Abstract
Cognitive impairment is common in heart failure patients. Poor dietary habits are associated with reduced neurocognitive function in other medical populations, including diabetes and Alzheimer’s disease. This study examined whether dietary habits help moderate the relationship between heart failure severity and cognitive function. A total of 152 persons with heart failure completed neuropsychological testing and a fitness assessment. Dietary habits were assessed using the Starting the Conversation-Diet questionnaire, a nutrition measure suggested for use in primary care settings. Moderation analyses showed that better dietary habits attenuated the adverse impact of heart failure severity on frontal functioning (b =1.28, p < .05). Follow-up analyses revealed consumption of foods high in sodium was associated with reduced cognitive function (p < .05). This study suggests dietary habits can moderate the association between heart failure and performance on tests of attention and executive function. Longitudinal studies are needed to confirm and clarify the mechanisms for our findings.
Keywords: Cognitive impairment, Dietary habits, Frontal functioning, Heart failure, Nutrition
1. Introduction
Heart failure (HF) affects nearly 6 million Americans and poses as a major public health concern due to the increased prevalence of obesity, hypertension and type 2 diabetes mellitus (1). An estimated 660,000 new cases of HF are diagnosed each year and HF has become the most common reason for re-hospitalization, accounting for $17.4 billion in Medicare costs per year (2-4).
HF patients are at elevated risk for Alzheimer’s disease (5, 6), vascular dementia (7), and other forms of cognitive impairment found in as many as 75% of persons with HF (8). Cognitive dysfunction in HF is an independent predictor of mortality, reduced instrumental activities of daily living, and poorer quality of life (9-14). The exact mechanisms of cognitive impairment are not entirely clear, perhaps due to a lack of prospective studies that permit causal relationships to be determined. In contrast, several cross-sectional studies have identified important correlates of cognitive impairment in HF that may underlie poor neurocognitive outcomes in this population if confirmed by longitudinal investigation. Specifically, cognitive impairment is commonly proposed to be a manifestation of reduced cardiac function resulting in cerebral hypoperfusion and subsequent cerebrovascular disease (15,16). Other important correlates of cognitive impairment in HF include demographic factors, such as being a male (17), older age, and lower education (18-21). Medical and clinical comorbidities (e.g., hypertension, depression) are also associated with reduced cognitive function in this population (22-25).
Because nutrition has a significant impact on the listed factors for cognitive dysfunction (e.g., cardiac function) (26), poor dietary habits may also contribute to poor cognitive performance in HF patients. Indeed, patients with HF are at significant risk for malnutrition (27), as they often do not adhere to dietary recommendations, consume excessive amount of sodium and inadequate amounts of other important nutrients (26). In turn, poor diet (e.g., lack of fruits and vegetables, high fat and sodium intake) has been linked with reduced cognitive function among persons with medical illnesses that are frequently co-morbid with HF. For instance, lack of fruits and vegetables, reduced adherence to Mediterranean diets, and diets high in fat intake have been shown to contribute to reduced cognitive functioning in vascular dementia, type-2 diabetes, Alzheimer’s disease, and mild cognitive impairment (28-32). In contrast, a diverse diet consisting of recommended foods from Dietary Guidelines (e.g., fruits, vegetables, lean meats, fish, low-fat dairy products, and whole grains) has been suggested to attenuate age-related cognitive decline among the healthy elderly (33).
Despite these findings, the potential contribution of dietary intake to cognitive function among patients with HF remains poorly understood. The current study used a cross-sectional design to examine whether diet quality affects the relationship between heart failure severity and cognitive function in older adults with HF. We hypothesized that poorer dietary habits would be associated with greater cognitive impairment among patients with HF. If confirmed, this study would identify dietary intake as an important correlate of cognitive impairment in HF, though prospective studies would be needed to fully elucidate the causal role of diet in poor neurocognitive outcomes in this population. Follow up analyses examining the relationship between dietary habits and other psychosocial outcomes (i.e., quality of life, depression) were also performed in this analysis.
2. Methods
2.1 Participants
A sample of 152 consecutive persons with HF was selected from a database of a large-scale research study of neurocognitive function in persons with heart failure. Participants were recruited from Summa Health System in Akron, Ohio, and reflect the heart failure population receiving treatment at that facility. The inclusion criteria were age of 50-85 years, English as a primary language, and a diagnosis of New York Heart Association (NYHA) class II or III HF at the time of enrollment. Potential participants were excluded for history of significant neurological disorder (e.g. dementia, stroke), head injury with >10 minutes loss of consciousness, severe psychiatric disorder (e.g. schizophrenia, bipolar disorder), substance use, and renal failure. Participants averaged 67.59 ± 10.79 years of age, were 39.5% female, 13.2% African-American, and 2.0% Native American/Alaskan Eskimo. See Table 1 for additional demographic and medical information.
Table 1.
Demographic and Clinical Characteristics of 152 Older Adults with Heart Failure.
| Demographic Characteristics | |
|---|---|
| N | 152 |
| Age, mean (SD) | 67.59 (10.79) |
| Female (%) | 39.5 |
| Race (% Caucasian) | 83.6 |
| Education, mean (SD) | 13.34 (3.05) |
| Clinical Characteristics | |
| CABG/Bypass Surgery (%) | 35.5 |
| Diabetes (%) | 33.6 |
| Hypertension (%) | 71.7 |
| 2MST Males, mean (SD) | 65.47 (25.10) |
| 2MST Females, mean (SD) | 54.97 (22.54) |
| 2MST Overall Sample, mean (SD) | 61.32 (24.59) |
CABG = Coronary artery bypass graft; 2MST = 2-minute step test; BDI-II = Beck Depression Inventory-II
2.2 Measures
2.2.1 Dietary Habits
The Starting the Conversation- Diet assessment tool [34-36] was used to assess participant’s dietary habits. This is a brief, 7-item, easy to interpret questionnaire that measures healthful and problematic dietary behaviors. Previous work has shown this instrument to be a valid tool for dietary assessment and intervention and useful for identifying healthy vs. unhealthy dietary patterns [35]. In addition, this measure has been shown to be more sensitive relative to other dietary screening measures and has also been correlated with serum carotenoid levels [35]. Higher scores on this questionnaire indicate a poorer diet (i.e., a diet high in cholesterol, fat, and sodium). This scale has been validated using a three-day dietary recall (34). The questionnaire asks participants to indicate how many servings or how often they consume the following foods on a weekly basis: fast food for meals or snacks; beans, chicken or fish; regular snack chips or crackers (not low-fat); desserts and other sweets; fruits or vegetables; regular sodas or glasses of sweet tea; margarine, butter, or meat fat they use to season vegetables or put on potatoes, bread or corn.
2.2.2 Quality of Life
The Short Form-12 Quality of Life Measure (SF-12) (37) measures health-related quality of life. The two primary composite scores, Physical Composite Score (PCS; physical functioning, role-physical, bodily pain, and general health) and Mental Composite Scale (MCS; vitality, social functioning, role-emotional, and mental health) were used in the analyses.
2.2.3 Depressive Symptoms
The Beck Depression Inventory-II (38) was used to assess depressive symptomatology in the current sample. The BDI-II is a commonly used measure of depressive symptoms with excellent psychometric properties in persons with medical conditions (39). BDI-II scores range from 0-63 with increased score indicative of increased symptomatology.
2.2.4 Neuropsychological Measures
All neuropsychological tests used in the current study demonstrate strong psychometric properties including excellent reliability and discriminant and construct validity. The domains and neuropsychological tests administered are as follows:
Global Cognitive Function: Modified Mini Mental State Examination (3MS) (40).
Frontal (attention and executive function): Trail Making Test A (41), Trail Making Test B (42), Digit Symbol Coding (43), Stroop Color Word Test Interference Effect (44, 45), and Letter Number Sequencing (LNS) (46).
Memory: The California Verbal Learning Test-II (CVLT-II) total learning, short delay free recall, long delay free recall, and total hits (47).
Language: Boston Naming Test (BNT) (48) and Animal Fluency (49).
2.2.5 Demographic and Medical History
Demographic characteristics and medical history were collected through a review of the medical charts and self-report. Refer to Table 1.
2.2.6 HF Severity
The 2-minute step test (2MST) is an assessment of cardiovascular endurance and was used to serve as an estimate of heart failure severity (50). The 2MST requires the patient to march in place for 2 minutes. The patient is asked to bring each knee up to a marked target set on the wall at the individual’s own midpoint between the kneecap and crest of the iliac. The number of times the right knee met the marked target was counted. Higher step count within the 2-minutes was reflective of greater cardiovascular fitness. Average step count for females between the ages of 50-85 ranges from 71to 115 steps, while males for this age group range from 60to 107 steps (50). The 2MST is practical for patients with orthopedic devices and reduced leg strength, as it permits the patient to use the wall or a chair to provide stabilization. In addition, the 2MST has been used to estimate HF severity in previous samples of HF patients (51) and is suggested to be an alternative to the 6-minute walk test, which is associated with functional work capacity and prognosis in HF (52,53).
2.3 Procedures
The local Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. Participants completed demographic, medical and psychosocial self-report measures. A brief neuropsychological test battery was then administered to all heart failure participants to assess attention, executive function, memory, and language. Individuals then completed the 2MST under supervision.
2.4 Statistical Analyses
To facilitate clinical interpretation, all raw scores of the neuropsychological measures assessing cognitive function were transformed to T-scores (a distribution with a mean of 50, and a standard deviation of 10) using existing normative data correcting for age (as well as gender, in the case of memory tasks). Composite scores for frontal functions, memory, and language were means of the t-scores within each cognitive domain.
Separate moderation analyses using a multiple linear hierarchical regression model was performed for each cognitive domain. All continuous variables were transformed to z-scores, gender was recoded as 1 for males 0 for females, and medical history was recoded as 1 for a positive and 0 for negative. For analyses of frontal functioning and language as the dependent variables, demographic and medical variables including gender, education, depressive symptomatology (as assessed by the BDI-II), and history of diabetes and hypertension were entered into the first block of the model. Only the medical variables and education were entered in the first block of the model that examined memory, as memory measures were already adjusted for age, and gender using normative data. For all analyses, cardiovascular fitness (2MST), the estimate of HF severity, was entered into the second block. A global indicator of dietary habits was a composite of the 7-item eating pattern questionnaire. For all analyses, dietary habits composite was entered into the third block. The cross product of dietary habits score by 2MST was calculated and entered into the fourth block of each model. After these analyses, partial correlations adjusting for the appropriate demographic and medical variables were computed to determine the association between each item of the Eating Patterns Questionnaire and cognitive function.
Finally, the relationship between dietary habits score and psychosocial outcomes (i.e., quality of life, and depression) was examined using partial correlations adjusting for age, gender, education, depressive symptomatology, HF severity, and medical history of hypertension and diabetes.
3. Results
Cognitive Impairment is Prevalent in the Current Sample of Older Adults with HF
The 3MS was used to characterize cognitive impairment in the current sample, as it provides an assessment of global cognitive functioning (40). The current sample had an average 3MS score of 92.66± 5.42. Specifically, 25% of the sample had a 3MS score below 90, 38.8% had a 3MS score between 90 and 95, and 36.2% had a 3MS score between 95 and 100. See Table 2.
Table 2.
Descriptive statistics of cognitive and psychosocial tests (raw means ± standard deviations)
| Test Performance, mean (SD) | |
|---|---|
| Global Cognitive Function | |
| 3MS | 92.66 (5.42) |
| Frontal Functions | |
| TMTA | 41.72 (16.79) |
| TMTB | 129.60 (76.90) |
| Digit Symbol | 50.66 (14.68) |
| LNS | 8.89 (2.51) |
| Stroop Interference | .63 (8.08) |
| Memory | |
| CVLT Total Raw | 40.01 (11.26) |
| CVLT SDFR | 7.51 (3.25) |
| CVLT LDFR | 7.98 (3.35) |
| CVLT Recognition | 13.48 (2.30) |
| Language | |
| Boston Naming Test | 53.33 (5.90) |
| Animals | 19.27 (4.93) |
| Quality of Life | |
| SF-12 PCS | 44.39 (51.08) |
| SF-12 MCS | 51.08 (10.61) |
| BDI-II, mean (SD) | 8.13 (7.87) |
3MS = Modified Mini Mental State Examination; TMTA = Trail Making Test A; TMTB = Trail Making Test B; LNS = Letter Number Sequencing; CVLT = California Verbal Learning Test; SDFR = Short Delay Free Recall; LDFR = Long Delay Free Recall; SF-12 PCS = Short Form-12 Physical Component Scale; SF-12 MCS = Short Form-12 Mental Component Scale; BDI-II = Beck Depression Inventory-II
Dietary Habits and 2MST are Associated with Cognitive Impairment
The model examining demographic and medical variables significantly predicted frontal (F(5, 141) = 13.52, R2 = .32, p < .01) and language functioning (F(5, 141) = 6.16, R2 = .18, p < .01). A trend emerged for memory (F(5, 142) = 2.42, R2 = .06, p = .05). After adjusting for the appropriate demographic and medical variables for each cognitive domain, the 2MST was significantly associated with frontal (ΔF(1,140) = 8.64, ΔR2 = .04, p < .01) and language functioning (ΔF(1,140) = 4.15, ΔR2 = .02, p < .05). In each case, increased step count (i.e., better fitness) was associated with better cognitive function in these domains (p < .05).
After adjusting for 2MST and the demographic and medical variables, the association between dietary habits and frontal function reached marginal significance (ΔF(1,139) = 3.85, ΔR2 = .02, p = .05). Poorer dietary habits were associated with reduced frontal functioning (p = .05). No such pattern emerged for memory (ΔF(1,140) = .56, ΔR2 = .00, p > .05) or language (ΔF(1,139) = 2.12, ΔR2 = .00, p > .05). See Table 3.
Table 3.
Association of Dietary Habits and the 2MST with Cognitive Functions in Older Adults with Heart Failure (N = 152): A summary of hierarchical regressions.
| Frontal | Memory | Language | |
|---|---|---|---|
| Variable | b(SE b) | b(SE b | b(SE b) |
| Block 1 | |||
| Gender | −1.00(1.25) | --- | − .39(1.64) |
| Education | 2.80(.63)** | 1 .77(.76)* | 3 .47(.83)** |
| BDI-II | −2.69(.61)** | − .87(.75) | −1.86(.81)* |
| Diabetes | −3.27(1.30)* | −1.38(1.60) | −1.77(1.72) |
| Hypertension | −1.33 | 2 .86(1.71) | .53(1.84) |
| R2 | .32 | .06 | .18 |
| F | 13.52** | 2.42 | 6.16** |
|
| |||
| Block 2 | |||
| 2MST | 1.83** | − .46 | 1.70* |
| R2 | .36 | .07 | .20 |
| F for ΔR2 | 8.64** | .34 | 4.15* |
|
| |||
| Block 3 | |||
| Dietary Habits | −1.20 | −.57 | −1 .20 |
| R2 | .38 | .07 | .21 |
| F for ΔR2 | 3.85(p = .05) | .56 | 2.12 |
|
| |||
| Block 4 | |||
| Interaction | 1.27* | −.54 | .71 |
| R2 | .40 | .07 | .22 |
| F for ΔR2 | 4.27* | .45 | .71 |
Note.
denotes p<0.05
denotes p<.001; Interaction = cross product of 2MST and dietary habits
Abbreviations: b – unstandardized regression coefficients, SE – standard error, BDI-II = Beck Depression Inventory-II; 2MST = 2-minute step test
Dietary Habits Moderate the Association between Heart Failure and Cognitive Function
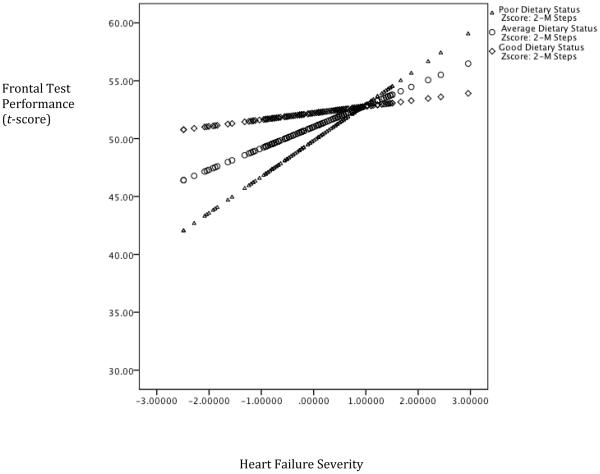
The interaction between dietary habits and the 2MST demonstrated incremental predictive validity above and beyond demographic and medical variables, 2MST, and dietary habits for frontal functioning (ΔF(1,138) = 4.27, ΔR2 = .02, p = .04). Better dietary habits were associated with an attenuation on frontal functioning (b =1.27, p < .05). See Figure 1. No such pattern emerged for memory (ΔF(1,139) = .45, ΔR2 = .00, p = .50) or language (ΔF(1,138) = .71, ΔR2 = .00, p = .40).
Figure 1. Dietary Habits Moderate the Association Between Heart Failure Severity and Frontal Functioning.
Note. Lower scores on the x-axis is reflective of worse heart failure severity and higher scores on the y-axis represents better test performance
Partial Correlations between Eating Pattern Questionnaire Items and Cognitive Function
To clarify which dietary habits were associated with cognitive test performance, we conducted item-by-item partial correlation analyses, adjusting for gender, education, depressive symptomatology, heart failure severity, and history of diabetes and hypertension. The results revealed that an increased number of sugary beverages was associated with poorer performance on measures of executive functioning (r (144) = −.27, p < .01). Similarly, after controlling for the same demographic and medical variables, greater reported consumption of snack chips or crackers per week was associated with poorer language functioning (r (144) = −.29, p < .01). A trend also emerged between poorer memory performance and increased consumption per week of snack chips or crackers (r (145) = −.16, p = .05).
Poor Diet is Associated with Reduced Quality of Life, and Depression
After adjusting for all demographic and medical variables, partial correlations revealed that poorer dietary habits were associated with reduced quality of life as indicated by SF-12 PCS (r (144) = −.24, p < .01) and more depressive symptoms endorsed on the BDI-II (r (144) = .19, p < .05).
4. Discussion
Previous work has shown dietary habits to be associated with cognitive dysfunction in other medical populations, including persons with diabetes mellitus (29), and Alzheimer’s disease (30-32). Our study extends these findings by suggesting that poor dietary habits are linked to exacerbation of cognitive impairment in persons with HF.
The current results suggest that better dietary habits might help to moderate the relationship between HF and performance on cognitive tests that is mediated by frontal brain regions. Current guidelines for HF patients recommend a diet low in saturated fat, trans fat, cholesterol and sodium (54-56). Deficits in attention and executive functions in persons with HF are common (17, 23, 57), and are associated with increased mortality (58) and impaired self-care as a result of poor decision-making (23, 59). The current findings raise the possibility that dietary interventions may help improve executive functioning in older adults with HF; however, this awaits empirical test.
A growing literature links nutrition and cognitive function in a variety of patient samples. Higher consumption of saturated and trans fats and lower intake of polyunsaturated fats has been linked to reduced cognitive performance in persons with type-2 diabetes (29). In contrast, a high intake of fruits, vegetables and fish has been associated with a decreased risk of dementia and Alzheimer’s disease (30-32). The current study shows that dietary patterns may similarly influence cognitive dysfunction in patients with HF. Notably, in spite of sodium restriction being a key dietary requirement in persons with HF (54), adherence to a low sodium diet is very poor in this population (27). Consistent with this pattern, we found that reported intake of foods high in sodium was associated with reduced cognitive function in our sample of older adults with HF. Further work is much needed to better understand the possible nutritional contributors to cognitive function in persons with HF, including non-adherence to a subscribed therapeutic diet.
It is important to keep in mind several limitations of this study. One is a problem that is common to all correlational cross-sectional studies, which is the directionality of findings and impossibility to infer causality. Although our study suggests that better dietary habits attenuate the cognitive deficits associated with HF, it is also possible that intact cognitive function may be responsible for better adherence to the recommended diet in persons with HF. If so, reported dietary habits may serve as a proxy measure for patients’ adherence to their medical regimen (e.g. medication, physical activity) rather than producing an independent effect on cognitive function. Given that HF patients are often advised to follow rigorous dietary recommendations, this possibility requires further evaluation (60).
Another limitation of the study is that the directionality of the diet habits/poor outcome relationship cannot be fully confirmed. Diminished quality of life and depression are common among older adults with HF (61, 62). Depression is a major contributor to poor outcomes in this population, including an increased risk of cognitive impairment (25), reduced independence in daily living (63), and increased risk of morbidity and mortality (64). Our study suggests that many poor dietary habits may be another contributor to reduced quality of life and depression in older adults with HF. However, as above, it is also possible that depressive symptoms lead to poor dietary habits and not vice versa. Yet, circumstantial evidence from several recent studies supports the contention that nutritional deficiencies contribute to depression. For example, omega-3 supplementation among persons with Alzheimer’s disease ameliorates depressive symptoms (65); conversely, vitamin D deficiencies are linked to negative mood in healthy older adults (66). Vitamin intake is strongly influenced by dietary choices; we suggest that future studies should examine the influence of vitamin deficiency on psychosocial outcomes, and cognitive function in older adults with HF.
Dietary habits were assessed in this study with a brief self-report instrument, which allows for quick evaluations in a clinical setting. However, self-report among a population with possible cognitive deficits may introduce concerns regarding the reliability or validity of these reports. Similarly, the present study does not offer potential mechanistic explanations of the effects of dietary consumption on neurocognitive function. A recent study found a low saturated fat/low-glycemic dietary intervention to modulate Alzheimer’s disease risk among a sample of persons with amnestic mild cognitive impairment through its effect on lipoproteins, insulin, oxidative stress, and Aβ42 (67). Future studies should explore the potential mechanisms by which diet influences cognitive function in patients with HF, particularly longitudinal studies. Larger more diverse samples are needed to increase the external validity of our findings. For instance, the current study consisted of HF patients ranging from 50-85 years old; future work should delineate underlying differences in the etiology of HF and cognitive impairment between elderly and non-elderly individuals. Future studies should also use sensitive measures of cardiac function (e.g., cardiac index, ejection fraction) to examine the association among HF severity, dietary habits, and cognitive function.
Finally, we have no information about the genetic peculiarities of the participants in this study. The extant studies in healthy adults indicate that detrimental cognitive effects of known vascular risk factors, such as fasting glucose levels and hypertension may be exacerbated or attenuated by common genetic polymorphisms (68, 69). Investigation of the attenuating and exacerbating role played by genetic variants in the effects of HF on cognition merits further investigation.
Despite these limitations, findings from the current study raise the proactive possibility that dietary habits may modulate the relationship between HF and cognitive function. Our findings indicate that better dietary habits may attenuate the cognitive deficits observed in this population. Future longitudinal studies are needed to clarify the directionality of the relationship.
Key Points.
Our findings suggest that poor dietary habits may contribute to reduced quality of life and depression in older adults with heart failure.
Poor dietary habits (i.e., intake of foods high in sodium) were associated with reduced cognitive function in the current sample of older adults with heart failure.
Cognitive impairment is prevalent in heart failure and better dietary habits may attenuate the cognitive deficits observed in this population.
Acknowledgements
Support for this work included National Institutes of Health (NIH) grants DK075119 and HL089311. NIH did not place any requirements on study design, data collection, analysis, or write-up. There are no conflicts of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association . Heart Disease and Stroke Statistics – 2010 Update. American Heart Association; Dallas, Texas: 2010. ©2010, American Heart Association. [Google Scholar]
- 4.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 5.Acanfora D, Trojano L, Iannuzzi GI, Furgi G, Picone C, Rengo C, et al. The brain in congestive heart failure. Arch Gerontol Geriatr. 1996;23:247–256. doi: 10.1016/s0167-4943(96)00733-9. [DOI] [PubMed] [Google Scholar]
- 6.Qiu C, Xu W, Winblad B, Fratiglioni L. Vascular risk profiles for dementia and Alzheimer’s disease in very old people: a population-based longitudinal study. J Alzheimers Dis. 2010;20:293–300. doi: 10.3233/JAD-2010-1361. [DOI] [PubMed] [Google Scholar]
- 7.Roman G. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20:91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 8.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Bennett SJ, Oldridge NB, Eckert G, Embree JL, Browning S, Hou N, et al. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bennett SJ, Cordes DK, Westmoreland G, Castro R, Donnelly E. Self-care strategies for symptommanagement in patients with heart failure. Nurs Res. 2000;49:139–145. doi: 10.1097/00006199-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Alosco ML, Spitznagel MB, Cohen R, Lawrence S, Colbert LH, Josephson R, et al. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. J Cardiovas Nurs. 2011 doi: 10.1097/JCN.0b013e318216a6cd. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan RS, Pressler SJ. Cognitive deficits in heart failure: Re-cognition of vulnerability as a strange new world. J Cardiovasc Nurs. 2009;24:241–248. doi: 10.1097/JCN.0b013e3181a00284. [DOI] [PubMed] [Google Scholar]
- 13.Putzke JD, Williams MA, Daniel FJ, Bourge RC, Boll TJ. Activities of daily living among heart transplant candidates: Neuropsychological and cardiac function predictors. J Heart Lung Transplant. 2000;19:995–1006. doi: 10.1016/s1053-2498(00)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson A, Poppas A, Paul R, Cohen R. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoth KF. Heart Failure and Cognitive Function. In: Cohen RA, Gunstad J, editors. Neuropsychology and Cardiovascular Disease. Oxford University Press; Oxford: 2010. [Google Scholar]
- 17.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacciatore F, Pasquale A, Ferrara N, Calabrese C, Napoli C, Maggi S, et al. Congestive heart failure and cognitive impairment in an older population. J Am Geriatr Soc. 1998;46:1343–1348. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 19.Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabel R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuccala G, Onder G, Pedone C, Carosella L, Pahor M, Bernabel R. Gifa-Onlus Study Group. Hypotension and cognitive impairment. Selective association in patients with heart failure. Neurology. 2001;57:1986–1992. doi: 10.1212/wnl.57.11.1986. [DOI] [PubMed] [Google Scholar]
- 21.Rengo F, Acanfora D, Trojano L, Scognamiglio P, Ciaburri F, Ceriello A. Congestive heart failure and cognitive impairment in the elderly. Arch Gerontol Geriatr. 1995;20:63–68. doi: 10.1016/0167-4943(94)00607-9. [DOI] [PubMed] [Google Scholar]
- 22.Vogels RLC, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, et al. Profile of cognitive impairment in heart failure. J Am Geriatr Soc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 23.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 24.Waldstein SR. The relation of hypertension to cognitive function. Curr Dir Psychol Sci. 2003;12:9–12. [Google Scholar]
- 25.Pullicino PM, Wadley YG, McClure LY, Safford MM, Lazar RM, Klapholz M, et al. Factors contributing to global cognitive impairment in heart failure: Results from a population-based cohort. J Card Fail. 2008;14:290–295. doi: 10.1016/j.cardfail.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemon SC, Olendzki B, Magner R, Li W, BPham ALC, Ockene I, et al. The dietary quality of persons with heart failure in NHANES 1999-2006. J Gen Intern Med. 2010;25:135–140. doi: 10.1007/s11606-009-1139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilotto M, Addante F, Franceschi M, Leandro G, Rengo G, D’Ambrosio P, et al. Multidimensional prognostic index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circulation. 2010;3:14–20. doi: 10.1161/CIRCHEARTFAILURE.109.865022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crichton GE, Bryan J, Murphy KJ, Buckley J. Review of dairy consumption and cognitive performance in adults: findings and methodological issues. Dement Geriatr Cogn Disord. 2010;30:352–361. doi: 10.1159/000320987. [DOI] [PubMed] [Google Scholar]
- 29.Devore EE, Stampfer MJ, Breteler MM, Rosner B, Hee Kang J, Okereke O, et al. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes Care. 2009;32:635–640. doi: 10.2337/dc08-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, et al. Dietary patterns and risk of dementia: the three-city cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 31.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. J Nutr. 2009;139:1944–1949. doi: 10.3945/jn.109.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammerman A, Haines P, DeVellis R, Strogatz D, Keyserling T, Simpson R. Brief dietary assessment to guide cholesterol reduction in low income individuals: Design and validation. J Am Dent Assoc. 1991;91:1385–90. [PubMed] [Google Scholar]
- 35.Paxton AE, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med. 2011;40:67–71. doi: 10.1016/j.amepre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Glasgow RE, Ory MG, Klesges LM, Cifuentes M, Fernald DH, Green LA. Practical and relevant self-report measures of patient health behaviors for primary care research. Am Fam Med. 2005;3:73–81. doi: 10.1370/afm.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd The Psychological Corporation; San Antonio (TX): 1996. [Google Scholar]
- 39.Amau R, Meagher M, Norris M, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 40.Teng E, Chui H. The Modified Mini-Mental (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 41.Spreen O, Strauss E. A compendium of Neuropsychological tests. Oxford University Press; New York (NY): 1991. [Google Scholar]
- 42.Dikmen S, Heaton R, Grant I, Temkin N. Test-retest reliability of the Expanded Halstead Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc. 1999;5:346–356. [PubMed] [Google Scholar]
- 43.Smith A. Clinical psychological practice and principals of neuropsychological assessment. In: Walker C, editor. Handbook of clinical psychology: Theory, Research, and practice. Dorsey Press; Homewood (IL): 1983. [Google Scholar]
- 44.Lezak MD, Howieson DB, Loring DW. 4th Oxford University Press; New York (NY): 2004. Neuropsychological assessment. [Google Scholar]
- 45.Utl B, Graf P. Color-Word Stroop test performance acrossthe adult life span. J Clin Exp Neuropsychol. 1997;19:405–420. doi: 10.1080/01688639708403869. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Third Edition. The Psychological Corporation; San Antonio (TX): 1997. [Google Scholar]
- 47.Delis D, Kramer J, Kaplan E, Ober B. Manual. Psychological Corporation; San Antonio (TX): 2000. California Verbal Learning Test-Second Edition: Adult Version. [Google Scholar]
- 48.Hawkins KA, Sledge WH, Orlean JE, Quinlan DM, Rakfeldt J, Huffman RE. Normative implications of the relationship between reading vocabulary and Boston Naming Test performance. Arch Clin Neuropsychol. 1993;8:525–537. [PubMed] [Google Scholar]
- 49.Morris J, Heyman A, Mohs R, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimers disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 50.Jones CJ, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging. 2002 March April;:24–30. [Google Scholar]
- 51.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Waechter D, Hughes J, Rosneck J, Gunstad J. The 2-minute step test is independently associated with cognitive function in older adults with heart failure. Aging Clin Exp Res. 2011 doi: 10.3275/8186. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nixon PA, Joswiak ML, Fricker FJ. A six-minute walk test for assessing exercise tolerance in severly ill children. J Pediatr. 1996;129(3):362–366. doi: 10.1016/s0022-3476(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 53.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270(14):1702–1707. [PubMed] [Google Scholar]
- 54.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–93. doi: 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- 56.Duda MK, O’Shea KM, Stanley WC. {omega}-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp169. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rains GD. Principles of human neuropsychology. McGraw-Hill; Boston (MA): 2002. [Google Scholar]
- 58.Pressler SJ, Kim J, Riley P, Ronis DL, Gradus-Pizlo I. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Card Fail. 2010;16:750–760. doi: 10.1016/j.cardfail.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickson VV, Tkacs N, Riegal B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–431. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 60.Carlson B, Riegel B, Moser D. Self-care abilities of patients with heart failure. Heart Lung. 2001;30:351–359. doi: 10.1067/mhl.2001.118611. [DOI] [PubMed] [Google Scholar]
- 61.Campbell RL, Banner B, Konick-McMahan J, Naylor MD. Discharge planning and home follow up of the elderly patient with heart failure. Nurs Clin North Am. 1998;33:497–513. [PubMed] [Google Scholar]
- 62.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: A meta-analytic review of prevalence, intervention effects, and association with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 63.Friedman B, Lyness JM, Delavan RL, Li C, Barker WH. Major depression and disability in older primary care patients with heart failure. J Geriatr Psychiatry Neurol. 2008;21:111–122. doi: 10.1177/0891988707311563. [DOI] [PubMed] [Google Scholar]
- 64.Yu DSF, Lee DTF, Woo J, Thompson DR. Correlates of psychological distress in elderly patients with congestive heart failure. J Psychosom Res. 2004;57:573–581. doi: 10.1016/j.jpsychores.2004.04.368. [DOI] [PubMed] [Google Scholar]
- 65.Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, et al. Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2008;23:161–169. doi: 10.1002/gps.1857. [DOI] [PubMed] [Google Scholar]
- 66.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 67.Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raz N, Dahle C, Rodrigue KM, Kennedy KM, Land S, Jacobs BS. Brain-Derived Neurotrophic Factor Val66Met polymorphism, blood glucose, and memory in healthy adults: The synergy of genetic and vascular risks. Front Hum Neurosci. 2008;2:1–6. doi: 10.3389/neuro.09.012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiol Aging. 2011;2011;32:1124–1137. doi: 10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]