Abstract
Background:
As the clinical value of cytokeratin-19 (CK19) and thymidine kinase-1 (TK1) in advanced gastrointestinal cancer remains controversial, we investigated their expression and clinical significance in this disease.
Methods:
A total of 171 advanced gastrointestinal cancer patients were prospectively enrolled in this study. The mRNA level of CK19 was detected using quantitative real-time reverse transcription-polymerase chain reaction (PCR) in all patients, along with a control group of fifty healthy individuals. Furthermore, detection of TK1 protein was carried out in 96 patients using a chemiluminescence dot blot assay. The primary endpoint was overall survival (OS) time.
Results:
Positive CK19 mRNA expression was detected in 74 (43.3%) of the 171 patients and positive TK1 expression was detected in 66 (68.8%) of the 96 patients. Furthermore, of the 96 patients, 36 (37.5%) were positive for both TK1 protein and CK19 mRNA, 30 (31.3%) were negative for TK1 protein, and 15 (15.6%) were negative for TK1 protein and positive for CK19 mRNA. The results indicated that patients who were positive for CK19 mRNA expression had significantly shorter OS times than those who were negative for it (median OS 7.7 vs. 9.7 months, respectively; P = 0.02). Moreover, patients who were positive for CK19 mRNA and TK1 protein expression had shorter OS times (median OS 6.1 months) than those who were positive for CK19 mRNA and negative for TK1 protein expression (median OS 9.1 months; P = 0.028). Positive CK19 mRNA expression was significantly associated with shorter OS in the univariate analysis (P = 0.027). Based on a multivariate Cox regression analysis, CK19 mRNA together with TK1 protein expression (P = 0.024) was an independent predictor for OS in gastrointestinal cancer patients.
Conclusions:
Our results suggest that positive expression of CK19 mRNA and TK1 protein is closely correlated with poor prognosis in advanced gastrointestinal cancer. Furthermore, both CK19 and TK1 are possible gastrointestinal cancer biomarkers.
Keywords: Advanced Gastrointestinal Cancer, Cytokeratin-19, Overall Survival, Thymidine Kinase-1
INTRODUCTION
Gastrointestinal cancer is a common digestive system tumor, accounting for 30% of the global incidence and 40% of the global malignant tumor mortality.[1] Tumor recurrence and metastases are still the major causes of disease development and death.[2] Therefore, prognostic and predictive factors are urgently needed for a better-personalized treatment.
Circulating tumor cells (CTCs) play an important role in the initial stage of cancer metastasis. CTCs can be released from a primary site through the bloodstream to the distant organs, contributing to metastasis.[3] Previous studies reported that the presence of CTCs was directly related to the prognosis of patients with metastatic breast cancer.[4,5,6] The detection of CTCs in the blood requires highly sensitive, specific, and reproducible methods. To date, several methods including immunocytochemistry, real-time reverse transcription-polymerase chain reaction (PCR), and flow cytometry have been used for the detection of these rare CTCs in the peripheral blood.[7]
Cytokeratin-19 (CK19) is a cytoskeletal protein that is expressed on epithelial but not on mesenchymal cells.[8,9,10] It is the most commonly used marker for CTCs.[11,12] To the best of our knowledge, not all CTCs arrive at a secondary site and develop into metastases.[13,14,15] If CTCs proliferate, it is likely that they will develop into metastases, but if they do not, they are cleared from the blood, cannot be detected, and will not develop into metastases. Thymidine kinase-1 (TK1) is internationally recognized as a marker of abnormal cell proliferation, which enables the dynamic monitoring of the proliferation of cancer cells and can indicate tumor recurrence and metastasis.[16] TK1 is usually detected using a chemiluminescence dot blot assay.[16]
To the best of our knowledge, rare studies have yet reported on the expression of both CK19 and TK1 in gastrointestinal cancer. In the present study, we used quantitative real-time PCR and chemiluminescence dot blot assay methods to specifically quantify the expression levels of CK19 and TK1, respectively, in the peripheral blood, and followed up patients with recurrence and death, to explore the clinical value and application of CK19 and TK1 as prognostic indicators of gastrointestinal cancer.
METHODS
Patients
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University. Written informed consent was obtained from all of the patients. A total of 171 patients with advanced gastrointestinal cancer attending the Department of Oncology of the First Affiliated Hospital of Anhui Medical University from May 2013 to January 2014 were enrolled in this study. None of the patients had received any treatment before the sample collection, including surgery, radiotherapy, or chemotherapy. All cases were pathologically confirmed as gastrointestinal cancer, and no patients had a history of any other type of cancers. Patient characteristics are shown in Table 1. There were 112 male and 59 female patients with a mean age of 59 years (range: 23–86 years). The gastrointestinal cancer specimens originated from esophageal cancer (n = 23), gastric cancer (n = 90), colorectal cancer (n = 31), and rectal cancer (n = 27). A total of fifty healthy people without a history of other systemic diseases, consisting of graduate students and staff of the First Affiliated Hospital of Anhui Medical University, served as controls.
Table 1.
Characteristics of enrolled patients with advanced gastrointestinal cancer
| Characteristics | All patients (n = 171) | CK19 mRNA positive (n = 74) |
|---|---|---|
| Age (years), median (range) | 59 (23–86) | 61 (25–86) |
| Gender, n (%) | ||
| Male | 112 (65.9) | 30 (26.8) |
| Female | 59 (34.5) | 44 (74.6) |
| Metastatic site, n (%) | ||
| 1–2 | 147 (86.0) | 62 (42.2) |
| 3–4 | 24 (14.0) | 12 (50.0) |
| Differentiation, n (%) | ||
| Well | 56 (32.8) | 21 (37.5) |
| Moderate | 83 (48.5) | 37 (44.6) |
| Poor | 32 (18.7) | 13 (40.6) |
| Classification of diseases, n (%) | ||
| Esophageal cancer | 23 (13.5) | 9 (39.1) |
| Gastric cancer | 90 (52.6) | 36 (40.0) |
| Colorectal cancer | 31 (18.1) | 16 (51.6) |
| Rectal cancer | 27 (15.8) | 13 (48.1) |
CK19: Cytokeratin 19
Collection of serum samples
Pretreatment fasting blood samples were collected from the peripheral vein into ethylenediaminetetraacetic acid-containing tubes. The first 3 ml of blood was discarded to prevent the epidermal contamination. Sample processing was performed within 1 h of blood withdrawal. Blood was transferred into a 10 ml centrifuge tube and centrifuged at 10,000 ×g at room temperature for 10 min. The plasma was removed, and the peripheral blood mononuclear cell (PBMC) fraction was stored at −80°C for future use.
RNA extraction and quantitative real-time reverse transcription-polymerase chain reaction
CK19 mRNA levels were detected in all patients and healthy controls. The CK19 mRNA assay was performed using a nucleic acid detection kit, based on the PCR fluorescence probe method (Pu Yuan Biotech Ltd., Anhui, China). Total RNA from the PBMC fraction was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer's protocol. The RNA was pretreated with RNAase-free DNase and 2 μg of RNA from each sample was used for cDNA synthesis with random hexamers. cDNA synthesis was carried out as described previously.[17] The PCR assay conditions and the primers for CK19 have also been described in detail previously.[18] All samples were measured in triplicate. The following primers were used: CK19, forward: 5′-GAA ATC AGT ACG CTG AGG GG-3′ and reverse: 5′-CCG GCT GGT GAA CCA GGC TT-3′ and glyceraldehyde-3-phosphate dehydrogenase, forward: 5′-GAA GGT GAA GGT CGG AGT CA-3′ and reverse: 5′-TTG AGG TCA ATG AAG GGG TC-3′. PCR reactions were performed in an ABI PRISM 7700 Sequence Detector (Perkin Elmer/Applied Biosystems, CA, USA). PCR amplifications were performed in a total volume of 50 μl consisting of 5 μl of template cDNA and 15 μl of PCR master mixture containing Tag DNA polymerase, dNTP mixture, and reaction buffer.
Cycling conditions were 5 min at 95°C for initial denaturation, followed by 45 cycles of 15 s at 95°C for denaturation, 60 s at 60°C for combined annealing, and 15 s at 72°C for primer extension. The cutoff level of CK19 mRNA copies was set at 102 copies/ml.
Thymidine kinase-1 assay
TK1 protein levels were detected in 96 patients (some of the patients refused to have the test) and the healthy controls. The TK1 assay was performed using a commercial kit, based on an enhanced chemiluminescence dot blot assay (Huarui Tongkang Bio-Tech Ltd., Shenzhen, China).[19] Briefly, 3 μl of serum was directly applied onto a nitrocellulose membrane. The serum samples were probed with human anti-TK1 chicken immunoglobulin Y antibody. Varying concentrations of TK1-peptide (20.0, 6.6 and 2.2 pmol/L) were used as an extrapolation standard. The spot intensities on the membrane were determined using a CIS-l Imaging System (Huarui Tongkang Bio-Tech Ltd., Shenzhen, China). From the intensities of the TK1 standard, the TK1 concentrations were calculated and expressed as pmol/L. The coefficient of variation was <10%. The threshold value of TK1 was set at 2.0 pmol/L. TK1 values of <2.0 pmol/L were defined as negative and considered as a lower risk for developing malignancy. TK1 values of >2.0 pmol/L were defined as positive and likely to represent individuals with an increased risk of premalignancy/malignancy progression.
Follow-up
The follow-up information was obtained from the medical records of the clinic and by telephone or written contact with the patients. The follow-up period was from the date of diagnosis to death or the last follow-up contact. The follow-up investigation was closed on January 10, 2015.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc., IL, USA). Differences in expression of CK19 mRNA and TK1 protein according to different clinicopathological characteristics were analyzed using the Chi-square test. Overall survival (OS) was analyzed using the Kaplan-Meier method, and differences were examined using the log-rank test. Univariate and multivariate analyses were performed using Cox regression analysis. A P < 0.05 was considered statistically significant.
RESULTS
Expression of cytokeratin-19 mRNA and thymidine kinase-1 protein in gastrointestinal cancer
We first examined expression levels of CK19 mRNA in 171 patients with gastrointestinal cancer by real-time PCR. Seventy four (43.3%) of the 171 patients were found to be positive for CK19 mRNA. None of the fifty healthy individuals had CTCs positive for CK19 mRNA. The positive rate of CK19 mRNA in healthy patients was significantly lower than that in samples from the advanced gastrointestinal cancer patients (P < 0.001). CK19 mRNA was observed in 39.1% of esophageal cancers (9/23), 40.0% of gastric cancers (36/90), 51.6% of colon cancers (16/31), and 48.1% of rectal cancers (13/27). Among the 171 patients, 26.8% of male patients (30/112) and 74.6% of female patients (44/59) were positive for CK19 mRNA.
We next examined TK1 protein expression in 96 patients with gastrointestinal cancer by chemiluminescence dot blot assay. Sixty-six (68.8%) were positive for TK1 protein. TK1 expression was observed in 81.8% of esophageal cancers (9/11), 74.5% of gastric cancers (38/51), and 55.9% of colorectal cancers (19/34).
Both TK1 protein and CK19 mRNA were detected in 36 (37.5%) of the 96 patients. Thirty (31.3%) of the 96 patients were negative for TK1. Fifteen (15.6%) of the 96 patients were TK1 protein-negative and CK19 mRNA-positive.
Survival analysis
The association of CK19 mRNA expression with OS in patients with gastrointestinal cancer was analyzed using the Kaplan-Meier method [Figure 1]. The results indicated that patients with positive CK19 mRNA expression had significantly shorter OS times than those who were negative for CK19 mRNA expression (median OS 7.7 vs. 9.7 months, respectively; P = 0.02).
Figure 1.
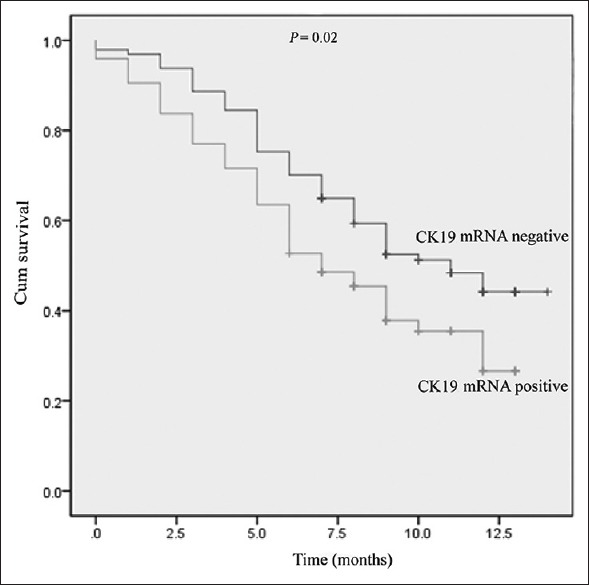
Kaplan-Meier survival curves of patients with positive or negative expression of CK19 mRNA. CK19: Cytokeratin-19.
Patients who were positive for CK19 mRNA and TK1 protein expression had significantly shorter OS times than patients who were positive for CK19 mRNA and negative for TK1 protein expression. Accordingly, patients who were positive for CK19 mRNA and TK1 protein expression had shorter OS times than those who were positive for CK19 mRNA and negative for TK1 protein expression (median OS 6.1 vs. 9.1 months, respectively; P = 0.028; Figure 2).
Figure 2.
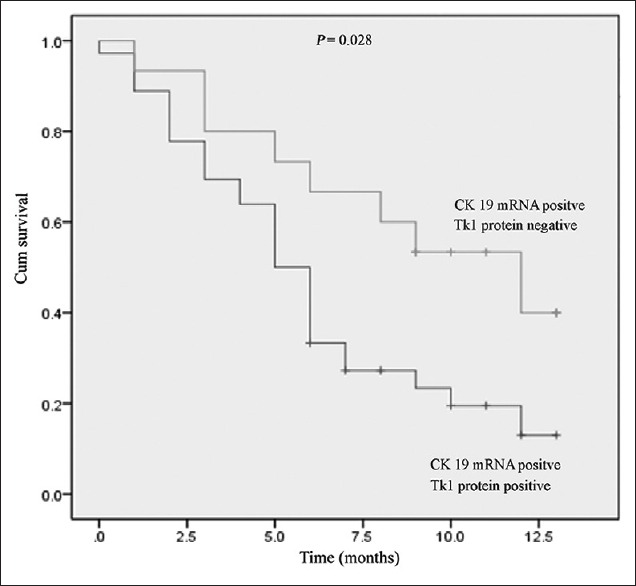
Kaplan-Meier survival curves of patients with CK19 mRNA positive and TK1 protein positive or CK19 mRNA positive but TK1 protein negative. CK19: Cytokeratin-19; TK1: Thymidine kinase-1.
Univariate and multivariate analyses
In the univariate analysis, positive CK19 mRNA expression was significantly associated with unfavorable outcomes (P = 0.027), and CK19 mRNA associated with TK1 was significantly related to OS (P = 0.031). Based on a multivariate Cox regression analysis, CK19 mRNA together with TK1 protein expression (P = 0.024) was an independent predictor for OS in gastrointestinal cancer patients [Table 2].
Table 2.
Multivariate analysis for overall survival in patients with advanced gastrointestinal cancer (n = 96)
| Variables | HR (95% CI) | P |
|---|---|---|
| Gender (male vs. female) | 0.801 (0.390–1.642) | 0.544 |
| Age (<50 vs. ≥50 years) | 0.976 (0.948–1.006) | 0.117 |
| Metastatic site (1–2 vs. 3–4) | 1.393 (0.570–3.832) | 1.478 |
| Route of metastasis (lymph nodes vs. blood) | 0.910 (0.550–2.403) | 1.150 |
| Differentiation (poor vs. well/moderate) | 0.870 (0.605–1.250) | 1.548 |
| CK19 and TK1 (CK19 positive and TK1 positive vs. CK19 positive but TK1 negative) | 0.366 (0.152–0.876) | 0.024 |
CK19: Cytokeratin-19; TK1: Thymidine kinase-1; CI: Confidence interval; HR: Hazard ratio.
DISCUSSION
The presence of CTCs has been analyzed in breast[6,14] and bladder cancer as has the presence of TK1 protein in breast and lung cancer.[17] However, the significance of CTCs and TK1 expression in the clinical setting and their prognostic value in gastrointestinal cancer has not yet been established. Therefore, the current study investigated whether CTCs were associated with TK1 in terms of protein levels and whether CTCs and TK1 levels had prognostic significance in patients with gastrointestinal cancer.
Malignant cells detached from primary tumors become invasive. These cells might then intravasate into the blood or lymphatic circulation and subsequently extravasate from the circulation and establish a secondary tumor in another organ far from the primary tumor.[20,21] The detection of CTCs has been well demonstrated in breast, lung, colon, prostate, and bladder cancers, melanoma, and other malignancies.[22,23,24,25,26] Although CTC detection can contribute to tumor diagnosis and help identify patients with advanced bladder cancer, such assays cannot be used as initial screening diagnostic tests due to their low sensitivity.[27] PCR-based methods are commonly used, rapid, and low-cost methods for detecting CTCs.[28] The utility of CTCs as biomarkers for predicting the clinical outcomes of patients with various cancers has been revealed in many studies.[29,30]
TK is a key enzyme in the pyrimidine salvage pathway, catalyzing the transfer of the terminal phosphate from adenosine triphosphate to the 5’-hydroxyl group of thymidine, producing deoxyadenosine triphosphate, which is subsequently incorporated into DNA. There are two forms of cellular TK enzymes; the cytoplasmic form, TK1, and the mitochondrial form, TK2. TK2 is cell-cycle independent whereas the level of TK1 protein is tightly correlated with the S and G2 phases of the cell-cycle and thus to overall cell proliferation. TK1 plays a role in regulating the intracellular thymidine pools throughout the cell-cycle but is probably also involved in the DNA repair process.[18] In proliferating normal cells and tumor cells, the synthesis of TK1 starts at the latter stage of the G1 phase and significantly increases until the late S and G2 phases.[31] TK1 in nonproliferating cells and in the serum of healthy people is minimal or undetectable.[31] TK1 also provides information on recurrence, on the 5-year survival of cancer patients and is useful for monitoring the outcome of tumor therapy in lung, rectal, breast, and cervical cancer patients.[18,31] Thus, elevated TK1 is an important risk factor indicating a high tumor proliferation capacity, leading to shorter survival.
In the study, we revealed the frequency of CK19 and TK1 expression in patients with metastatic and recurrent gastrointestinal cancer. At the same time, we investigated the value of CK19 and TK1 as prognostic indicators of advanced gastrointestinal cancer. CK19 mRNA was observed in 74 (43.3%) of all 171 advanced gastrointestinal cancer patients. TK1 was positive in 66 (68.8%) of 96 patients. The results indicate that positive CK19 expression predicts poor OS in patients with advanced gastrointestinal cancer; however, that patients who are positive for CK19 but negative for TK1 might have a better prognosis. In other words, patients who are positive for both CK19 and TK1 expression have worse OS than those who are positive for CK19 but negative for TK1 expression in advanced gastrointestinal cancer.
In conclusion, co-expression of CK19 and TK1 might be crucial for the progression of tumors and, therefore, for the prognosis of patients with gastrointestinal cancer, indicating that the expression of these might be considered as independent prognostic markers. More studies are urgently required to test the clinical utility of using CTCs and TK1 in liquid biopsies to contribute to personalized treatment strategies in gastrointestinal cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Rokavec M, Li H, Jiang L, Hermeking H. The p53/microRNA connection in gastrointestinal cancer. Clin Exp Gastroenterol. 2014;7:395–413. doi: 10.2147/CEG.S43738. doi: 10.2147/CEG.S43738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu D, Li XF, Zheng S, Jiang WZ. Quantitative real-time RT-PCR detection for CEA, CK20 and CK19 mRNA in peripheral blood of colorectal cancer patients. J Zhejiang Univ Sci B. 2006;7:445–51. doi: 10.1631/jzus.2006.B0445. doi: 10.1631/jzus.2006.B0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwatsuki M, Toyoshima K, Watanabe M, Hayashi N, Ishimoto T, Eto K, et al. Frequency of HER2 expression of circulating tumour cells in patients with metastatic or recurrent gastrointestinal cancer. Br J Cancer. 2013;109:2829–32. doi: 10.1038/bjc.2013.680. doi: 10.1038/bjc.2013.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 5.Reinholz MM, Kitzmann KA, Tenner K, Hillman D, Dueck AC, Hobday TJ, et al. Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central Cancer Treatment Group trials, N0234/336/436/437. Clin Cancer Res. 2011;17:7183–93. doi: 10.1158/1078-0432.CCR-11-0981. doi: 10.1158/1078-0432.CCR-11-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magbanua MJ, Carey LA, DeLuca A, Hwang J, Scott JH, Rimawi MF, et al. Circulating tumor cell analysis in metastatic triple-negative breast cancers. Clin Cancer Res. 2015;21:1098–105. doi: 10.1158/1078-0432.CCR-14-1948. doi: 10.1158/1078-0432.CCR-14-1948. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi H, Kitagawa Y. Circulating tumor cells in gastrointestinal cancer. J Hepatobiliary Pancreat Sci. 2010;17:577–82. doi: 10.1007/s00534-009-0193-4. doi: 10.1007/s00534-009-0193-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, et al. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: Significance of the prediction of postoperative metastasis. World J Surg. 2006;30:1007–13. doi: 10.1007/s00268-005-0485-z. doi: 10.1007/s00268-005-0485-z. [DOI] [PubMed] [Google Scholar]
- 9.Sun GR, Dong XY, He QS, Qu H, Wang YM. Expression and clinical significance of CK19 and CK20 expressions in transverse mesocolon biopsies from patients with gastric carcinoma. Cell Biochem Biophys. 2012;62:361–4. doi: 10.1007/s12013-011-9293-2. doi: 10.1007/s12013-011-9293-2. [DOI] [PubMed] [Google Scholar]
- 10.Saloustros E, Mavroudis D. Cytokeratin 19-positive circulating tumor cells in early breast cancer prognosis. Future Oncol. 2010;6:209–19. doi: 10.2217/fon.09.147. doi: 10.2217/fon.09.147. [DOI] [PubMed] [Google Scholar]
- 11.Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14:2593–600. doi: 10.1158/1078-0432.CCR-07-4758. doi: 10.1158/1078-0432.CCR-07-4758. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Wang Y, Liu Y, Cheng M, Wu X, Wei H. Flow cytometric analysis of CK19 expression in the peripheral blood of breast carcinoma patients: Relevance for circulating tumor cell detection. J Exp Clin Cancer Res. 2009;28:57. doi: 10.1186/1756-9966-28-57. doi: 10.1186/1756-9966-28- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69(Suppl 1):14–6. doi: 10.1159/000086626. doi: 10.1159/000086626. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar N, Hu P, Bedard P, Clemons M, McCready D, et al. Identification of genomic signatures in circulating tumor cells from breast cancer. Int J Cancer. 2015;137:332–44. doi: 10.1002/ijc.29399. doi: 10.1002/ijc.29399. [DOI] [PubMed] [Google Scholar]
- 15.Ismail MS, Wynendaele W, Aerts JL, Paridaens R, Gaafar R, Shakankiry N, et al. Detection of micrometastatic disease and monitoring of perioperative tumor cell dissemination in primary operable breast cancer patients using real-time quantitative reverse transcription-PCR. Clin Cancer Res. 2004;10:196–201. doi: 10.1158/1078-0432.ccr-0515-2. doi: 10.1158/1078-0432.CCR-0515-2. [DOI] [PubMed] [Google Scholar]
- 16.Bjöhle J, Bergqvist J, Gronowitz JS, Johansson H, Carlsson L, Einbeigi Z, et al. Serum thymidine kinase activity compared with CA 15-3 in locally advanced and metastatic breast cancer within a randomized trial. Breast Cancer Res Treat. 2013;139:751–8. doi: 10.1007/s10549-013-2579-x. doi: 10.1007/s10549-013-2579-x. [DOI] [PubMed] [Google Scholar]
- 17.He E, Xu XH, Guan H, Chen Y, Chen ZH, Pan ZL, et al. Thymidine kinase 1 is a potential marker for prognosis and monitoring the response to treatment of patients with breast, lung, and esophageal cancer and non-Hodgkin's lymphoma. Nucleosides Nucleotides Nucleic Acids. 2010;29:352–8. doi: 10.1080/15257771003738535. doi: 10.1080/15257771003738535. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, He C, Li L, Lin A, Zheng X, He E, et al. Nuclear TK1 expression is an independent prognostic factor for survival in pre-malignant and malignant lesions of the cervix. BMC Cancer. 2013;13:249. doi: 10.1186/1471-2407-13-249. doi: 10.1186/1471-2407-13- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZH, Huang SQ, Wang Y, Yang AZ, Wen J, Xu XH, et al. Serological thymidine kinase 1 is a biomarker for early detection of tumours – A health screening study on 35,365 people, using a sensitive chemiluminescent dot blot assay. Sensors (Basel) 2011;11:11064–80. doi: 10.3390/s111211064. doi: 10.3390/s111211064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidler IJ. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 21.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 22.Young R, Pailler E, Billiot F, Drusch F, Barthelemy A, Oulhen M, et al. Circulating tumor cells in lung cancer. Acta Cytol. 2012;56:655–60. doi: 10.1159/000345182. doi: 10.1159/000345182. [DOI] [PubMed] [Google Scholar]
- 23.Armakolas A, Panteleakou Z, Nezos A, Tsouma A, Skondra M, Lembessis P, et al. Detection of the circulating tumor cells in cancer patients. Future Oncol. 2010;6:1849–56. doi: 10.2217/fon.10.152. doi: 10.2217/fon.10.152. [DOI] [PubMed] [Google Scholar]
- 24.Katseli A, Maragos H, Nezos A, Syrigos K, Koutsilieris M. Multiplex PCR-based detection of circulating tumor cells in lung cancer patients using CK19, PTHrP, and LUNX specific primers. Clin Lung Cancer. 2013;14:513–20. doi: 10.1016/j.cllc.2013.04.007. doi: 10.1016/j.cllc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Nezos A, Lembessis P, Sourla A, Pissimissis N, Gogas H, Koutsilieris M. Molecular markers detecting circulating melanoma cells by reverse transcription polymerase chain reaction: Methodological pitfalls and clinical relevance. Clin Chem Lab Med. 2009;47:1–11. doi: 10.1515/CCLM.2009.009. doi: 10.1515/CCLM.2009.009. [DOI] [PubMed] [Google Scholar]
- 26.Davis JW. Circulating tumor cell assays for the prognosis of prostate and colon cancers. Expert Opin Med Diagn. 2009;3:293–301. doi: 10.1517/17530050902791598. doi: 10.1517/17530050902791598. [DOI] [PubMed] [Google Scholar]
- 27.Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: Systematic review and meta-analysis. BMC Cancer. 2011;11:336. doi: 10.1186/1471-2407-11-336. doi: 10.1186/1471-2407-11- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nezos A, Pissimisis N, Lembessis P, Sourla A, Dimopoulos P, Dimopoulos T, et al. Detection of circulating tumor cells in bladder cancer patients. Cancer Treat Rev. 2009;35:272–9. doi: 10.1016/j.ctrv.2008.11.003. doi: 10.1016/j.ctrv. 2008.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, et al. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. doi: 10.3892/ijo.28.2.297. [PubMed] [Google Scholar]
- 30.Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, He E, Skog S. The proliferation marker thymidine kinase 1 in clinical use. Mol Clin Oncol. 2013;1:18–28. doi: 10.3892/mco.2012.19. doi: 10.3892/mco. 2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


