Abstract
Background:
Nontuberculous Mycobacterium (NTM) bloodstream infection (BSI) is relatively rare. We aimed in this study to evaluate the clinical characteristics, laboratory evaluation, and outcomes of patients with NTM BSI.
Methods:
We retrospectively reviewed the clinical records of inpatients with NTM BSI at our institution between January 2008 and January 2015 and recorded clinical parameters including age, gender, underlying disease, clinical manifestation, organs involved with NTM disease, species of NTM, laboratory data, treatment and outcome of these patients. We also reviewed the reported cases and case series of NTM BSI by searching PubMed, EMBASE, and Wanfang databases. Data of normal distribution were expressed by mean ± standard deviation (SD). Data of nonnormal distribution were expressed by median and interquartile range (IQR).
Results:
Among the ten patients with NTM BSI, the median age was 51 years (IQR 29–57 years) and three patients were males. Eight patients were immunocompromised, with underlying diseases including human immunodeficiency virus (HIV) infection (one patient), rheumatic diseases (two patients), breast cancer (one patient), myelodysplastic syndrome (two patients), and aplastic anemia (two patients). Other organ(s) involved were lung (two patients), endocardium (two patients), brain, spinal cord, and soft tissue (one each patient). The median lymphocyte was 0.66 × 109/L (IQR 0.24–1.93 × 109/L). The median cluster of differentiation 4 (CD4) cell count was 179/mm3 (IQR 82–619/mm3). Five patients died (three with hematological diseases, one with breast cancer, and one with rheumatic disease), three recovered, and two were lost to follow-up.
Conclusions:
We reported all cases in our hospital diagnosed with bloodstream NTM infection that was rarely reported. In this group of patients, patients usually had a high fever and could have multiple organ involvements. All patients with poor prognosis had underlying diseases.
Keywords: Bloodstream Infection, Hematogenous Disseminated, Nontuberculous Mycobacterium
INTRODUCTION
Mycobacterium includes Mycobacterium tuberculosis (MTB) and nontuberculous Mycobacterium (NTM). NTM are ubiquitous microorganisms in the environment. Most NTM is found in wet soil, natural waters, and even in tap water.[1] They are not common causes of invasive diseases in immunocompetent individuals. Diseases and therapies that reduce cell-mediated immunity increase the risk of NTM disease. Acquired immunodeficiency syndrome (AIDS), cancer, and hematologic and solid organ transplants have been identified as associated with NTM disease. More recently, immunosuppressive drugs including anti-tumor necrosis factor biologics have been associated with NTM disease in population-based studies.[2] NTM disease has also been reported in patients with rheumatic and autoimmune diseases, such as in those with systemic lupus erythematosus (SLE).[3]
Extrapulmonary NTM disease, including disseminated, skin, and catheter-related disease, is more common in immunosuppressed patients compared to immunocompetent patients.[2] However, hematogenous disseminated NTM or NTM bloodstream infection (BSI) is a severe form of NTM disease as overall in-hospital mortality was reported to be 41.7% in AIDS patients.[4] However, relevant reports on NTM BSI are scarce with most published data emerging from the human immunodeficiency virus (HIV) infected patients.[4,5,6] To add to the knowledge regarding NTM BSI among the general population and immunocompromised patients admitted to the general hospital and to arouse clinicians to pay more attention to this severe form of NTM disease, we retrospectively evaluated all patients with NTM BSI who were admitted to our institution, a major national referral hospital in Beijing, China, between January 2008 and January 2015.
METHODS
Study subjects
We searched the electronic database in the clinical microbiology laboratory at our institution and found 58 reports of positive Mycobacterium species from peripheral blood samples between January 2008 and January 2015. Forty-eight cases of MTBwere excluded. For the remaining ten patients included in the final analysis, information including demographics, underlying diseases, clinical presentation, laboratory data, time of positive culture results, type of antimicrobial directed NTM therapy, and outcomes was collected from the medical records. This study was reviewed and approved by the Institutional Review Board at our institution and waiver of consent was granted because this was a retrospective and observational study.
We also reviewed the past case reports and case series of NTM BSI by searching the PubMed, EMBASE, and Wanfang databases up to February 29, 2016. We used the search terms “Non-tuberculous Mycobacterium,” and “bloodstream infection,” or “bacteremia” or “blood culture” or “hematogenous disseminated.” The inclusion criterion was open published full texts, English or Chinese, and NTM BSI diagnosed by positive blood cultures.
Laboratory diagnosis
The laboratory diagnosis of NTM from blood was performed as following. Peripheral blood cultures were collected and inoculated into a liquid culture method (BacT ALERT MP [BioMerieux] from 2008 to 2010 and BD MGIT 960 [BD] after 2010). When a positive culture of Mycobacterium species was confirmed, MTB colloidal gold method of kabely kit was used to identify MTB or NTM. Then, chip hybridization was performed for species identification.
Statistical analysis
We used the Kolmogorov-Smirnov test to check if variants followed normal distribution. Measurement data of normal distribution were expressed by mean ± standard deviation (SD), and measurement data of nonnormal distribution were expressed by median and interquartile range (IQR). Statistical analysis was performed by the SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients’ characteristics are summarized in Table 1. Among the ten patients included, three patients were males and the median age was 51 years (IQR 29–57 years). Eight patients were immunocompromised, with underlying conditions including HIV infection (one patient, with a cluster of differentiation 4 [CD4] cell count of 15/mm3), rheumatic diseases (two patients), breast cancer (one patient), aplastic anemia (AA, two patients), and myelodysplastic syndrome (MDS, two patients). The median peak temperature was 39.8°C (IQR 39.3–40.0°C). Three patients had night sweats, and eight patients had weight loss. Six patients had other organ(s) involvement, and they all had underlying diseases. Other organ(s) involvement included the lung in three patients, endocardium in two patients, brain, spinal cord, and soft tissue in one each patient. Two patients had more than one organ involved.
Table 1.
Demographics, underlying diseases, organs involved, laboratory data, treatment, and outcome of the ten patients in our hospital
| Cases | Age (years)/gender | Underlying conditions | Other organs involved | Identification of NTM | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | 58/male | HIV-infected | Lung, brain, and spinal cord | NA | Isoniazid, clarithromycin, levofloxacin, ethambutol, amikacin | Improved |
| 2 | 21/female | AA | Endocardium | M. fortuitum | Amikacin, cefoxitin, SMZ/TMP, minocycline, clarithromycin, and linezolid | Died |
| 3 | 45/male | None | No | NA | Clarithromycin, ethambutol, amikacin, and levofloxacin | Lost to follow-up |
| 4 | 58/female | MDS | Lung, marrow | NA | Rifampicin, isoniazid, ethambutol, pyrazinamide, amikacin, and moxifloxacin | Died |
| 5 | 32/female | SLE | No | M. kansasii | Ethambutol, clarithromycin, and moxifloxacin (resistant to INH) | Improved |
| 6 | 56/female | Breast cancer | No | NA | Moxifloxacin, amikacin, and clarithromycin | Died |
| 7 | 21/female | AA | Soft tissue | M. intracellulare | Azithromycin, rifampicin, ethambutol, amikacin, and moxifloxacin | Died |
| 8 | 57/female | MDS | Lung | M. intracellulare | Isoniazid, rifampicin, ethambutol, and levofloxacin | Lost to follow-up |
| 9 | 41/male | TA | Endocardium | M. abscessus | Clarithromycin, ethambutol, imipenem, levofloxacin, and amikacin | Died |
| 10 | 56/female | None | No | M. chelonae/ M. abscessus | Clarithromycin, ethambutol, imipenem, and moxifloxacin | Improved |
NTM: Nontuberculous Mycobacterium; HIV: Human immunodeficiency virus; NA: Not available; AA: Aplastic anemia; SMZ/TMP: Sulfamethoxazole/trimethoprim; INH: Isoniazid; MDS: Myelodysplastic syndrome; SLE: Systemic lupus erythematosus; TA: Takayasu arteritis; M. fortuitum: Mycobacterium fortuitum; M. kansasii: Mycobacterium kansasii; M. intracellulare: Mycobacterium intracellulare; M. chelonae: Mycobacterium chelonae; M. abscessus: Mycobacterium abscessus.
The laboratory examinations are shown in Table 2. The median leukocyte was 9.72 × 109/L, (IQR 0.86–21.08 × 109/L). The median lymphocyte was 0.66 × 109/L, (IQR 0.24–1.93 × 109/L). The median thrombocyte was 94 × 109/L, (IQR 41–303 × 109/L). The median hemoglobin was 65 g/L, (50–89 g/L). All patients had increased erythrocyte sedimentation rate with a median of 135 mm/h (IQR 59–140 mm/h). Eight patients had hypersensitive C-reactive protein examination, and the median value was 171.15 mg/L (IQR 61.31–202.73 mg/L). Eight patients had T-cell and B-cell subsets tests, and the median CD4 counts were 179/mm3 (IQR 82–619/mm3) and the median CD8 counts were 422/mm3 (IQR 167–517/mm3).
Table 2.
Laboratory examinations (routine blood test, ESR, and hsCRP) of the ten patients in-hospital
| Cases | Leukocyte (×109/L) | Lymphocytes (×109/L) | Hemoglobin (g/L) | Platelet (×109/L) | ESR (mm/h) | hsCRP (mg/L) |
|---|---|---|---|---|---|---|
| 1 | 19.10 | 0.57 | 51 | 364 | 140 | 177.34 |
| 2 | 0.50 | 0.28 | 47 | 6 | 140 | NA |
| 3 | 27.00 | 2.98 | 71 | 790 | 140 | 226.52 |
| 4 | 0.98 | 0.19 | 56 | 71 | 140 | NA |
| 5 | 11.28 | 0.76 | 78 | 283 | 130 | 16.32 |
| 6 | 7.30 | 1.84 | 88 | 82 | 28 | 59.21 |
| 7 | 12.00 | 0.25 | 59 | 51 | 140 | 174.30 |
| 8 | 0.25 | 0.02 | 46 | 9 | 39 | 168.00 |
| 9 | 8.16 | 0.74 | 109 | 105 | 66 | 67.59 |
| 10 | 47.25 | 2.20 | 92 | 275 | 108 | 211.20 |
ESR: Erythrocyte sedimentation rate, normal range: <20 mm/h; hsCRP: Hypersensitive C-reactive protein, normal range: <3 mg/L; NA: Not available.
Among the eight patients who had a T-SPOT.TB assay (Oxford Immunotec, Abingdon, UK) done before diagnosis, one patient of Mycobacterium kansasii BSI had a positive result with 32 spots forming cells/106 peripheral blood mononuclear cells.
Mycobacterium intracellulare was identified in two patients, Mycobacterium abscessus/chelonae complex was also identified in two patients, Mycobacterium fortuitum and Mycobacterium kansasii were identified in one patient. The median time to positive culture was 11.5 days (IQR 6.8–18.8 days) in the study. Three species were rapidly grown mycobacteria (RGM).
All the patients were diagnosed and treated in-hospital. Till the last follow-up in November 2015 (the median follow-up time was 43 months, [IQR 16–63 months]), two patients were lost to follow-up, five patients died, three patients improved after anti-NTM treatment. One patient received 2.5 years of anti-NTM drugs, one patient received one year of anti-NTM drugs, and one patient was still on treatment which started one year ago. Among the three patients, one had no underlying diseases and one had SLE with the SLE disease activity index of zero at diagnosis of NTM BSI. The remaining one with HIV infection received empiric anti-tuberculosis (TB) treatment before the result of the blood culture. All the patients who died during follow-up had underlying diseases, with hematological diseases in three patients (AA in two patients and MDS in one patient) and breast cancer and rheumatic disease (Takayasu Arteritis, TA) in one patient. The species of NTM among patients who died were Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium intracellulare.
DISCUSSION
In the study, we reported all cases diagnosed in our institution with NTM BSI which were rarely reported before, especially in China. The data showed that patients with NTM BSI were mostly immunocompromised and usually had multiple organ involvement. The prognosis was poor.
It is known that biological agents such as infliximab increase the risk of tuberculosis. Less is known about its association with the development of Mycobacterium other than tuberculosis (MOTT) infection.[7] Besides the use of infliximab, a rapidly increasing number of people face challenging conditions such as HIV infection, rheumatic diseases, and cancers. This growing proportion of the immunocompromised population is at risk for severe forms of Mycobacterium infection, especially dissemination and BSI with poor outcomes.[1] The biological variation among NTM is great regarding the potential to cause clinical diseases in predisposed hosts and to affect various target organs or tissues.[1] The course of disease and development of infection depends on the characteristics of the NTM species, the presence of predisposing host factors, and the clinical setting.[8] Autosomal mutations and X-linked mutations have been reported to confer susceptibility to disseminated nontuberculousmycobacterial infection. GATA binding protein 2 deficiency and anti-interferon-γ autoantibodies also give rise to disseminated infection.[9]
T-cell and B-cell subsets examined in our group of patients showed a low median CD4 count (179/mm3), and routine blood test showed low median lymphocytes (0.66 × 109/L), which might be the reason why these patients were susceptible to NTM infections.
We conducted a literature review to obtain a broader perspective of the topic. A total of 179 papers were initially spotted out, six duplicate papers were excluded first. Thirty-nine papers were excluded by title screening for no relation with NTM BSI. Three were excluded for being written in neither English nor Chinese. During abstract viewing, another 57 papers of MTB BSI were excluded, 41 papers which were not cases or case series were excluded, and five papers which were meeting abstracts with no full texts were then excluded. After full-paper evaluation, 16 were excluded because they did not meet the diagnostic criterion. Finally, 12 papers including 22 cases were included [Table 3]. The process was shown as a flow chart in Figure 1. Table 3 summarized the 22 cases reported[7,10,11,12,13,14,15,16,17,18,19,20] which were compared with patients in our hospital.
Table 3.
Demographics, underlying diseases, organs involved, laboratory data, treatment, and outcome of the 22 patients in the literature review
| Cases | Age (years)/gender | Underlying conditions | Other organs involved | Identification of NTM | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1[7] | 36/female | Crohn’s disease | Lung | M. avium complex | Rifampin, ethambutol, ciprofloxacin, and azithromycin | Improved |
| 2[10] | 30/male | Intravenous drug use | Endocardium | M. neoaurum | Tobramycin, azithromycin, and moxifloxacin | Lost to follow-up |
| 3[11] | 76/male | Interstitial pneumonia | Lung | M. abscessus | Imipenem, cilastatin, amikacin, levofloxacin, and clarithromycin | Died |
| 4[12] | 62/female | NHL | None | M. mucogenicum | Multiple including clarithromycin | Died |
| 5[12] | 46/female | AML | None | M. mucogenicum | Ceftazidime, gentamicin, and teicoplanin | Improved |
| 6[12] | 41/male | AML | None | M. neoaurum | Meropenem | Improved |
| 7[12] | 42/male | NHL | None | M. mucogenicum | Ciprofloxacin and clarithromycin | Improved |
| 8[12] | 40/female | AML | None | M. mucogenicum | Ciprofloxacin | Improved |
| 9[13] | 16/male | ALL | None | M. mucogenicum | Meropenem, teicoplanin, and gentamicin | Improved |
| 10[14] | 22/male | HIV-infected | Gut | MAC | Azithromycin and ethambutol | Improved |
| 11[14] | 32/female | HIV-infected | Gut, lung | MAC | Azithromycin, amikacin, ethambutol, and sparfloxacin | Improved |
| 12[15] | 59/female | None | Subcutaneous ICD insertion site | M. mageritense | Ciprofloxacin and clarithromycin | Improved |
| 13[16] | 41/female | Rheumatic heart disease | Endocardium | M. chelonae | Rifampicin, clarithromycin, amikacin, and levofloxacin | Died |
| 14[17] | 26/male | Sickle cell disease; T1DM | None | M. terrae complex | Imipenem and amikacin | Improved |
| 15[18] | 55/male | HIV-infected | Lung | M. arupense | Clarithromycin, ethambutol, and rifabutin | Improved |
| 16[18] | 40/male | HIV-infected | None | M. arupense | Clarithromycin, ethambutol, and rifabutin | Died |
| 17[19] | 30/male | HIV-infected | Gut | M. avium | None (lost to follow-up when diagnosed) | Lost to follow-up |
| 18[20] | 15/male | ALL, Burkitt lymphoma | None | M. mucogenicum | Amikacin | Improved |
| 19[20] | 3/male | ALL | None | M. mucogenicum | Clarithromycin and ciprofloxacin | Improved |
| 20[20] | 5/male | Rhabdomyosarcoma | None | M. mucogenicum | None (died before the culture results were available) | Died |
| 21[20] | 5/female | Neuroblastoma | None | M. mucogenicum | Clarithromycin and ciprofloxacin | Improved |
| 22[20] | 17.5/male | AA | None | M. mucogenicum | Clarithromycin | Improved |
NTM: Nontuberculous Mycobacterium; NHL: Non-Hodgkin lymphoma; AML: Acute myelocytic leukemia; ALL: Acute lymphoblastic leukemia; ICD: Implantable cardioverter defibrillator; HIV: Human immunodeficiency virus; T1DM: Type 1 diabetes mellitus; AA: Aplastic anemia; MAC: Mycobacterium avium complex; M. mucogenicum: Mycobacterium mucogenicum; M. avium: Mycobacterium avium; M. arupense: Mycobacterium arupense; M. terrae: Mycobacterium terrae; M. neoaurum: Mycobacterium neoaurum; M. chelonae: Mycobacterium chelonae; M. mageritense: Mycobacterium mageritense; M. abscessus: Mycobacterium abscessus.
Figure 1.
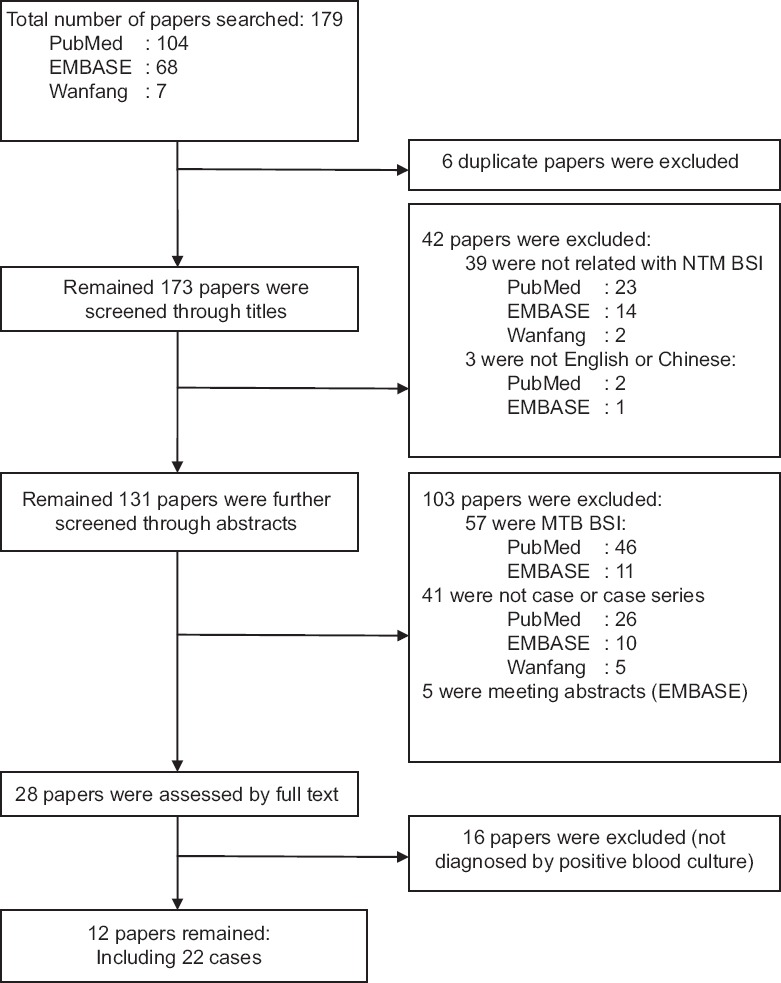
The flowchart for the result of literature search. NTM: Nontuberculous Mycobacterium; BSI: Bloodstream infection; MTB: Mycobacterium tuberculosis.
Among the two groups of patients, most patients were immunocompromised in both groups (80% and 91%). Among patients in our hospital, lung was the most common organ involved, followed by endocardium, brain, spinal cord, and soft tissue. Among cases previously reported, lung was also the most common organ involved, followed by gut, endocardium, and subcutaneous implantable cardioverter defibrillator insertion site. Some patients had more than one organ involved. The most common species of NTM was Mycobacterium chelonae/abscessus in our group, and Mycobacterium mucogenicum in cases reported, and distribution of other species of NTM varied in the two groups. After the treatment, the proportion of patients died in the study was higher than cases reported. One reason to explain this might be the sample size of both groups. In addition, Peking Union Medical College Hospital is the national center for complicated and severe diseases in China. Patients admitted to our hospital often had severe and complicated conditions, which might be another reason for the poorer prognosis. Overall, in previously reported case group, 23–50% of patients died. We also observed that all patients who died had underlying diseases and/or received immunosuppressive therapy in both groups, including hematological diseases, rheumatic diseases, solid tumors, HIV infection, and interstitial pneumonia. In the study, patients who died had Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium intracellulare BSI. In reported cases, among the five patients who died, two patients had Mycobacterium mucogenicum BSI, one each patient had Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium arupense BSI. As Kotilainen et al.[21] showed in a study of 167 non-HIV adult patients with NTM disease, patients with pulmonary Mycobacterium avium complex (MAC) had a significantly lower risk of death as compared to patients with pulmonary infection of other NTM (hazard ratio [HR] 0.50, 95% confidence interval [CI] 0.33–0.77, P = 0.002). Moreover, when compared to patients with pulmonary infection caused by RGM, the risk of death was lower with pulmonary MAC patients (HR 0.47, 95% CI 0.25–0.87, P = 0.020). In another study by Hsieh et al.[6] of AIDS patients, 22 cases of disseminated TB and 15 cases of disseminated MAC were analyzed, the patients with disseminated TB survived much longer than patients with disseminated MAC (mean survival, 96 vs. 22 weeks, P = 0.008). However, these were all AIDS patients, which were different from the study subjects. Therefore, the final outcomes might vary due to different forms of NTM disease, different species of NTM, and different health conditions of the patients. Further studies might be done to analyze possible risk factors related to poor prognosis.
Ten patients with central venous catheters in haemato-oncology unit were considered to be caused by contaminated water supply. The species identified were Mycobacterium mucogenicum in nine patients and Mycobacterium neoaurum in one patient.[12,20] This usually occurred in immunocompromised hosts, especially in patients with underlying malignancies. Water is the main source of NTM, which can be controlled by chlorination and monitoring of bacterial growth. In hospitals with hematology and oncology departments where patients are immunocompromised and with intravascular catheters, adequate disinfection of medical devices and water supply system should be ensured to avoid nosocomial outbreaks.[12,22]
In the study, eight patients had a T-SPOT.TB assay before the diagnosis of NTM BSI. As interferon-γ releasing assays (IGRAs) examine the MTB specific antigen early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10), all NTM infected samples would have negative results, but one patient had false positive result due to Mycobacterium kansasii. According to Adams et al.,[23] two NTM that affect humans, Mycobacterium marinum and Mycobacterium kansasii, contain the ESAT-6 or CFP-10 antigens used in the IGRA assays. Infection with either of these NTM has been shown to produce positive results in assays using these antigens.
There are also some limitations in the study. This is a retrospective study of small samples in a single center. The institution is a center for complicated and severe diseases in China. Patients in the hospital are often difficult to diagnose when admitted to hospital and in more severe conditions. Therefore, the findings might not be representative of patients in other places.
In summary, as the immunocompromised population is increasing, MOTT infection should be paid attention to including BSI. Physicians should pay attention to this severe form of NTM infection, especially in patients with underlying diseases, high fever, and multiple organ involvement. Not only blood culture of aerobic and anaerobic bacteria but also blood culture of Mycobacterium should be performed.
Financial support and sponsorship
This study was supported by grants from the National Major Science and Technology Research Projects for the Control and Prevention of Major Infectious Diseases in China (No. 2014ZX10003003), and Health Research and Special Projects Grant (No. 201402001).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all health-care providers who had participated in taking care of patients.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Petrini B. Non-tuberculous mycobacterial infections. Scand J Infect Dis. 2006;38:246–55. doi: 10.1080/00365540500444652. doi: 10.1080/00365540500444652. [DOI] [PubMed] [Google Scholar]
- 2.Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36:91–9. doi: 10.1016/j.ccm.2014.11.002. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok MY, Wong SS, Chan TM, Fong DY, Wong WS, Lau CS. Non-tuberculous mycobacterial infection in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2007;46:280–4. doi: 10.1093/rheumatology/kel206. doi: 10.1093/rheumatology/kel206. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos RP, Scheid K, Goldani LZ. Disseminated nontuberculous mycobacterial disease in patients with acquired immune deficiency syndrome in the South of Brazil. Trop Doct. 2010;40:211–3. doi: 10.1258/td.2010.100019. doi: 10.1258/td.2010.100019. [DOI] [PubMed] [Google Scholar]
- 5.Miguez-Burbano MJ, Flores M, Ashkin D, Rodriguez A, Granada AM, Quintero N, et al. Non-tuberculous mycobacteria disease as a cause of hospitalization in HIV-infected subjects. Int J Infect Dis. 2006;10:47–55. doi: 10.1016/j.ijid.2004.11.005. doi: 10.1016/j.ijid.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh SM, Hung CC, Chen MY, Hsueh PR, Chang SC, Luh KT. Clinical features and outcome in disseminated mycobacterial diseases in AIDS patients in Taiwan. AIDS. 1998;12:1301–7. doi: 10.1097/00002030-199811000-00011. doi: 10.1097/00002030-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Salvana EM, Cooper GS, Salata RA. Mycobacterium other than tuberculosis (MOTT) infection: An emerging disease in infliximab-treated patients. J Infect. 2007;55:484–7. doi: 10.1016/j.jinf.2007.08.007. doi: 10.1016/j.jinf.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen VO, Andersen AB, Miörner H. Incidence and clinical significance of non-tuberculous mycobacteria isolated from clinical specimens during a 2-y nationwide survey. Scand J Infect Dis. 2002;34:648–53. doi: 10.1080/00365540210147813. doi: 10.1080/00365540210147813. [DOI] [PubMed] [Google Scholar]
- 9.Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–80. doi: 10.1016/S1473-3099(15)00089-4. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Pazhayattil GS, Das A, Conte HA. Mycobacterium neoaurum causing prosthetic valve endocarditis: A case report and review of the literature. Braz J Infect Dis. 2014;18:235–7. doi: 10.1016/j.bjid.2013.05.012. doi: 10.1016/j.bjid.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Ito W, Takeda M, Kobayashi N, Uek S, Sato K, et al. Detection of Mycobacterium abscessus from blood cultures during treatment of interstitial pneumonia: A case study. Rinsho Byori. 2011;59:852–7. [PubMed] [Google Scholar]
- 12.Baird SF, Taori SK, Dave J, Willocks LJ, Roddie H, Hanson M. Cluster of non-tuberculous mycobacteraemia associated with water supply in a haemato-oncology unit. J Hosp Infect. 2011;79:339–43. doi: 10.1016/j.jhin.2011.07.006. doi: 10.1016/j.jhin.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Marshall C, Samuel J, Galloway A, Pedler S. Mycobacterium mucogenicum from the Hickman line of an immunocompromised patient. J Clin Pathol. 2008;61:140–1. doi: 10.1136/jcp.2007.049486. doi: 10.1136/jcp.2007.049486. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Yasuoka A, Tachikawa N, Teruya K, Genka I, Yamaguchi M, et al. Two cases of long lasting bacteremia due to Mycobacterium avium complex despite new macrolides-containing regimens in patients with acquired immunodeficiency syndrome. Intern Med. 2001;40:454–8. doi: 10.2169/internalmedicine.40.454. doi: 10.2169/internalmedicine.40.454. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga M, Goya M, Ogawa M, Fukuda K, Taniguchi H, Ando K, et al. Implantable cardioverter defibrillator infection due to Mycobacterium mageritense. J Infect Chemother. 2016;22:180–3. doi: 10.1016/j.jiac.2015.09.010. doi: 10.1016/j.jiac.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Jagadeesan N, Patra S, Singh AP, Nagesh CM, Reddy B, Badnur SC, et al. Spontaneous endocarditis caused by rapidly growing non-tuberculous Mycobacterium chelonae in an immunocompetent patient with rheumatic heart disease. J Cardiovasc Dis Res. 2013;4:254–6. doi: 10.1016/j.jcdr.2013.06.003. doi: 10.1016/j.jcdr.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esnakula AK, Mummidi SK, Oneal PA, Naab TJ. Sepsis caused by Mycobacterium terrae complex in a patient with sickle cell disease. BMJ Case Rep. 2013;2013:1–3. doi: 10.1136/bcr-2013-009159. doi: 10.1136/bcr-2013-009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidarieh P, Hashemi-Shahraki A, Khosravi AD, Zaker-Boustanabad S, Shojaei H, Feizabadi MM. Mycobacterium arupense infection in HIV-infected patients from Iran. Int J STD AIDS. 2013;24:485–7. doi: 10.1177/0956462412472818. doi: 10.1177/0956462412472818. [DOI] [PubMed] [Google Scholar]
- 19.Narang R, Narang P, Jain AP, Mendiratta DK, Joshi R, Das R, et al. Mycobacterium avium bacteremia and dual infection with Mycobacterium avium and Mycobacterium wolinskyi in the gut of an AIDS patient – First case report. Indian J Tuberc. 2010;57:148–51. [PubMed] [Google Scholar]
- 20.Livni G, Yaniv I, Samra Z, Kaufman L, Solter E, Ashkenazi S, et al. Outbreak of Mycobacterium mucogenicum bacteraemia due to contaminated water supply in a paediatric haematology-oncology department. J Hosp Infect. 2008;70:253–8. doi: 10.1016/j.jhin.2008.07.016. doi: 10.1016/j.jhin. 2008.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in non-tuberculous mycobacterial infections: Patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34:1909–18. doi: 10.1007/s10096-015-2432-8. doi: 10.1007/s10096-015-2432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis. 2001;33:1363–74. doi: 10.1086/323126. doi: 10.1086/323126. [DOI] [PubMed] [Google Scholar]
- 23.Adams LV, Waddell RD, Von Reyn CF. T-SPOT. TB Test (R) results in adults with Mycobacterium avium complex pulmonary disease. Scand J Infect Dis. 2008;40:196–203. doi: 10.1080/00365540701642179. doi: 10.1080/00365540701642179. [DOI] [PubMed] [Google Scholar]


