) increased IL-10 in CHF mice (Figure 3C). These data demonstrate that IL2/JES6-1 treatment increases Tregs and blunts pro-inflammatory cytokine expression in the lung of mice with existing LV failure.
Figure 3. IL2/JES6-1 treatment significantly reduces lung inflammatory cytokines and mildly affects LV inflammatory cytokines in mice with existing left ventricle (LV) failure.
Data were collected from mice under basal conditions (Ctr), or treated with IL2/JES6-1 or PBS under TAC conditions (CHF-IL2/JES6-1 or CHF-PBS). A, Flow cytometry plots and quantitative data represent the percentage of Tregs (CD25+Foxp3+) within the CD4+ T-cell population of lungs. B, Quantitative data of flow cytometry represent the total number of Tregs in the lung. C, Quantitative RT-PCR results of IL-1β, TNF-α, MCP-1 and IL-10 mRNA levels in lung lysates. D, Quantitative RT-PCR results of IL-1β, TNF-α, IL-10 and Foxp3 mRNA levels in LV lysates. n=4–6 per group.
Furthermore as shown in Figure 3D, Foxp3 mRNA level was similar in the LV of CHF and control mice. IL2/JES6-1 treatment markedly increased Foxp3 mRNA level in CHF mice. The mRNA level of IL-1β in the LV was up-regulated 1.8-fold; while TNF-α did not change in CHF mice. IL2/JES6-1 treatment did not alter IL-1β or TNF-α mRNA level. However, LV IL-10 mRNA level was significantly increased by IL2/JES6-1 treatment. These data suggest that IL2/JES6-1 treatment did not significantly affect the contents of pro-inflammatory cytokine IL-1β or TNF-α mRNA, but increased anti-inflammatory IL-10 mRNA in mice with existing LV dysfunction.
IL2/JES6-1 Treatment Suppressed Macrophage, CD45+, CD4+, and CD8+ Cell Infiltration in the Lung of Mice with Existing LV Failure
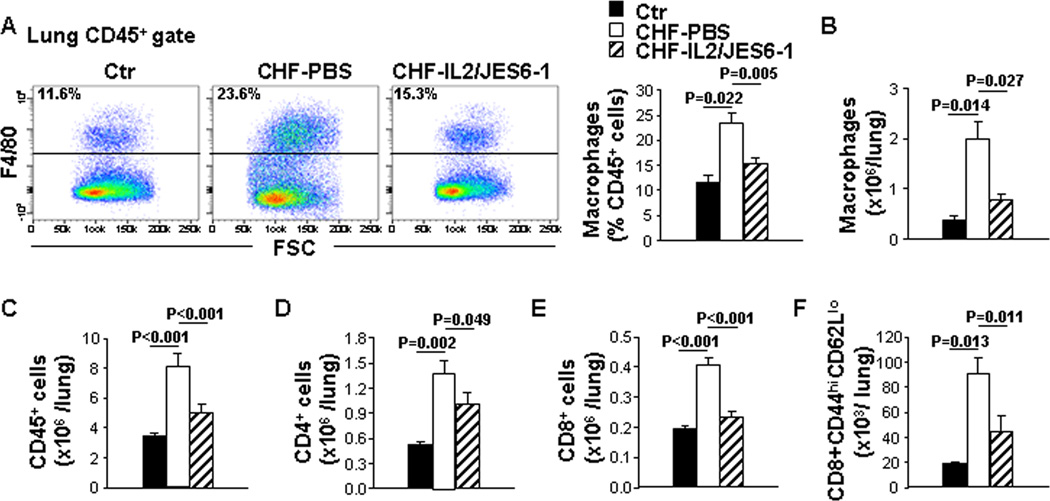
Inflammatory stimuli accumulate a variety of immune cells including macrophages and cytotoxic CD8+ T cells, which generate inflammatory cytokines that are important for the outcome of tissue inflammation. In order to understand the potential mechanism for beneficial effects of IL2/JES6-1 on the lung inflammation due to CHF, we examined macrophages, CD4+ and CD8+ T cells in the lung using flow cytometry analysis. CHF mice exhibited dramatic macrophage accumulation in the lung. IL2/JES6-1 treatment significantly reduced macrophage accumulation in the lung with existing LV failure (Figure 4A and 4B), which was also consistent with the observation of Mac2 staining in the lung (Figure S5A). Besides, leukocyte infiltration including CD45+ cells, CD4+, CD8+ T cells and effector memory CD8+ T cells was markedly increased in the lung of CHF mice. IL2/JES6-1 treatment significantly attenuated these increases in the lung of mice with existing LV failure (Figure 4C–4F). These data reveal that IL2/JES6-1 treatment reduces immune-cell accumulation in the lung of mice with existing LV failure.
Figure 4. IL2/JES6-1 treatment suppresses immune-cell infiltration in the lung of mice with existing left ventricle (LV) failure.
Data were collected from mice under basal conditions (Ctr), or treated with IL2/JES6-1 or PBS under TAC conditions (CHF-IL2/JES6-1 or CHF-PBS). A, Flow cytometry plots and quantitative data represent the percentage of macrophages (F4/80+) within the CD45+ cell population of lungs. B, Quantitative data of flow cytometry represent the total number of macrophages in the lung. C to F, Quantitative data of flow cytometry represent the total number of CD45+ cells, CD4+ T, CD8+ T cells and effector memory (CD44highCD62Llow) CD8+ T cells in the lung. n=4–6 per group.
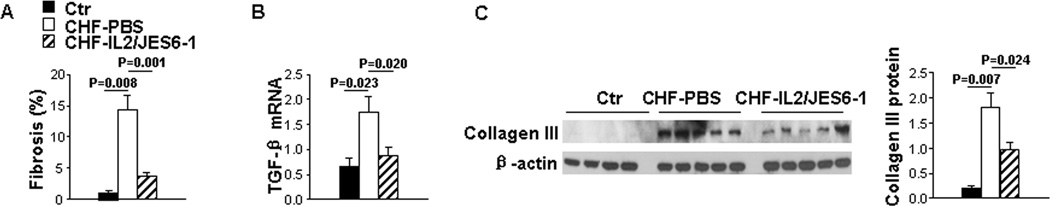
IL2/JES6-1 Treatment Attenuated Pulmonary Fibrosis in Mice with Existing LV Failure
Pro-inflammatory cytokines are known to accelerate the accumulation of collagen following the inflammatory response, which causes pulmonary fibrosis. As shown in Figure 5A and Figure S5B, lung fibrosis as identified by Sirius red and Fast green staining was remarkably increased (7.2-fold) in the lung of CHF mice as compared to that in control mice. IL2/JES6-1 treatment significantly attenuated TAC-induced profound lung fibrosis. Consistently, the increased TGF-β, a pro-fibrogenic cytokine mRNA in the lung of CHF mice was also significantly reduced by IL2/JES6-1 treatment (Figure 5B), which was in line with collagen-III deposition results (Figure 5C). These data demonstrate that IL2/JES6-1 treatment attenuates lung fibrosis in mice with existing LV failure.
Figure 5. IL2/JES6-1 treatment attenuates lung fibrosis in mice with existing left ventricle (LV) failure.
Data were collected from mice under basal conditions (Ctr), or treated with IL2/JES6-1 or PBS under TAC conditions (CHF-IL2/JES6-1 or CHF-PBS). A, Quantitative data of Sirius red/Fast green staining for detection of fibrosis in lungs. B, Quantitative RT-PCR results of TGF-β mRNA levels in lung lysates. C, Western blot of collagen III and β-actin (loading control) in lung lysates. n=5 per group.
IL2/JES6-1 Treatment attenuated TAC-Induced LV Inflammatory Responses and Hypertrophy
Since IL2/JES6-1 treatment tended to decrease the LV hypertrophy in mice with existing LV failure (Figure 2H), we subsequently determined whether IL2/JES6-1 treatment attenuated TAC-induced LV inflammatory responses and hypertrophy. Briefly, IL2/JES6-1 treatment was commenced 3 days before a moderate TAC created with a 26G needle, and final studies were performed 12 weeks after TAC. As expected, IL2/JES6-1 treatment significantly attenuated TAC-induced increases in ratios of LV, LA and lung weight to tibial length (Figure 6A–6C). IL2/JES6-1 treatment also attenuated TAC-induced decrease of LV EF (Figure 6D) and the increase of LVESD (Figure 6E), while LVEDD was not significantly affected (Figure 6F). Furthermore, IL2/JES6-1 treatment prevented TAC-induced increases of LV atrial natriuretic peptide (ANP) and β-myosin heavy chain protein content (Figure 6G). In addition, IL2/JES6-1 treatment significantly attenuated TAC-induced increases of LV CD45+ cell infiltration (Figure 6H) and mRNA levels of IL-1β and TNF-α, as well as the decrease of IL-10 mRNA level (Figure 6I). Foxp3 mRNA was remarkably up-regulated in the LV of IL2/JES6-1-treated mice (Figure 6I). These data indicate that IL2/JES6-1 treatment has attenuated TAC-induced LV hypertrophy and dysfunction, as well as LV inflammatory response.
Figure 6. IL2/JES6-1 treatment attenuates the development of transverse aortic constriction (TAC)-induced left ventricular (LV) hypertrophy and cardiac dysfunction.
The treatment was commenced 3 days before TAC. Data were collected from mice under basal conditions (Ctr), or treated with IL2/JES6-1 or PBS under TAC conditions (CHF-IL2/JES6-1 or CHF-PBS). A to C, The ratio of LV, left atria (LA) and lung weight to tibial length of mice. D to F, Echocardiographic measurements of LV ejection fraction (EF), LV end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD) from hearts. G, Western blot of ANP, β-MHC and vinculin (loading control) in LV lysates. H, Quantitative data of CD45 immunostaining of LVs. I, Quantitative RT-PCR results of IL-1β, TNF-α, IL-10 and Foxp3 mRNA levels in LV lysates. n=5 per group. J, Diagram of the proposed underlying mechanism.
Discussion
We demonstrate that induction of Tregs with IL2/JES6-1 treatment is effective in attenuating cardiac and lung inflammation, RV hypertrophy and further LV functional deterioration in mice with existing LV failure. Mechanistically, we demonstrated that end-stage CHF is characterized by a profound immune-cell accumulation in the lung and relatively mild inflammation in the heart. Cytokine therapy with IL2/JES6-1 results in significant increases of Tregs, consequently reduces infiltration of macrophages and CD8+ T cells, and decreases pro-inflammatory cytokine expression in the lung of mice with existing LV failure. IL2/JES6-1 treatment prior TAC also attenuates the development of LV hypertrophy and dysfunction. The potential underlying mechanism is summarized in Figure 6J and online Figure S6. These findings indicate that therapeutic approaches to increase Tregs are useful in treating CHF by attenuating cardiac, lung and/or systemic inflammation.
In clinical conditions, patients usually do not seek treatment until LV-dysfunction symptoms occur. Therefore, therapies to attenuate the transition from LV failure to RV hypertrophy/failure are clinically significant. We found that IL2/JES6-1 treatment continuously caused systemic Treg induction in mice under control conditions and after TAC. IL2/JES6-1 treatment significantly reduced pulmonary inflammation and fibrosis, and attenuated RV hypertrophy in mice with existing LV failure. IL2/JES6-1 also significantly attenuated further decrease of LV dysfunction in mice with existing LV failure. These data indicate that increasing Tregs can be a promising therapeutic approach to attenuate the transition from LV failure to RV hypertrophy/failure. The findings that IL2/JES6-1 treatment dramatically reduced lung inflammation/remodeling and RV hypertrophy without significant attenuated LV hypertrophy in mice with existing LV failure suggest that IL2/JES6-1 treatment might exert more impact on the lung and RV remodeling in these mice. The decreased lung remodeling after IL2/JES6-1 treatment in mice with existing LV failure is likely a collective effect of the increased expression of anti-inflammatory cytokine IL-10, the reduced accumulation of inflammatory leukocytes (such as CD4+ T cells, CD8+ T cells and macrophages), and the reduced expression of pro-inflammatory cytokines (such as IL-1β, TNF-α and MCP-1) in lung tissues. The reduced RV hypertrophy is likely a result of reduced lung inflammation and remodeling, as well as the reduced ventricular inflammatory response due to the induction of Tregs.
In addition, IL2/JES6-1 treatment prior to TAC also effectively attenuated TAC-induced LV hypertrophy and dysfunction, suggesting that IL2/JES6-1 treatment attenuates CHF progression not only by attenuating lung inflammation observed in mice with end-stage CHF, but also by attenuating the low grade LV inflammation at least partially through the collective effect of the increased expression of IL-10, the reduced accumulations of CD3+ T and macrophages, and the reduced expression of pro-inflammatory cytokines in LV tissues. Our findings that induction of Tregs attenuates tissue inflammation and CHF progression are conceptually consistent with the important role of Tregs in suppressing inflammation in other diseases, such as type-1 diabetes15, organ transplantation-induced inflammation and rejection16, myocardial infarction-induced cardiac remodeling17 and high fat diet-induced atherosclerosis18. Acute depletion of Tregs resulted in a rapid development of autoimmune diseases and mice died within 3 weeks.19 Depletion of Tregs with Foxp3DTR transgene in adult mice resulted in an increased infarct size, exacerbated myocardial inflammation, and worse clinical outcome.20 Depletion of Tregs using anti-CD25 antibodies also resulted in increases of infarction-induced LV dilation and remodeling.21 While the detailed molecular mechanism by which Tregs exert their suppressor/regulatory activity has not been definitively characterized, studies indicate that Tregs exert their biological actions by suppressing proliferation and cytokine production of T helper cells, reducing the accumulation of activated T cells, inhibiting the maturation and function of antigen-presenting cells, and raising the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β.19, 22
IL-2 is required for the proliferation, functional competence and stability of Tregs and plays a critical role in regulating the immune tolerance.9, 10 Inhibition of IL-2 signaling by IL-2 neutralization, IL-2 gene deletion, or IL-2 receptor deletion causes autoimmune diseases such as diabetes and colitis by reducing proliferation of Tregs.9, 10 IL-2 administration drives Treg expansion both in vitro and in vivo. However, due to a rapid enzymatic degradation of IL-2, administration of IL-2 alone in vivo could only lead to a mild increase of Tregs.9,10 Nevertheless, the biological activity of IL-2 in vivo could be greatly enhanced by administation of IL-2 with a particular IL-2 monoclonal antibody JES6-1, as JES6-1 binds to a site on IL-2 that could reduce IL-2 degradation and selectively lead to vigorous stimulation of CD25+ cells.11, 12 The ~6 fold increase of Tregs in response to IL2/JES6-1 treatment is largely consistent with the previous reports in other disease models.
It should be mentioned that severe LV failure results in an increase of pulmonary venous pressure, a critical factor in CHF-induced lung remodeling and RV hypertrophy.1, 2 Previous studies have reported lung inflammation in swine after pulmonary venous banding23 and in patients with increased pulmonary venous pressure secondary to mitral valve diseases or LV failure24. In the present study, induction of Tregs in mice with existing LV failure only partially inhibits the further decrease of LV EF and lung inflammation in these mice, indicating that ameliorating inflammation alone can’t totally block CHF progression.
We demonstrate that end-stage CHF is associated with a profound lung leukocyte infiltration and a relatively mild LV leukocyte infiltration, suggesting an important role of lung inflammation in CHF progression and the transition from LV failure to RV hypertrophy/failure. Unfortunately, there are no available experimental models to unambiguously define the precise impact of lung inflammation/remodeling on CHF progression at the present time.
In summary, cytokine therapy using IL2/JES6-1 treatment is effective in selective induction of Tregs, and in attenuating the progression of CHF by reducing cardiac, pulmonary and/or systemic inflammation.
Supplementary Material
Table 1.
Outline of experiment design.
| Study purpose * |
Mice background |
Needle used for TAC |
Time to give IL2/JES6-1 |
Time to collect data |
|---|---|---|---|---|
| Protocol-1 | Balb/c | 27G | 10 days after TAC | 4W after TAC |
| Protocol-2 | C57/B6 | 26G | 3 days before TAC | 12W after TAC |
The purpose of protocol-1 was to determine the effect of induction of Tregs in treating mice with existing LV failure. The purpose of protocol-2 is to determine the effect of induction of Tregs in attenuating or preventing the development of LV failure. The degree of TAC is calibrated by a 27G needle (severe TAC) or a 26G (moderate TAC).
Perspectives.
End-stage congestive heart failure (CHF) is often accompanied by severe inflammatory responses in lungs and moderate inflammation in hearts. CD4+CD25+Foxp3+ regulatory T cells (Tregs) suppress activation of the immune system and contribute to the maintenance of immunologic self-tolerance. Cytokine therapy with Interleukin-2/anti-interleukin-2 antibody (JES6-1) complexes (IL2/JES6-1) selectively expands and maintains 5 to 6-fold increase of Tregs in vivo. In this article, we demonstrate that IL2/JES6-1 treatment suppresses up-regulated inflammation and fibrosis in hearts and lungs due to pressure overload, and consequently attenuates both development and progression of systolic overload-induced CHF. Our study provides the first direct evidence that treatment with IL2/JES6-1 may be an attractive new therapeutic approach for treating CHF.
Novelty and Significance.
What Is New?
Induction of regulatory T cells (Tregs) is effective in attenuating lung inflammation and the transition from left ventricular (LV) to right ventricular hypertrophy in mice with existing LV failure.
What Is Relevant?
End-stage congestive heart failure (CHF) is often accompanied by severe inflammatory responses in lungs and moderate inflammation in hearts. Thus, induction of Tregs may be an attractive new therapeutic approach for treating end-stage CHF.
Summary
Selective induction of Tregs is effective in attenuating lung inflammation and fibrosis, RV hypertrophy and the further decrease of LV function in mice with existing LV failure, suggesting strategies to properly expand Tregs may be useful in treating CHF.
Acknowledgments
None.
Sources of funding
This study was supported by U.S. Public Health Service Grants HL021872, HL098669, HL098719, HL102597, HL089249, R01HL105406, HL11879, and T32HL069764 from the National Institutes of Health, and Research Grant 09GRNT2260175 from the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8:776–787. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–2124. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haribhai D, Williams JB, Jia S, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A, Agrotis A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 12.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 13.Singer BD, King LS, D'Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46. doi: 10.3389/fimmu.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Xu X, Fassett J, Kwak D, Liu X, Hu X, Falls TJ, Bell JC, Li H, Bitterman P, Bache RJ, Chen Y. Double-stranded RNA-dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure. Circulation. 2014;129:1397–1406. doi: 10.1161/CIRCULATIONAHA.113.002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl. Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edozie FC, Nova-Lamperti EA, Povoleri GA, Scotta C, John S, Lombardi G, Afzali B. Regulatory T-cell therapy in the induction of transplant tolerance: the issue of subpopulations. Transplantation. 2014;98:370–379. doi: 10.1097/TP.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 17.Sharir R, Semo J, Shimoni S, Ben-Mordechai T, Landa-Rouben N, Maysel-Auslender S, Shaish A, Entin-Meer M, Keren G, George J. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS. One. 2014;9:e113653. doi: 10.1371/journal.pone.0113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 20.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 21.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307:H1233–H1242. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Immunology: Conditional stability of T cells. Nature. 2010;468:41–42. doi: 10.1038/468041a. [DOI] [PubMed] [Google Scholar]
- 23.Pereda D, Garcia-Alvarez A, Sanchez-Quintana D, Nuno M, Fernandez-Friera L, Fernandez-Jimenez R, Garcia-Ruiz JM, Sandoval E, Aguero J, Castella M, Hajjar RJ, Fuster V, Ibanez B. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl. Res. 2014;7:494–506. doi: 10.1007/s12265-014-9564-6. [DOI] [PubMed] [Google Scholar]
- 24.Wagenvoort CA. Lung biopsy findings in secondary pulmonary hypertension. Heart Lung. 1986;15:429–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.