Abstract
The marine environment represents approximately half of the global biodiversity and could provide unlimited biological resources for the production of therapeutic drugs. Marine seaweeds comprise few thousands of species representing a considerable part of the littoral biomass. Extracts of the Cystoseira indica and Cystoseira merica were subjected to phytochemical and cytotoxicity evaluation. The amount of total phenol was determined with Folin-Ciocalteu reagent. Cytotoxicity was characterized by IC50 of human cancer cell lines including MCF-7 (human breast adenocarcinoma), HeLa (cervical carcinoma), and HT-29 (human colon adenocarcinoma) using Sulforhodamin assay. Antioxidant activities were evaluated using 2,2-diphenylpicrylhydrazyl (DPPH) method. The analysis revealed that tannins, saponins, sterols and triterpenes were the most abundant constituents in these Cystoseira species while cyanogenic and cardiac glycosides were the least ones. C. indica had the higher content of total phenolics and also showed higher antioxidant activity. Cytotoxic results showed that both species inhibited cell growth effectively, especially against MCF-7 cell line. The present findings suggest potential pharmacological applications of selected seaweeds but require further investigation and identification of their bioactive principles.
Keywords: Antioxidant, Cytotoxic, Cystoseira indica, Cystoseira merica, seaweed, Persian Gulf
INTRODUCTION
The marine environment is an exceptional reservoir of bioactive natural products, many of which exhibit structural/chemical features not found in terrestrial natural products. Because of the physical and chemical conditions in the marine environment, almost every class of marine organism exhibits a variety of molecules with unique structural features (1). Seaweeds can be divided into the brown (Phaeophytes), green (Chlorophytes), and red (Rhodophytes) seaweeds. Red and brown algae have been used as human food sources and traditional medicine since ancient times (2).
Seaweeds have caused an emerging interest in the biomedical area, mainly due to their contents of bioactive substances which show great potential as anti-inflammatory, antimicrobial, antiviral, antioxidant, anti-Alzheimer and antitumor activities (2,3). Substances currently received most attention from pharmaceutical companies or from academic researchers for drug development or for drug design include fucoidans, a group of sulfated polysaccharides purified from brown algae, possessing a variety of pharmacologic effects, including anticancer and anti-inflammatory properties (4). Other substances biosynthesized by algae with economic impact in food science and in human health include carotenoids, natural pigments, used as antioxidant compounds reducing the incidence of many diseases especially those mediated by light (5).
Cystoseira (Cystoseiraceae) is a widely distributed genus of brown algae with antibacterial, antifungal, and cytotoxic activities (6). Many compounds such as terpenoids, alkaloids, polysaccharides and steroids have been isolated from different species of the Mediterranean brown algae of the genus Cystoseira but few studies on pharmacological properties of these compounds have been published (7). Recent data has shown more than 150 species of marine algae from coastlines of Iranian islands and Hormozgan Province (8). There have been only a few studies on the pharmacological effects and especially phytochemistry of the marine algae in this region of Iran. Hence, it is necessary to conduct a comprehensive study on screening of the pharmaceutical activities of marine algae. In this study some properties of two Cystoseira extracts including antioxidant activity, cytotoxic potential and phytochemical screening were investigated.
MATERIAL AND METHODS
Authentication of plant material
The seaweeds were collected from the Persian Gulf coasts of Iran, Bushehr Province. Voucher specimens (No. 2665 and 2666) were deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences and were identified by Agricultural and Natural Resources Research Center of Bushehr.
Preparation of the extracts
The plant samples were cut into small pieces, completely air-dried and stored in glass containers until extraction. About 100 g of the dried plant material was macerated for five days with methanol. The extracts were filtered through 2 layers of cotton fabric and evaporated at room temperature, under reduced pressure to the dry residue and stored in sterile vial pending phytochemical and cytotoxic tests (9).
In vitro cytotoxicity assay
The extracts were tested using MCF-7 (human breast adenocarcinoma), HeLa (cervical carcinoma), HT-29 (human colon adenocarcinoma) cells and human gingival fibroblast (normal cell). The cancer cell lines and normal cell were grown in Dulbeccos Modified Eagle Medium (D-MEM) supplemented with 10% fetal bovine serum (FBS). Cells were seeded in 96-well (cancer cells 3500 cells/well, normal cell 5000 cells/well) and allowed to adhere for 24 h at 37ᴼ C with 5% CO2 in fully humidified incubator. Then 100 µl of serially diluted concentration of samples in medium were dispensed into the wells of the cell plates and incubated further for 72 h. After removal of the sample medium, the cells were topped up with 200 µl D-MEM medium and incubated. After 72 h cells were fixed with cold 40% trichloroacetic acid and in 4 ᴼC for 1 h and washed with tap water. The cells were determined by sulforhodamin assay. The absorbance was measured at 492 nm using a microplate reader (BioTeck, Germany). Percentage of dead cells was calculated in comparison to the control. The concentration of the extract that inhibited 50% cells growth (IC50) was determined from the graph plotted by the concentration vs percentage of dead cells. The cytotoxic activities of all the extracts against breast cancer cell lines were labeled according to the National Cancer Institute (NCI, USA) criteria (highly inhibiting activity means IC50≤ 20 μg/mL) (10,11).
Phytochemical screening
The phytochemical analyses of the seaweed extracts were carried out using the methods of Harborne (12). Following phytochemicals were evaluated.
Alkaloids
About 0.2 g of extract was warmed with 1% of aqueous hydrochloric acid for two minutes. The mixtures were filtered and few drops of Dragendorff's reagent (Sigma, USA) were added. A reddish-brown color and turbidity with the reagent indicated the presence of alkaloids.
Flavonoids
Small quantities (2 g) of the extracts were dissolved in 10% of sodium hydroxide (NaOH) and hydrochloric acid (HCl). A yellow solution that turned colorless on addition of HCl indicated the presence of flavonoids.
Anthraquinones
5 g of the extracts was shaken with 10 mL of benzene. The solution was filtered and 5 mL of 10% NH4OH solution was added to the filtrate. A pink, red or violet color in the ammoniacal (lower) phase indicated the presence of anthraquinones.
Cardiac glycosides
The test method is referred to as Lieberman's test. A small quantity of the extracts was dissolved in 2 mL of acetic anhydride and cooled in ice. Sulphuric acid (conc.) was then carefully added. Color change from violet to blue to green indicated the presence of a steroidal nucleus (that is algycone portion of the cardiac glycoside).
Tannins
5 mg of the powdered extracts was stirred with 10 mL of hot distilled water, filtered and ferric chloride was added to the filtrate and observed for blue-black, blue-green or green precipitate.
Sterols and triterpens
The test for steroids was performed using the Lieberman acid test. A portion of the extract was treated with drops of acetic anhydride. Concentrated H2SO4 was carefully added to the side of the test tube. The presence of a brown ring at the boundary of the mixture was taken as positive result.
Saponins
0.1 g of the powdered extract was boiled in 10 mL of distilled water for 5 min and decanted while still hot. The filtrate was used for the following tests: frothing test (a); 1 mL of filtrate was diluted with 4 mL of distilled water and mixture was shaken vigorously and observed for persistent foam which lasted for at least 15 min, and emulsion test (b); this was performed by adding 2 drops of olive oil to the frothing solution and shaken vigorously. Formation of an emulsion indicated a positive test.
Cyanogenic glycosides
Cyanogenic glycosides were identified by subjecting 2 g extract in 10 mL sterile water with few drops of chloroform, and were filtered. Sodium picrate paper was added to the filtrate and heated to boiling. Change in color indicated the presence of cyanogenic glycosides.
Determination of total phenolics
The powdered plant material (20 g of each sample) were weighed in to 50 mL flask, extracted with 30 mL of ethanol 40% using sonicator for about 30 min and shaking for about 10 min. After allowing the extracts cool down to room temperature, the flasks were filled to full volume with extraction solvent.
Preparation of standard
20 mg of gallic acid and 30 mL EtOH 40% were added into 50 mL volumetric flask and sonicated until no solid was present in the flask. After allowing the solution cool down to room temperature, the flask was filled with extraction solvent. The standard solution was diluted several times.
1 mL of standard solution transferred to 100 mL volumetric flask with 60-70 HPLC grade water. The contents swirled to mix. 5 mL of Folin-Ciocalteu's phenol reagent (Merck, Germany) was added and mixed again. After 1 min and before 8 min, 15 mL of sodium carbonate solution was added, the time recorded as time zero. The volume was made up to 100 mL exactly with HPLC grade water. The flask stoppered and mixed thoroughly by inverting it several times. After 2 h the UV absorption range at 550-850 nm and maximum absorbance about 760 nm recorded. Same solution without the extraction solution used as blank solution (12).
2,2-diphenyl-1- picrylhydrazil free radical scavenging assay
The free radical scavenging activity was measured using the 2,2-diphenyl-1-picrylhydrazil (DPPH) assay. Sample stock solutions (1.0 mg/mL) of the extracts were diluted to final concentrations of 243, 81, 27, 9, 3, and 1 μg/mL, in ethanol. One mL of a 50 μg/mL DPPH ethanol solution was added to 2.5 mL of sample solutions of different concentrations, and allowed to react at room temperature. After 30 min the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using the following equation:

Ethanol (1.0 mL) plus plant extracts solutions (2.5 mL) were used as the blank. DPPH solution (1.0 mL) plus ethanol (2.5 mL) was used as a negative control. The positive controls (ascorbic acid, Butylated hydroxyanisole (BHA), and Butylated hydroxytoluene (BHT) were those using the standard solutions. Assays were carried out in triplicate (13)
Statistical analysis
One-way analysis of variance (ANOVA version 16) and Scheffe post hoc were used for data analysis.
RESULTS
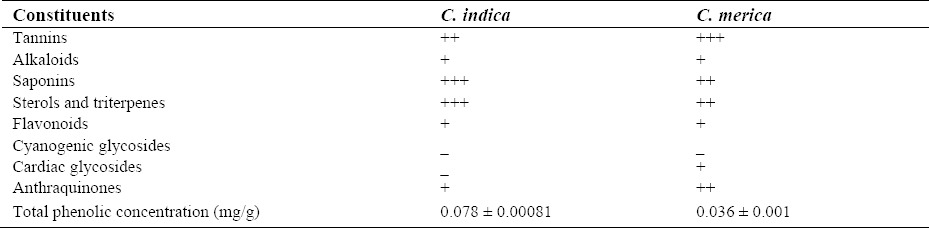
Phytochemical analysis of the extracts of seaweeds tested showed that the most abundant constituents of the C. indica were saponins, sterols, and triterpenes fallowed by tannins, alkaloids, flavonoids, and anthraquinones. Cyanogenic and cardiac glycosides were absent in this alga. In C. merica tannins were the most abundant compounds fallowed by saponins, tannins, sterols, and triterpenes. Cyanogenic glycosides were not present. Table 1 demonstrates the presence of secondary metabolites.
Table 1.
Phytochemical constituents of Cystoseira species
The amount of total phenol was determined with the Folin-Ciocalteu reagent. Gallic acid was used as a standard compound and the total phenols were expressed as mg/g gallic acid equivalent using the standard curve equation:
y = 1.1771x – 0.0252, R2 = 0.9958 (2)
where, y is the absorbance at 760 nm and x is the total phenolic content in the extracts of different algae expressed in mg/l. Table 1 shows the contents of total phenols measured by Folin-Ciocalteu reagent in terms of gallic acid equivalent. The total phenol in selected seaweeds was 0.078, and 0.036 mg in 1 gram dry extract in C. indica and C. merica, respectively.
The cytotoxicity data for the extracts against MCF-7, HeLa, and HT-29 cells are displayed in Figs. 1–3. Cytotoxic results showed that all species could inhibit cell growth effectively (Table 2), especially MCF-7 cell line (IC50 = 83.9 and 69.9 for C. indica and C. merica, respectively).
Fig. 1.
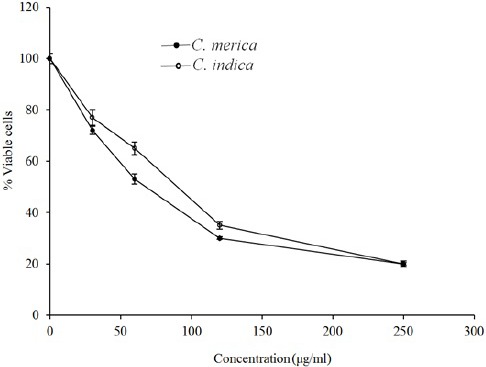
The cytotoxic effect of C. indica and C. merica on HeLa cell line.
Fig. 3.
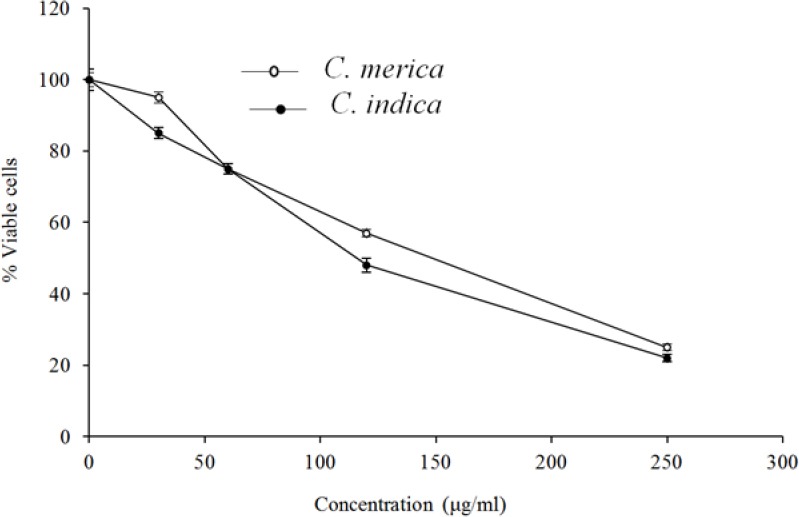
The cytotoxic effect of C. indica and C. merica on HT-29 cell line.
Table 2.
Cytotoxic activity shown as IC50 of the tested seaweeds on three different cell lines

Fig. 2.
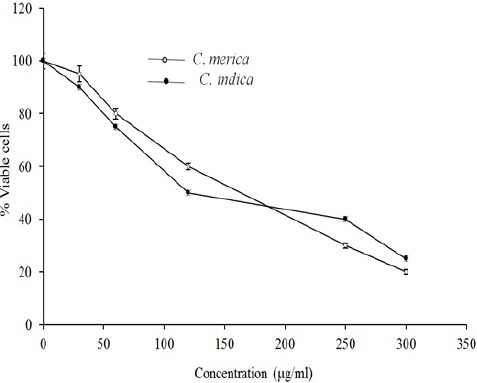
The cytotoxic effect of C. indica and C. merica on MCF-7 cell line
The antioxidant activity of the seaweed extracts was measured on the basis of the scavenging activity of the stable DPPH free radical. IC50 value is inversely related to the antioxidant activity. The extracts of two algae were found to have different levels of antioxidant activity. The results of DPPH free radical scavenging activity on the two extracts are shown in Table 3.
Table 3.
Antioxidant activity of the seaweeds presented as IC50

DISCUSSION
Pharmacognosy, which literally means studying medications of natural sources, has been a part of medical arts and sciences since mankind first began to treat illnesses (14). Up till now, more than 2400 marine natural products have been isolated from seaweeds of subtropical and tropical populations (15). Certain algae have long been used in traditional Chinese herbal medicine in the treatment of cancer (16). Many studies have been developed in order to determine the bioactive compounds produced by marine algae. Antioxidant and cytotoxic activities are one of the most important specificities of marine algae. Some metabolites such as bromophenols, carotene and steroids have so far been isolated and purified from some algae and their activity against some cancer cell lines have been demonstrated (17). Species of Cystoseira are the most widespread marine flora along the Mediterranean coasts and represent one of the most important elements of the ecosystem. The genus is also one of the most studied marine genera, from both biological and chemical viewpoints, and has been used as a model system for chemotaxonomic studies (18).
In this research an effort was made to study the similarity and differences in two Cystoseira species in terms of phytochemical analysis and bioevaluation. Phytochemical tests revealed many similarities than variations. The major difference between the two plants was the presence of cardiac glycosides in C. merica. The amount of tannins, sterols and triterpenes, saponins and flavonoids were also different. There are several reports demonstrating the phytochemical constituents of seaweeds, mangroves and other marine life forms, but only limited information is available for seaweeds especially from Iran.
Regarding cytotoxic activities both species were more effective and equipotent against MCF-7 cell lines than HT-29 and HeLa cell lines. The results of the present investigation are in line with the earlier reports (19,20).
In the current study the sulforhodamine B (SRB) protein stain assay was used instead of methylthiazol tetrazolium (MTT) calorimetric assay for in vitro chemosensitivity testing of various human tumour cell lines. The SRB assay provides a better linearity with cell number than the MTT assay, even at a suboptimal wavelengths. The SRB assay is, because of its large linearity range, suitable for studying chemosensitivity of subconfluent monolayers and multilayer cell clusters containing large amounts of cells. In contrast to the MTT assay, SRB staining is stable and plates can be stored for several weeks up to several months. In addition, the protein assay can be interrupted at several steps during the protocol (21). The SRB assay provides not only a higher sensitivity but also a lower variation between cell lines. This underlines the advantage of the SRB assay, which does not depend on enzymatical activity but on protein content. The SRB assay has now been used as a chemosensitivity test in different laboratories with more than 25 cell lines such as squamous cell carcinoma and colon, ovarian, breast, and prostate cancer, fibroblasts, and leukaemia, and bone marrow cells with a different histological origin from man, rat, and mouse. The variation in optical density between these cell lines is less than in a comparable panel of cell lines tested with the MTT assay (22,23)
The result of the present study showed that the extract of C. indica, which contains highest amount of phenolic compounds exhibits a great antioxidant activity. The high scavenging property of C. indica is related to hydroxyl groups existing in the phenolic compounds.
Marine algae, including Cystoseira species are considered to be healthy food items, especially in Asia due to their low content of lipids, and high levels of polysaccharides, vitamins, and polyunsaturated fatty acids. Besides their primary metabolites, they are capable of producing interesting secondary metabolites that help them to survive in complex habitats. These seaweeds are characterized by polyphenols (phlorotannins), but they also contain other secondary metabolites such as halogenated terpenes, alkaloids, carotenoids, and sulfated sterols (24,25). Several studies have proved the antioxidant, antitumor, antimicrobial, antiviral, and antiinflammatory activities of brown algae and/or their secondary metabolites (26,27). However, only a few studies have been performed on cytotoxic and antioxidant activities and especially phytochemistry of these marine plants in Iran.
The current work highlights the potential of Cystoseira species from Persian Gulf, as excellent sources of novel natural products with cytotoxic and antioxidant activities. Since the starting materials (algal extracts) are devoid of high toxicity, brown algae-derived natural products could prove real cytotoxic hits. To the best of our knowledge, this is the first report about comparison of these two Cystoseira species of Iranian coastlines. The extracts will be undergoing further fractionation in order to isolate and characterize their active principals.
CONCLUSION
The extracts of two cystoseira seaweeds from Persian Gulf, Iran were screened for their antioxidant, cytotoxic, total phenolic content and phytochemical analysis. Phytochemical and cytotoxic results were similar, with few differences, in both algae. C. indica, however, indicated higher amount of phenolics and higher antioxidant activity. Both species inhibited cell growth effectively, especially against MCF-7 cell line. These seaweed extracts and their active components could emerge as natural and alternative antioxidants or serve as starting points for synthesizing more effective cytotoxic drugs.
ACKNOWLEDGEMENTS
This research project was sponsored by Research Council of Isfahan University of Medical sciences, Isfahan, Iran. Also the authors would like to thank Dr. Zandi and Mr. Sartavi for their assistance in collection of the samples.
REFERENCES
- 1.Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Pro Rep. 2007;24(1):31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- 2.Smith AJ. Medicinal and pharmaceutical uses of seaweed natural products [a review] J Appl Phycol. 2004;16(4):245–262. [Google Scholar]
- 3.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res Pharm Sci. 2013;8(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 4.Yegdaneh A, Putchakan S, Yueyongsawad S, Ghannadi A, Plubrukarn A. 3-Oxoabolene and 1- oxocurcuphenol, aromatic bisabolanes from the sponge Myrmekioderma sp. Nat Pro Com. 2013;8(10):1355–1357. [PubMed] [Google Scholar]
- 5.Kusumoto IT, Nakabayashi T, Kida H, Miyashrio H, Hattori M, Namba T, et al. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus-I (HIV-I) protease. Phyto Res. 1995;9(3):180–184. [Google Scholar]
- 6.Abourriche A, Charrouf M, Berrada M, Bennamara A, Chaib N, Francisco C. Antimicrobial activities and cytotoxicity of the brown alga Cystoseira tamariscifolia. Fitoterapia. 1999;70(6):611–614. [Google Scholar]
- 7.Banaigs B, Francisco C, Gonzalez E, Fenical W. Diterpenoid metabolites from the marine alga Cystoseira elegans. Tetrahedron. 1983;39(4):629–638. [Google Scholar]
- 8.Sohrabipour J, Rabiei R. The checklist of green algae of the Iranian coastal lines of the Persian Gulf and Gulf of Oman. Iran J Bot. 2007;13(2):146–149. [Google Scholar]
- 9.Yegdaneh A, Putchakan S, Yueyongsawad S, Ghannadi A, Plubrukarn A. 3-Oxoabolene and 1-oxocurcuphenol, Aromatic Bisabolanes from the Sponge Myrmekioderma sp. Nat Prod Commun. 2013;8(10):1355–1357. [PubMed] [Google Scholar]
- 10.Kamba AS, Hassan LG. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr J Pharm Pharmacol. 2010;4:645–652. [Google Scholar]
- 11.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemoth Rep. 1972;3:1–103. [Google Scholar]
- 12.Harborne JB. Phytochemical methods a guide to modern techniques of plant analysis. London: 1973. pp. 49–188. [Google Scholar]
- 13.Braca A, DeTommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Pro. 2001;64(7):892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 14.Kazemi M, Eshraghi A, Yegdaneh A, Ghannadi A. “Clinical pharmacognosy”- A new interesting era of pharmacy in the third millennium. DARU J Pharm Sci. 2012;20:18. doi: 10.1186/2008-2231-20-18. DOI: 10.1186/2008-2231-20- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, et al. Biopotentials of seaweeds collected from southwest coast of India. J Marine Sci Technol. 2009;17(1):67–73. [Google Scholar]
- 16.Yamamoto I, Takahashi M, Tamura E, Maruyama H, Mori H. Antitumor activity of edible marine algae: effect of crude fucoidan fractions prepared from edible brown seaweeds against L-1210 leukemia. Hydrobiologia. 1984;22:145–148. [Google Scholar]
- 17.Xu N, Fan X, Yan X, Tseng C. Screening marine algae from China for their antitumor activities. J Appl Phycol. 2004;16:451–456. [Google Scholar]
- 18.Amico V. Marine brown algae of family Cystoseiraceae: chemistry and chemotaxonomy. Phytochemistry. 1995;39(6):1257–1279. [Google Scholar]
- 19.Khanavi M, Nabavi M, Sadati N, Shams M, Sohrabipour J, Nabavi SM, et al. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43(1):31–37. [PubMed] [Google Scholar]
- 20.Erfani M, Nazenosadat Z, Moein M. Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D. Pharmacognosy Res. 2015;7(2):133–137. doi: 10.4103/0974-8490.150539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skehan P, Storeng P, Scudiero D, Monks A, McMahon J, Vistica D, et al. New calorimetric cytotoxicity: assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein LV, Shoemaker RH, Paul KD, Simon RM, Tosini S, Skehan P, et al. Comparison of invitro anticancer-drug screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human umor cell lines. J Natl Cancer Inst. 1990;82(13):1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 23.Panjehpour M, Movahedian A, Yekdaneh A. Adenosine receptors expression in the human lung adenocarcinoma cell line (Calu-6) J Thor Onc. 2007;2(8):p S549. [Google Scholar]
- 24.Sridhar KR, Vidyavathi N. Antimicrobial activity of seaweeds. Acta Hydrochim Hydrobiol. 1991;19(5):455–496. [Google Scholar]
- 25.Wright JT, Nys R, Poore AGB, Steinberg PD. Chemical defense in a marine alga: heritability and the potential for selection by herbivores. Ecology. 2004;85(11):2946–2959. [Google Scholar]
- 26.González del VA, Platas G, Basilio A, Cabello A, Gorrochategui J, Suay I, et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain) Int Microbiol. 2001;4(1):35–40. doi: 10.1007/s101230100006. [DOI] [PubMed] [Google Scholar]
- 27.Campanella L, Martini E, Tomassetti M. Antioxidant capacity of the algae using a biosensor method. Talanta. 2005;66(4):902–911. doi: 10.1016/j.talanta.2004.12.052. [DOI] [PubMed] [Google Scholar]


