Abstract
A family of structurally related LOX enzymes present in human cells which catalyse the metabolism of released arachidonic acid from phospholipids by inflammatory stimuli, to biologically active mediators. Mainly, expression of three types of LOXs occurs in cells, which catalyse the insertion of molecular oxygen into the molecule of arachidonic acid at carbon 5, 12, and 15. According to this chemical reaction, the LOXs are named 5-, 12-, and 15-LOX, amongst which, 15-LOX with isoforms 15-LOX-1 and 15-LOX-2 have critical role in neoplastic diseases. 15-LOX-1 is overexpressed in some neoplastic conditions. Hence, in this research, we focused on the synthesis of naphthalimide analogs as potential 15-LOX-1 inhibitors. Fortunately, the most of synthesized compounds demonstrated remarkable inhibitory potency towards 15-LOX-1 in nanomolar ranges. Naphthalimide derivatives could be suggested as potential LOX inhibitors with likely applications of anticancer activity.
Keywords: Synthesis, Lipoxygense, Naphthalimide, Arachidonic acid
INTRODUCTION
Arachidonic acid and linoleic acid are produced due to hydrolysis of phospholipids in mammalian cell membrane via function of phospholipases. Arachidonic acid acts as a critical cellular signaling mediator, the precursor of eicosanoids and an essential component of cellular membranes. Arachidonic acid is metabolized mainly by LOXs (LOX), including 5-LOX, 12-LOX, and 15-LOX and cyclooxygenase (COX) (1,2,3).
A family of structurally related LOX enzymes presents in human cells which catalyses the metabolism of released arachidonic acid from the phospholipids by inflammatory stimuli, to biologically active mediators. Mainly, expression of three types of LOXs occurs in cells, which catalyse the insertion of molecular oxygen into the molecule of arachidonic acid at carbon 5, 12, and 15. According to this chemical reaction, the LOXs are named 5-, 12-, and 15-LOX. 5-LOX is the main enzyme in the synthetic pathway of leukotriene synthesis and catalyses the leukotriene A4 synthetic process from arachidonic acid. The role of 5-LOX has not clearly defined in human cells. 15-LOX is presented in two different forms 15-LOX-1 and 15-LOX-2. Only, 40% of similarity in amino acid sequences is observable in these two isoforms and consequently the biological function of these two isoforms is very different. The 15-LOX-1 enzyme is particularly expressed in airway epithelial cells, eosinophils, alveolar macrophages, dendritic cells and reticulocytes. The enzyme performs a critical role in the metabolism of polyunsaturated fatty acids such as arachidonic acid to various metabolites. In contrast to 5-LOX and 12-LOX, 15-LOX-1 can also, oxygenate fatty acids attached to membrane phospholipids (4).
Recently reported literatures revealed that human 15-LOX-1 has antitumor activities in human airway carcinomas and also promote apoptosis. By contrast, 15-LOX-1 has demonstrated an overexpression in prostatic tumors compared to normal adjacent tissues and 15-LOX-2 has shown little expression in prostate tissue. In PC3 cells, 13-S-hydroxyoctadecadienoic acid (13(S)-HODE), one of the 15-LOX-1 metabolites, upregulated MAP kinase, whereas in contrast 15-S-hydroxyeicosatetraenoic acid (15(S)-HETE), the 15-LOX-2 metabolite, downregulated MAP kinase (5,6,7,8). Intense and irregular expression of the enzymes responsible for conversion of unsaturated fatty acid such as arachidonic acid and linoleic acid to bioactive lipid metabolites seems to be significantly correlate to the development of prostatic carcinoma. Others have reported that 15-LOX-2 is expressed in normal prostate tissue, but poorly expressed in prostate tumors. Thus 15-LOX-1 is highly expressed in prostate tumors while 15-LOX-2 is highly expressed in normal tissues. 15-LOX-1 in prostate cancer tumors converts linoleic acid, its preferred substrate to 13-(S)-HODE and other metabolites. These metabolites appear to alter cellular signaling pathways, and thus the inappropriate expression might alter biological events and contribute to tumor development (9,10,11).
Naphthalimide derivatives have been known as DNA intercalators and exhibited high anticancer activities against various cell lines. Some naphthalimide-based compounds, such as amonafide, mitonafide, elinafide, and bisnafide (Fig. 1) have demonstrated remarkable potency in clinical trials (12,13,14). However, the obtained results in trials were associated with severe side effects. The study of structure activity relationship revealed that naphthalimide core should be intact while adding other functional groups may decrease the systemic toxicity. Fusion of some aromatic rings like benzene, imidazole, pyrazine, furan, and thiophene was carried out to naphthalene nucleus that led to the significant improvement in cellular cytotoxic activity compared to amonafide (15,16,17). Besides, other series of naphthalimides with unfused benzene or furan ring was also investigated that displayed favourable cytotoxicity in cancerous cell lines. Amonafide as naphthalimide-based anticancer agents acts also as topo II poison and is in phase III clinical trials for the treatment of acute myeloid leukemia (18,19,20).
Fig. 1.
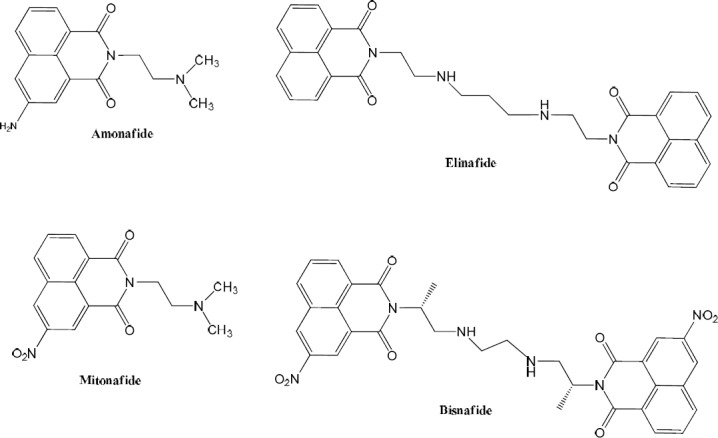
Some naphthalimide-based anticancer agents in clinical trial.
Based on above information, the role of 15-LOX-1 in the etiology of neoplastic disorders has been confirmed. Besides, according to the positive background of the naphthalimide derivatives as potential anticancer agents, in the current investigation we embarked on the synthesis and in vitro assessment of new naphthalimide analogs as potential anticancer agents via 15-LOX-1 inhibition.
MATERIALS AND METHODS
Chemistry
Chemicals and reagents were purchased from Kavoshgar Exir Co. Naphthalene-1,8-dicarboxylic anhydride, glycine, triethylamine, dicyclohexylcarbodiimide (DCC), aniline derivatives, toluene, tetrahydrofuran, n-hexane, silicagel (Merck, Germany), diethyl ether (Scharlou, Spain), hydroxybenzotriazole (Aldrich, USA). The purification of the prepared compounds was carried out by column chromatography using ethyl acetate/petroleum ether. Spectroscopic methods were applied for characterization of the synthesized compounds. 1HNMR spectra were acquired by Bruker 500 MHz in deutrated dimethylsulfoxide (DMSO-d6) and the obtained data were expressed as δ (ppm) compared to tetramethylsilane (TMS) as internal standard. Infrared (IR) spectra of the prepared compounds were obtained by Shimadzu 470 using potassium bromide (KBr) disk. The mass spectra were run on a Finigan TSQ-70 spectrometer (Finigan, USA) at 70 eV. Melting points were determined using electrothermal 9001 melting point analyzer apparatus and are uncorrected.
Synthesis of 2-(1,3-Dioxo-1H-benzo [de]isoquinolin-2(3H)-yl)acetic acid (2)
4 g (20 mmol) of naphthalene-1,8-dicarboxylic anhydride were mixed with 1.52 g (20 mmol) of glycine and 2.80 mL (20 mmol) triethylamine (Et3N) in 50 mL toluene. Reflux condition was performed to the reaction mixture for 20 h. Thin layer chromatography (TLC) was applied to determine the completion of the reaction. The obtained pink precipitate was washed by diethyl ether and n-hexane. Column chromatography (Ethylacetate/Petroleum ether, 80/20) was carried out for purification (21). 1HNMR (500 MHz, DMSO-d6) δ: 4.63 (s, 2H, -CH2-), 7.87 (m, 2H, H5,8-Naphthalimide), 8.50 (m, 4H, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 2250-3250 (Broad peak, O-H, Stretch, Acid), 1770 (C=O, Naphthalimide), 1739 (C=O, Amide). MS (m/z, %): 255 (M+, 10), 254 (5), 238 (35), 210 (100), 196 (45).
General procedure for synthesis of compounds 3a to -3m
Equimolar quantities of compound (2), dicyclohexylcarbodiimide (DCC), hydroxyl-benzotriazole (HOBt), and appropriate aniline derivative were mixed in 20 mL tetrahydrofuran (THF). The reaction mixture was stirred in ice bath for 1 h and then stirring was continued for 24 h at room temperature. TLC was utilized for reaction monitoring. After completion, the reaction mixture was filtered to discard the dicyclohexylurea (DCU) and filtered THF was evaporated under reduced pressure. Water/ethylacetate was added to the residue. Organic layer was separated and washed two times by diluted sulfuric acid (2%), sodium bicarbonate 5% and brine. After dryness by anhydrous sodium sulfate, ethylacetate was evaporated using rotary evaporator (22,23). All final compounds 3a to- 3m were purified by column chromatography (Ethylacetate /Petroleum ether, 60/40).
15-LOX-1 assay
The basis of this method is oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with 3 dimethyl-aminobenzoic acid (DMAB) in a hemoglobin catalyzed reaction. This reaction is initiated in the presence of LOX reaction product, linoleic acid hydroperoxide and results in a blue color formation which has an absorption peak at 590 nm (24). Quercetin was used as the reference compound. Linoleic acid and two stock solutions (A and B) were prepared first. Solution A contained 50 mM DMAB and l00 mM phosphate buffer (pH, 7.0). Solution B was prepared by mixing 10 mM MBTH (3 mL) and hemoglobin (5 mg/mL, 3 mL) in 50 mM phosphate buffer at pH 5.0 (25 mL). A linoleic acid solution (1 mg/mL) was prepared by diluting 5 mg linoleic acid (solubilised in 0.5 mL ethanol) with KOH 100 mM.
For each compound the samples were solved in ethanol (25 µL) and mixed in a test tube with soybean LOX (SLO) (4000 units/mL, prepared in 50 mM phosphate buffer pH = 7.0, 25 µL) and phosphate buffer (50 mM, pH = 7, 900 µL). After 5 min delay at room temperature, 50 µL linoleic acid was added to the mixture to start the hydroperoxidation reaction. After 8 min, solution A (270 µL) and solution B (130 µL) were added to the above mixture. 5 min later, 200 µl of sodium dodecyl sulfate (SDS) solution (2%) was added to stop the reaction. The absorbance at 590 nm was compared with control (ethanol without sample).
RESULTS
Chemistry
According to Fig. 2, naphthalene 1,8-dicarboxylic anhydride was reacted with glycine in toluene to proceed a Gabriel reaction. Reflux condition was performed and acidic derivative (2) was prepared with an acceptable 83% yield (Table 1). Application of dicyclohexyl carbodiimide (DCC) in tetrahydrofuran (THF) as coupling reagent assisted the direct coupling of prepared acidic derivative with various aniline derivatives to achieve the final compounds 3a-3m. Hydroxybenzotriazole (HOBt) was also added to the medium of coupling reaction to facilitate the coupling process as well as to prevent the side reaction of N-acylurea formation.
Fig. 2.
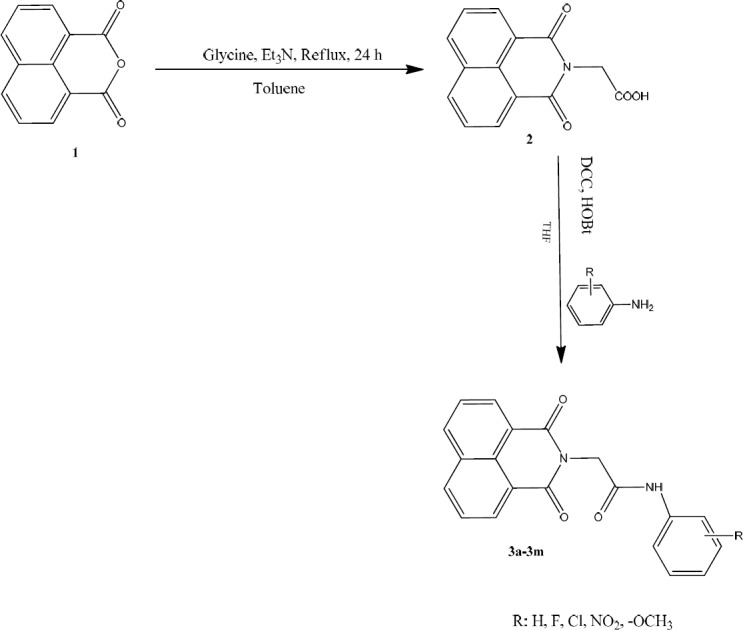
Synthetic pathway of compounds 3a-3m.
Table 1.
Physicochemical properties of compounds 2 and 3a-3m
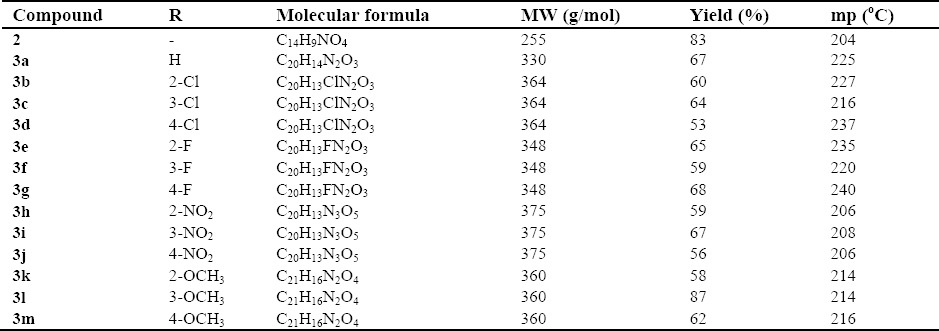
The coupling process afforded the final derivatives 3a-3m with moderate yields. Melting points of synthesized compounds were measured using melting point analyzer. Fluorinated derivatives exerted the highest melting points and nitro containing derivatives melted in lower thermal points. Compound 3l with meta methoxy moiety displayed the highest yield, whereas compound 3d with para chlorine substituent rendered the lowest yield in this series.
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-phenylacetamide (3a)
1HNMR (500 MHz, DMSO-d6) δ: 4.85 (s, 2H, -CH2-), 7.09-7.11 (m, 2H, Phenyl), 7.27 (t, 1H, J = 5 Hz, Phenyl), 7.36 (t, 1H, J = 5 Hz, Phenyl), 7.52-7.57 (m, 1H, Phenyl), 7.87 (t, 2H, J = 10 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, 5 Hz, H4,6,7,9-Naphthalimide), 10.35 (brs, NH). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 330 (M+, 15), 238 (30), 210 (100), 196 (45), 134 (55), 120 (25).
N-(2-Chlorophenyl)-2-(1,3-dioxo-1H-benzo [de]isoquinolin-2(3H)-yl)acetamide (3b)
1HNMR (500 MHz, DMSO-d6) δ: 5.52 (s, 2H, -CH2-), 7.5-7.8 (m, 4H, 2-Chlorophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, 5 Hz, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 366 (M++2, 5), 364 (M+, 12), 333 (25), 238 (20), 210 (100), 180 (35), 169 (25), 152 (25), 127 (45), 98 (15).
N-(3-Chlorophenyl)-2-(1,3-dioxo-1H-benzo [de]isoquinolin-2(3H)-yl)acetamide (3c)
1HNMR (500 MHz, DMSO-d6) δ: 4.86 (s, 2H, -CH2-), 7.31-7.75 (m, 3H, 3-Chlorophenyl), 7.73 (s, 1H, H2- 3-Chlorophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, 5Hz, H4,6,7,9-Naphthalimide), 10.51 (brs, NH). IR (KBr, cm-1) ῡ: 3329 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 366 (M++2, 2), 364 (M+, 5), 238 (35), 210 (100), 180 (15), 152 (35), 127 (40), 98 (25).
N-(4-Chlorophenyl)-2-(1,3-dioxo-1H-benzo [de]isoquinolin-2(3H)-yl)acetamide (3d)
1HNMR (500 MHz, DMSO-d6) δ: 4.85 (s, 2H, -CH2-), 7.08 (d, 2H, J = 10 Hz, 4-Chlorophenyl), 7.38 (d, 2H, J = 10 Hz, 4-Chlorophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.49 (dd, 4H, J = 10 Hz, 5 Hz, H4,6,7,9-Naphthalimide), 10.41 (brs, NH). IR (KBr, cm-1) ῡ: 3329 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 366 (M++2, 2), 364 (M+, 5), 333 (20), 238 (60), 210 (100), 180 (25), 169 (25), 152 (25), 127 (75), 98 (25).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(2-fluorophenyl)acetamide (3e)
1HNMR (500 MHz, DMSO-d6) δ: 4.92 (s, 2H, -CH2-), 7.12-7.53 (m, 4H, 2-Fluorophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, 5 Hz, H4,6,7,9-Naphthalimide), 10.25 (brs, NH). IR (KBr, cm-1) ῡ: 3329 (NH, Stretch), 3070 (C-H, Aromatic), 2924 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 348 (M+, 10), 317 (15), 248 (45), 246 (100), 238 (40), 210 (85), 176 (40), 152 (15).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(3-fluorophenyl)acetamide (3f)
1HNMR (500 MHz, DMSO-d6) δ: 4.86 (s, 2H, -CH2-), 7.27-7.29 (m, 2H, 3-Fluorophenyl), 7.64-7.66 (m, 2H, 3-Fluorophenyl), 7.88 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide), 10.57 (brs, NH). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3070 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 348 (M+, 12), 317 (12), 248 (65), 246 (100), 238 (20), 210 (35), 176 (60), 111 (20).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(4-fluorophenyl)acetamide (3g)
1HNMR (500 MHz, DMSO-d6) δ: 4.84 (s, -2H, -CH2-), 7.09-7.13 (m, 2H, 4-Fluorophenyl), 7.53-7.55 (m, 2H, 4-Fluorophenyl), 7.88 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.50 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide), 10.46 (brs, NH). IR (KBr, cm-1) ῡ: 3332 (NH, Stretch), 3070 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 348 (M+, 5), 317 (10), 248 (80), 246 (100), 238 (30), 210 (75), 176 (40), 152 (15), 137 (20), 111 (20).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(2-nitrophenyl)acetamide (3h)
1HNMR (500 MHz, DMSO-d6) δ: 5.52 (s, 2H, -CH2-), 7-10-8.12 (m, 4H, 2-Nitrophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 375 (M+, 5), 238 (65), 210 (100), 180 (50), 154 (25), 152 (10), 126 (70).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(3-nitrophenyl)acetamide (3i)
1HNMR (500 MHz, DMSO-d6) δ: 4.90 (s, 2H, -CH2-), 7.61-782 (m, 4H, 3-Nitrophenyl), 7.87 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, 5 Hz, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 375 (M+, 15), 238 (75), 210 (100), 180 (20), 154 (30), 152 (30), 126 (75).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(4-nitrophenyl)acetamide (3j)
1HNMR (500 MHz, DMSO-d6) δ: 4.92 (s, 2H, -CH2-), 6.55 (d, 2H, J = 10 Hz, H2,6-4-Nitrophenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 7.90 (d, 2H, J = 10 Hz, H3,5-4-Nitrophenyl), 8.51 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 3329 (NH, Stretch), 3070 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 375 (M+, 5), 238 (95), 210 (100), 180 (60), 154 (30), 152 (30), 126 (55).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(2-methoxyphenyl)acetamide (3k)
1HNMR (500 MHz, DMSO-d6) δ: 3.28 (s, 3H, -OCH3), 4.73 (s, -2H, -CH2-), 6.80-7.10 (m, 4H, 2-Methoxyphenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.49 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 360 (M+, 10), 329 (20), 248 (20), 246 (65), 238 (30), 210 (100), 176 (10), 165 (30), 152 (20), 137 (10), 123 (30).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(3-methoxyphenyl)acetamide (3l)
1HNMR (500 MHz, DMSO-d6) δ: 3.69 (s, 3H, -OCH3), 7.18-7.25 (m, 4H, 3-Methoxyphenyl), 7.87 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide), 10.46 (brs, NH). IR (KBr, cm-1) ῡ: 3325 (NH, Stretch), 3066 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1778 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 360 (M+, 10), 329 (30), 248 (30), 246 (25), 238 (35), 210 (100), 176 (10), 165 (15), 137 (40), 123 (45).
2-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-(4-methoxyphenyl)acetamide (3m)
1HNMR (500 MHz, DMSO-d6) δ: 3.28 (s, 3H, -OCH3), 4.82 (s, 2H, -CH2-), 6.94 (d, 2H, J = 10 Hz, H3,5-4-Methoxyphenyl), 7.06 (d, 2H, J = 10 Hz, H3,5-4-Methoxyphenyl), 7.89 (t, 2H, J = 5 Hz, H5,8-Naphthalimide), 8.51 (dd, 4H, J = 10 Hz, J = 5 Hz, H4,6,7,9-Naphthalimide), 9.31 (brs, NH). IR (KBr, cm-1) ῡ: 3329 (NH, Stretch), 3062 (C-H, Aromatic), 2927 (C-H, Aliphatic), 1774 (C=O, Naphthalimide), 1735 (C=O, Amide). MS (m/z, %): 360 (M+, 20), 329 (20), 248 (30), 246 (55), 238 (20), 210 (100), 176 (30), 165 (20), 152 (20), 137 (30), 123 (85).
Enzymatic assay
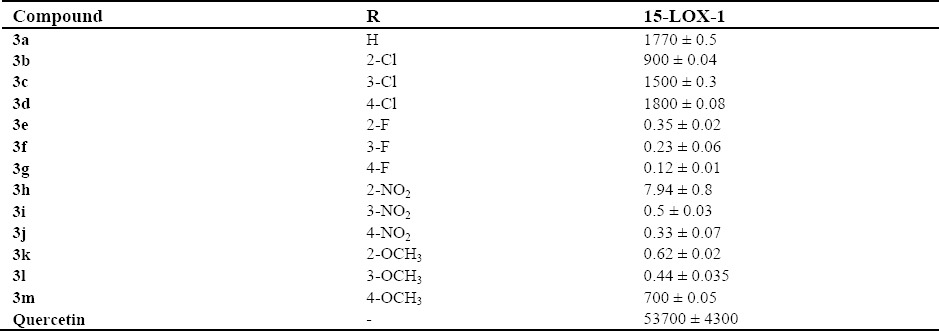
Results for LOX assay are listed in Table 2. Fluorinated derivatives indicated a remarkable inhibitory activity compared to other members in this series.
Table 2.
Enzymatic results (IC50 ± SD, nM) of compounds 3a-3m.
DISCUSSION
A new series of naphthalimide-based 15-LOX-1 inhibitors were synthesized and their inhibitory activity was investigated in vitro. Most of tested derivatives demonstrated higher inhibition activity on LOX compared to quercetin as the reference drug. Electron withdrawing as well as electron donating groups were introduced on the phenyl residue to explore the role of electronic effects at this region for binding. Amongst synthesized derivatives, chlorinated compounds demonstrated lowest inhibitory effect towards LOX enzyme. In contrast, fluorinated derivatives were the most active derivatives in this series. Compound 3a without any substituent on the phenyl residue did not exert remarkable enzyme inhibition but was more potent than quercetin. It could be concluded that electronic effects on the phenyl residue are important for exertion of enzyme inhibition. Substitution of chlorine atom on the phenyl ring is also beneficial for LOX inhibition in comparison to quercetin. But chlorinated compounds (3b, 3c, 3d) did not rendered remarkable activity compared to other tested compounds. Compared to fluorinated derivatives (3e, 3f, 3g) with significant enzyme inhibitory activity, it is likely that electron withdrawing effect of the moiety is not a satisfactory crucial parameter for activity. Namely, it is probable that lipophilicity of the chlorine atom is a limiting factor for enzyme inhibition activity. More hydrophilicity of the fluorine atom leads to more potency. Fluorine atom has also smaller size than chlorine and the role of steric effect is also necessary to investigate. Comparison of chlorinated compounds with nitro bearing derivatives show that steric effect of the nitro moiety is not limiting factor for enzyme inhibition. Electron withdrawing and hydrophilic properties of the nitro moiety are potentiating parameters in the phenyl zone. Detrimental effect of the chlorine moiety is more obvious at position para rather than ortho and meta positions. Electronic effect of the fluorine atom is more effective while substitution was carried out at position para. Fluorine with a significant electron withdrawing activity and low steric effect caused a strong enzyme inhibition. Nitro containing derivatives also exhibited more inhibitory activity when nitro group especially introduced at position para of the phenyl ring. Methoxylated derivatives (3k, 3l, 3m) also rendered favorable potency especially while methoxy substituent put on position meta of the phenyl residue. It could be supposed that electron donating effect of the methoxy group is not a favorable factor for enhancing activity.
Overall, addition of an aromatic phenyl ring in side chain of the naphthalimide-based derivatives led to the discovery of new 15-LOX-1 inhibitors. Exploration of different electron withdrawing as well as electron donating groups improved the enzyme inhibitory property of the synthesized compounds. Generally, electron receiving substituents with low lipophilicity (eg. fluorine) or high hydrophilicity (eg. nitro) had remarkable positive impact toward the enhancement of LOX inhibition. Methoxy moiety as electron releasing group also showed favourable increasing effect on the inhibitory activity of naphthalimide-based derivatives.
Literature survey have shown that incorporation of aromatic rings such as 1,2,3-triazole moiety as well as aliphatic side chain increases the bioactivity of the naphthalimide derivatives. So, the obtained results in this project also proved that the phenyl residue as aromatic moiety could assist the molecule for probable enzyme inhibitory or likely DNA binding interactions (17,19). Naphthalimides also demonstrated remarkable enzyme inhibitory activity towards various enzyme responsible in cell division and proliferation (18). As we prepared naphthalimides as high lipophilic compounds in this research with potential 15-LOX inhibition, the previous reports also confirmed that lipophilicity of the whole molecule is a beneficial parameter for enhancement of the activity (25).
CONCLUSION
The role of LOXs like 15-LOX-1 has been clarified in the origin of neoplastic disorders in the recent years. Naphthalimide derivatives synthesized in the current project could be proposed as novel 15-LOX-1 inhibitors.
ACKNOWLEDGEMENTS
Authors appreciate the research council of Kermanshah University of Medical Sciences for financial support of this project (approved proposal No. 93458). This work was performed in partial fulfillment of the requirement for Pharm.D of Mr. Arash Haqiqi.
REFERENCES
- 1.Matsuyama M, Yoshimura R. Relationship between arachidonic acid pathway and human renal cell carcinoma. Onco Targets Ther. 2008;1:41–48. doi: 10.2147/ott.s3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis. 1998;19(8):1393–1400. doi: 10.1093/carcin/19.8.1393. [DOI] [PubMed] [Google Scholar]
- 3.Tong WG, Ding XZ, Adrian TE. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2002;296(4):942–948. doi: 10.1016/s0006-291x(02)02014-4. [DOI] [PubMed] [Google Scholar]
- 4.Shi HY, Lv FJ, Zhu ST, Wang QG, Zhang ST. Dual inhibition of 5-LOX and COX-2 suppresses esophageal squamous cell carcinoma. Cancer Lett. 2011;309(1):19–26. doi: 10.1016/j.canlet.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon V, Chorny I, Carvajal WJ, Holman TR, Jacobson MP. Novel human lipoxygenase inhibitors discovered using virtual screening with homology models. J Med Chem. 2006;49(4):1356–1363. doi: 10.1021/jm050639j. [DOI] [PubMed] [Google Scholar]
- 6.Steele VE, Holmes CV, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8(5):467–483. [PubMed] [Google Scholar]
- 7.Shureiqi I, Chen D, Jack Lee J, Yang P, Newman RA, Brenner DE, et al. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-Induced apoptosis in colorectal cancer cells. J Natl Cancer Inst. 2000;92(14):1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 8.Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61(17):6307–6312. [PubMed] [Google Scholar]
- 9.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22(11):1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 10.Shureiqi I, Xu X, Chen D, Lotan R, Morris JS, Fischer SM, et al. Nonsteroidal anti-Inflammatory drugs induce apoptosis in esophageal cancer cells by restoring 15-Lipoxygenase-1 expression. Cancer Res. 2001;61(12):4879–4884. [PubMed] [Google Scholar]
- 11.Mahdavi M, Shirazi MS, Taherkhani R, Saeedi M, Alipour E, Moghadam FH, et al. Synthesis, biological evaluation and docking study of 3-aroyl-1-(4-sulfamoylphenyl)thiourea derivatives as 15-lipoxygenase inhibitors. Eur J Med Chem. 2014;82:308–313. doi: 10.1016/j.ejmech.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Milelli A, Tumiatti V, Micco M, Rosini M, Zuccari G, Raffaghello L, et al. Structure-activity relationships of novel substituted naphthalene diimides as anticancer agents. Eur J Med Chem. 2012;57:417–428. doi: 10.1016/j.ejmech.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Li W, Tian Z, Zhao J, Wang C. Mononaphthalimide spermidine conjugate induces cell proliferation inhibition and apoptosis in HeLa cells. Toxicol in Vitro. 2011;25(4):882–889. doi: 10.1016/j.tiv.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Wu A, Xu Y, Qian X. Novel naphthalimide-amino acid conjugates with flexible leucine moiety as side chain: Design, synthesis and potential antitumor activity. Bioorg Med Chem. 2009;17(2):592–599. doi: 10.1016/j.bmc.2008.11.080. [DOI] [PubMed] [Google Scholar]
- 15.Wu A, Xu Y, Qian X, Wang J, Liu J. Novel naphthalimide derivatives as potential apoptosis-inducing agents: Design, synthesis and biological evaluation. Eur J Med Chem. 2009;44(11):4674–4680. doi: 10.1016/j.ejmech.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Wang Y, Yan X, Chen H, Ma G, Zhang P, et al. DNA binding and anticancer activity of naphthalimides with 4-hydroxyl-alkylamine side chains at different lengths. Bioorg Med Chem Lett. 2012;22(2):937–941. doi: 10.1016/j.bmcl.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Xu S, Tang Y, Ding S, Zhang J, Wang S, et al. Synthesis, anticancer activity and DNA-binding properties of novel 4-pyrazolyl-1,8-naphthalimide derivatives. Bioorg Med Chem Lett. 2014;24(2):586–590. doi: 10.1016/j.bmcl.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Chen Z, Tong L, Tan S, Zhou W, Peng T, et al. Naphthalimides exhibit in vitro antiproliferative and antiangiogenic activities by inhibiting both topoisomerase II (topo II) and receptor tyrosine kinases (RTKs) Eur J Med Chem. 2013;65:477–486. doi: 10.1016/j.ejmech.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Lia X, Lina Y, Yuana Y, Liua K, Qiana X. Novel efficient anticancer agents and DNA-intercalators of 1,2,3-triazol-1,8-naphthalimides: design, synthesis, and biological activity. Tetrahedron. 2011;67(12):2299–2304. [Google Scholar]
- 20.Sk UH, Prakasha Gowda AS, Crampsie MA, Yun JK, Spratt TE, Amin S, et al. Development of novel naphthalimide derivatives and their evaluation as potential melanoma therapeutics. Eur J Med Chem. 2011;46(8):3331–3338. doi: 10.1016/j.ejmech.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi-Farani A, Ahmadi A, Nadri H, Aliabadi A. Synthesis, docking and acetylcholinesterase inhibitory assessment of 2-(2-(4-Benzylpiperazin-1-yl)ethyl)isoindoline-1,3-dione with potential anti-alzheimer effects. Daru. 2013;21(1):47. doi: 10.1186/2008-2231-21-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agamennone M, Campestre C, Preziuso S, Consalvi V, Crucianelli M, Mazza F, et al. Synthesis and evaluation of new tripeptide phosphonate inhibitors of MMP-8 and MMP-2. Eur J Med Chem. 2005;40(3):271–279. doi: 10.1016/j.ejmech.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi-Farani A, Foroumadi A, Rezvani Kashani M, Aliabadi A. N-Phenyl-2-p-tolylthiazole-4-carboxamide derivatives: Synthesis and cytotoxicity evaluation as anticancer agents. Iran J Basic Med Sci. 2014;17(7):502–508. [PMC free article] [PubMed] [Google Scholar]
- 24.Anthon GE, Barrett DM. Colorimetric method for the determination of lipoxygenase activity. J Agric Food Chem. 2001;49(1):32–37. doi: 10.1021/jf000871s. [DOI] [PubMed] [Google Scholar]
- 25.Sadeghian H, Attaran N, Jafari Z, Saberi MR, Seyedi SM, Eshghi H, et al. Design and synthesis of 4-methoxyphenylacetic acid esters as 15-lipoxygenase inhibitors and SAR comparative studies of them. Bioorg Med Chem. 2009;17(6):2327–2335. doi: 10.1016/j.bmc.2009.02.009. [DOI] [PubMed] [Google Scholar]


