Abstract
Leishmaniasis has a wide spectrum of signs and symptoms due to infection to numbers of Leishmania species and makes enormous mortality and morbidity. There are clues of antileishmanial effects of prenylated coumarins. Apiaceae family is one of the most important sources of coumarins. Air-dried aerial parts of Ferulago angulata and fruits of Prangos asperula were extracted with n-hexane, using a soxhlet apparatus. The solvents were evaporated under reduced pressure. Column chromatography and crystallization process resulted to isolation of three prenylated coumarins. 1H-nuclear magnetic resonance, electron ionization Mass and Infrared spectra were used for elucidation of isolated compounds. Leishmanicidal activity of isolated coumarins was assessed on Leishmania major strain (MRHO/IR/75/ER) for the first time. Suberosin epoxide and suberosin were isolated from aerial parts of F. angulata and osthol was extracted from grounded fruits of P. asperula. Osthol showed a significant antileishmanial effect on promastigotes in early hours of exposure with IC50 of 14.40 µg/mL but suberosin epoxide showed only a weak antileishmanial activity. IC50 of osthol and suberosin epoxide after 48 h were 10.79 and 54.0 µg/mL, respectively. Suberosin showed no remarkable effect in these concentrations. This is the first report on the pharmacological activity of suberosin epoxide. Substantial difference between efficacies of two isomers, osthol and suberosin remarks the importance of prenyl substituent location on C-8.
Keywords: Leishmania, Prenylated coumarins, Osthol, Suberosin epoxide, Suberosin
INTRODUCTION
Leishmaniasis includes a spectrum of infectious diseases caused by Leishmania species. Many people in many countries are affected by the parasite with high mortality and morbidity and high endemicity in developing countries (1). According to World Health Organization (WHO) report, leishmaniasis is threatening lives of about 350 million men, women and children in 88 countries all over the world. As many as 12 million people are believed to be currently infected, with about 1–2 million estimated new cases occurring yearly. About ninety percent of cutaneous leishmaniasis cases occur in Afghanistan, Brazil, Iran, Peru, Saudi Arabia, and Syria (2).
Leishmaniasis can be classified in different clinical forms as cutaneous, mucocutaneous and visceral forms of which cutaneous form is the most prevalent. L. tropica, L. major and L. aethiopica are the old species which cause cutaneous leishmaniasis (1). L. major, a unicellular protozoan parasite is the cause of an acute infection with a period of 3 to 6 months (3). L. major is endemic in rural, arid or desert regions of the Mediterranean littoral, Middle East, North Africa, Central Asia and India. Although variable, human lesions tend to be relatively large and wet, with overlaying exudates. Desert rodents are the reservoirs. Phlebotomus papatasi and other Phlebotomus spp. serve as vectors. L. major has also been a major problem for rural settlers in Iran (1).
Plants derived compounds are proposed to provide new sources for alternative drugs. Natural constituents such as alkaloids (4,5), benzophenones (6), coumarins (7,8), and terpenoids are proved to have antileishmanial effects (9,10).
Coumarins are the lactones of ortho hydroxycinnamic acid which is widely found in nature (11). Coumarins exist in plants in free or glycoside forms (12). Coumarin derivatives have been reported to have numerous therapeutic applications including anti-inflammatory, antioxidant (13) and anti-HIV effects (14), and some are also active as neuroprotective (15) and cancer preventive agents (16). Therefore, coumarins are excellent potential pharmaceutical agents. Several coumarins have rendered leishmanicidal effects (7,8), among them auraptene and umbelliprenin (Fig. 1), the prenylated coumarins, have shown significant antileishmanial effects (17,18). Investigations indicate that prenyl moieties (3-methyl-2-buten-1-yl; bolded side chain in Fig. 1) in the terpenoid coumarins have an important role in biological activities of this group of natural compounds (19,20). It is considered that the prenyl moiety facilitate attachment of the bioactive molecules to cysteine residues of intercellular proteins (21).
Fig. 1.
Chemical structures of aurapten and umbelliprenin where prenyl substitution is highlighted.
In the present study the antileishmanial properties of three other prenylated coumarins: osthol, suberosin and suberosin epoxide were investigated. Suberosin, suberosin epoxide and osthol have been previously isolated from Ferulago angulata and Prangos asperula respectively. F. angulata (Apiaceae) is an aromatic endemic plant (22) with wide use as food seasoning and antidiabetic agent in south-western (Kohgiluyeh-Boirahmad) and oil flavor and antioxidant in western of Iran (Kermanshah). The essential oil of the plant also possesses antimicrobial activity (23). P. asperula is also belongs to Apiaceae family and used as provender for mutton. The fruits of some other Prangos species are used as emollient, carminative and tonic in Iranian traditional medicine and proved to exert different pharmacological activities (24,25,26).
MATERIALS AND METHODS
General instrumental procedures
1H (500 MHz) and 13C (125 MHz) nuclear magnetic resonance (NMR) spectra were measured on a Bruker spectrometer (USA), using CDCl3 as solvent and TMS as internal standard (Merck, Germany). Mass spectra were obtained using a Hewlett-Packard 7890A mass spectrometer (USA). Infrared spectra were also recorded using an FTIR spectrometer (WQF-510, Rayleigh, China). Open column chromatography was performed using silica gel (70–230 mesh) and analytical grade solvents (Merck, Germany). Separations were monitored by thin layer chromatography on Merck 60 F254 (0.25 mm) plates and were visualized by UV inspection (Camag, Switzerland) and/or staining with Cerium Sulphate/molibdate (Merck, Germany) proceeding by heating.
Plant material
Aerial parts of F. angulata and the fruits of P. asperula were collected from Dena Mountains, west of Iran, in June and July 2011 respectively. The plant materials were identified by Department of Botany, Yasouj University and voucher specimens were deposited at the Herbarium of School of Pharmacy and Pharmaceutical Sciences, Isfahan, Iran (No. 1972 and 1126).
Extraction and isolation of prenylated coumarins
Air-dried aerial parts of F. angulata (200 g) were extracted with n-hexane, using a soxhlet apparatus (Duran, Germany) for 6 h. The solvent was evaporated under reduced pressure to 50 mL and was cooled to 4°C for several days to render a semi pure white to pale yellow crystals. For further purification, they were washed with chilled n-haxane for several times. Later, the sample was subjected for re-crystallization process until resulted pure crystals of compound 1 (suberosin epoxide).
The mother liquor was fractionated on an open silica column using a mixture of heptane and ethyl acetate to render a lump of crystals which were further purified through a recrystallization process to get compound 2 (suberosin).
Compound 3 (osthol) was also isolated from fruits of P asperula according to the method which we have previously described (25). Chemical structures of these three prenylated coumarins are illustrated in Fig. 2.
Fig. 2.
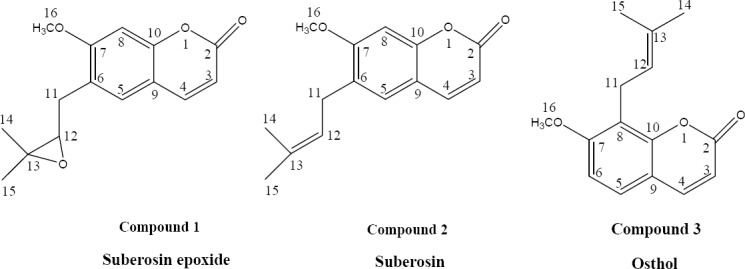
The chemical structures of three prenylated coumarins.
Compound 1
Suberosin epoxide; 7-methoxy-6-(3-methyl -2,3-epoxy)-1-benzopyran-2-one; pale yellow crystals; 1HNMR (CDCl3, 500 MHz, J in Hz): δ 7.66 (1H, d, J = 9.5, H-4), δ 7.33 (1H, s, H-5), δ 6.82 (1H, H-8), δ 6.28 (1H, d, J = 9.5, H-3), δ 3.93 (1H, H-16), δ 3.00 (2H, m, H-11), δ 2.77 (1H, dd, H-12), δ 1.42 (3H, s, H-14), δ 1.36 (3H, s, H-15).
13CNMR (CDCl3, 125 MHz): δ 161.7 (C-2), δ 161.1 (C-7), δ 155.3 (C-10), δ 143.9 (C-4), δ 128.9 (C-5), δ 124.5 (C-6), δ 113.5 (C-3), δ 112.5 (C-9), δ 99.2 (C-8), δ 63.6 (C-12), δ 59.3 (C-16), δ 56.4 (C-13), δ 29.6 (C-11), δ 25.2 (C-14), δ 19.4 (C-15).
Mass m/z 260 [M]+, 189 [M-((CH3)2CHCO)]+. Calculated for: C15H16O4.
Fourier transform infrared spectroscopy (FT-IR) (KBr): νmax = 3001, 2962, 2848, 1716, 1620, 1138, 1209, 831.
Compound 2
Suberosin; 7-methoxy-6-(3-methyl-2-butenyl)-1-benzopyran-2-one; Colorless crystals; 1HNMR (CDCl3, 500 MHz, J in Hz): δ 7.61(1H, d, J = 9.5, H-4), δ 7.17 (1H, s, H-5), δ 6.74 (1H, s, H-8), δ 6.20 (1H, d, J = 9.5, H-3), δ 5.28 (1H, t, J = 6.21, H-12), δ 3.84 (3H, s, H-16), δ 3.29 (2H, d, J = 6.21, H-11), δ 1.76 (3H, s, H-14), δ 1.70 (3H, s, H-15).
13CNMR (CDCl3, 125 MHz): δ 161.91 (C-2), δ 161.05 (C-7), δ 154.86 (C-10), δ 144.08 (C-4), δ 134.00 (C-12), δ 127.87 (C-5), δ 127.83 (C-6), δ 121.78 (C-13), δ 113.31 (C-3), δ 112.29 (C-9), δ 98.86 (C-8), δ 56.25 (C-16), δ 28.19 (C-11), δ 26.20 (C-14), δ 18.15 (C-15).
Mass m/z 244 [M]+, 229 [M-CH3]+, 213 [M-OCH3]+, 189 [M-CH=C(CH3)2]+.
FT-IR (KBr): νmax = 3087, 2933, 2854, 2634, 1738, 1650, 1136, 822. Calculated for: C15H16O3.
Compound 3
Osthol; 7-methoxy-8-(3-methyl-2-butenyl)-1-benzopyran-2-one; colorless needle like crystals; 1HNMR (CDCl3, 500 MHz, J in Hz): δ 7.64 (1H, d, J = 9.44, H-4), δ 7.32 (1H, d, J = 8.57, H-5), δ 6.86 (1H, d, J = 8.57, H-6), δ 6.26 (1H, d, J = 9.44, H-3), δ 5.26 (1H, t, J = 7.03, H-12), δ 3.95 (3H, s, H-16), δ 3.57 (2H, d, J = 7.03, H-11), δ 1.88 (3H, s, H-14), δ 1.70 (3H, s, H-15).
Mass m/z 244 [M]+, 213 [M-OCH3]+.
FT-IR (KBr): νmax = 1717, 1604, 1500, 1160, 830. Calculated for: C15H16O3.
Parasites
L. major strain MRHO/IR/75/ER was maintained with passage in BALB/c mice. Promastigotes on NNN medium, sub cultured in RPMI 1640 (PAA, Australia) containing 10% v/v heat inactivated FCS (Sigma, USA), L- glutamine (Sigma USA), 100 U/mL of penicillin (Jaber Ebne Hayan, Iran) and 100 mg/mL of streptomycin sulfate (Jaber Ebne Hayan, Iran) at 25°C (18). Antileishmanial assays were conducted using stationary-phase promastigotes.
Antileishmanial evaluation
For the in vitro assessment of leishmanicidal effect, stock solutions of the test coumarins were made at the concentrations of 5 and 20 mg/mL and further dilutions were prepared from stocks immediately prior to use. As these coumarins were not soluble in inorganic solvents, we dissolved them in 0.5% dimethyl sulfoxide (DMSO). At this concentration, DMSO does not affect morphology and growth rate of promastigotes.
Further dilutions were made using the stock solutions. In the next step, promastigotes of L. major; grown to the concentration of 5 × 105 parasite/mL were treated with mentioned diluted test solutions.
The treatment period was 48 h and sampling was done at 1, 3, 24 and 48 h of this period, then using a hemocytometer chamber the parasites in each sample were counted with microscope. The negative control used to evaluate samples, consisted of 0.5% DMSO and 5 × 105 parasite/mL cell suspension. Amphotericin B was used as a positive control. Stibogluconates, the first line treatment of leishmaniasis are not effective in vitro, so we applied amphotericin B instead.
Statistical analysis
All analyses were performed using ANOVA and Tukey's post hoc multiple comparison test. To assess the normality of variables, the Kolmogorov-Smirnov test was used. P-values less than 0.05 were considered statistically significant. SPSS version 16 was used for statistical analyses (SPSS Inc, Chicago, USA).
RESULTS
Identification of compounds
The isolated coumarins were identified by comparison of their NMR and MS data with those previously described in the literature (25,27,28). This is the first report of suberosin and suberosin epoxide from F. angulata, but osthol has been previously reported from P. asperula (25).
Suberosin showed an ion peak in the mass spectrum at m/z of 244 [M]+ with the base peak at 229 resulting from releasing a methyl. The structure of compound 2 was established from analysis of the 1H and 13C NMR spectra. Compound 2 displayed 15 carbon signals, nine being typical of a coumarin skeleton, five related to prenyl branch (including unsaturated bond, δ121.7 and 133.9) and the other one signal was ascribable to methoxyl substituent (δ 56). The downfield signal at δC 161.9 was assigned to the carbonyl carbon of the coumarin moiety, the functional group being confirmed by IR analysis at 1738 cm-1 in accordance with six-membered ring esteric carbonyl.
Suberosin epoxide showed a small molecular ion at m/z of 260 showing an unstable molecule confirming the epoxide moiety, in accordance with 13CNMR chemical shifts of 63.6 and 56.3 ppm and IR absorbance in 1100-1200 cm-1, as well. Compound 1 displayed 15 carbon signals, nine being related to coumarin skeleton, five related to methylated epoxide branch and the other one signal was ascribable to methoxyl substituent. The downfield signal at δ C 161.7 was assigned to the carbonyl carbon of the coumarin moiety.
Antileishmania evaluation
Among three isolated coumarins, osthol, showed a significant antileishmanial effect on promastigotes of L. major strain MRHO/IR/75/ER in early hours of exposure. IC50s of osthol were 14.40 and 10.79 µg/mL in 3 and 48 h after exposure to cell suspension, respectively.
After three hours exposure to test (osthol) solution with 20 µg/mL final concentration, about 71% of parasites were killed. This inhibition growth percentage for concentration of 50 µg/mL after three hours of addition of osthole was about 91%. After 48 h at 20 µg/mL 13% and in 50 µg/mL only 7 % of promastigotes were alive.
The other tested coumarin, suberosin epoxide showed a weak antileishmanial effect. It inhibited promastigote growth, with an IC50 of 54 µg/mL after 48 h of incubation. At concentration of 100 µg/mL after 48 h of incubation, 66% of promastigotes were killed.
Suberosin showed no considerable antileishmanial effect at 5 µg/mL, 20 µg/mL, 40 µg/mL, 50 µg/mL, 80 µg/mL and 100 µg/mL concentrations after 48 h (Fig. 3).
Fig. 3.
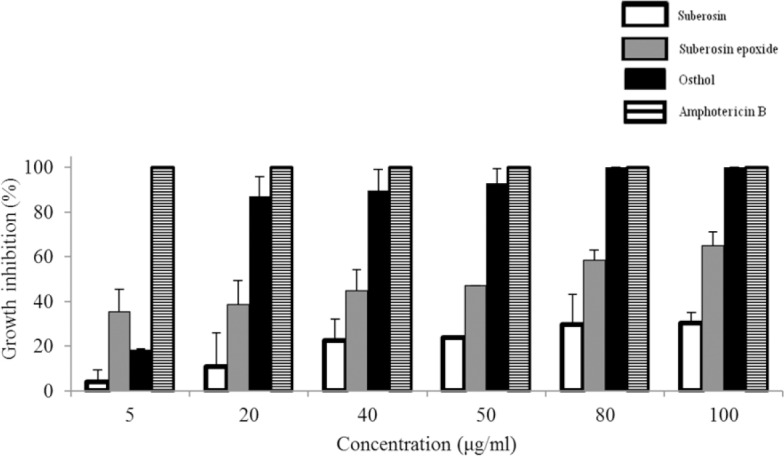
Antileishmanial activities of osthol, suberosin and suberosin epoxide against Leishmania major promastigotes. Cells were cultivated in the presence of different concentrations of osthol, suberosin and suberosin epoxide and counted after 48 h. The height of the bars indicates the percentage of growth inhibition at each concentration compared to the control containing only the solvent DMSO. The IC50 value for amphotericin B is reported to be 0.3 μg/mL (ref. 29). The experiments were performed four times independently for osthol and suberosin epoxide and 3 times independently for suberosin. Data are reported as mean ± SD.
DISCUSSION
Leishmania species are responsible for considerable morbidity and mortality especially in developing countries. Currently available drugs have unpleasant side effects and apt to resistance; so more efficacious drugs are urgently required (30). In this regard, medicinal plants offer promising prospects for discovering new compounds with therapeutic properties.
Earlier studies have shown antileishmanial effect of some coumarins. Coumarins have shown inhibitory effects against L. amazonensis, L. braziliensis and L. donovani (7,8). Mammea type coumarins isolated from Calophyllum brasiliense were active against both promastigotes and interacellular amastigote forms. They could also sharply decrease the parasite mitochondrial membrane potential which is an important organelle for the parasite (8). The other studies found that treating L. major promastigotes with prenylated coumarins, aurapten and umbelliprenin resulted in significant decrease in parasite count after 48 h (17,18).
In the present work, antileishmanial effect of three coumarins, osthol, suberosin and suberosin epoxide were studied. In early hours of exposure to osthol at 20 µg/mL, there was 71% reduction in parasite count, which is, in comparison with suberosin epoxide, suberosin and the coumarins studied in earlier investigations; a significant anti-parasitic activity. The morphology of the parasites was also significantly affected by osthol. Large vacuoles were seen and the flagella movement was limited. This rapid effect was seen only with osthol and the other two coumarins did not show such efficient activity.
As expected, suberosin epoxide showed more activity than suberosin because of its epoxide group. Anti-inflammatory effect of suberosin via modulating NF-κB and NF-AT has already been reported (31) but herewith, to the best of our knowledge, we report for the first time the pharmacological activity of a rare coumarin suberosin epoxide. It is known that alteration of substituents at position 7 and 8 is essential in leishmanicidal effects of the coumarins (32). Therefore, greater leishmanicidal activity of osthol compared to that of its isomer suberosin could be attributed to the substitution of prenyl at C-8 in osthol as opposed to C-6 in other coumarins.
Although osthol has been reported as potent anti-convulsant (33), neuroprotective, antioxidant (34), anti-tumor (35), anti-hyperlipidemic, anti-hypertensive (34,36), and antispasmodic agent (37), reports on its topical use are scarce. It has been shown that enhancers like chenopodium, menthol and azone can increase osthol penetration through skin via destroying the barrier function of stratum corneum (38).
On the other hand, suberosin, an isomer of osthol, failed to show activity in most biological tests including antiparasitic activity against Plasmodium falciparum (39) or antibacterial effect against Staphylococcus aureus (40). Nevertheless, suberosin could inhibit the aggregation and ATP release of rabbit platelets induced by arachidonic acid, collagen, ADP, platelet-activating factor (41). As a promising effect reported previously, suberosin could inhibit phytohemagglutinin-induced proliferation of human peripheral blood mononuclear cells mediated through reduction of [Ca2+], extracellular signal-regulated protein kinas, NF-AT, and NF-κB activation, and early gene expression in these cells including cyclins and cytokines, and arrest of cell cycle progression in the cells (31). The effective compounds should not have cytotoxic effects on human promonocytic cells (32), so this effect of osthol is proposed to be assessed in future.
CONCLUSION
Due to the resistance to currently available antileishmanial drugs such as antimonials and amphotericin B and sever adverse effects, search of new drug in this field is very valuable and important. The evaluation of antileishmanial activity of three prenylated coumarins yielded interesting results indicating that osthol could be considered as an attractive and potent natural antileishmanial agent. For the development of this prenylated coumarin as a new antileishmanial drug, further investigations of in vivo activity and toxicity of osthol are imperative.
ACKNOWLEDGEMENTS
The content of this paper is extracted from two Pharm.D theses, No. 389367 and 386245 submitted by N. Mohseni and H. Asgari-Nasab, which were financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Gillespie SH, Pearson RD. Chichester: John Wiley & Sons Ltd; 2001. Principles and practice of clinical parasitology; pp. 292–299. [Google Scholar]
- 2.WHO Communicable Diseases Surveillance and Response. Geneva: WHO; WHO, World Health Organization. The increase in risk factors for leishmaniasis worldwide. http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/index.html,2001: accessed 15.05.2010 . [Google Scholar]
- 3.Markell EK, Voge M, John DT. 6th ed. Philadelphia: W.B. Saunders Company; 1986. Medical Parasitology; pp. 121–125. [Google Scholar]
- 4.Sen R, Chatterjee M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine. 2011;18:1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Da Silva DB, Tulli EC, Militão GC, Costa-Lotufo LV, Pessoa C, De Moraes MO, et al. The antitumoral, trypanocidal and antileishmanial activities of extract and alkaloids isolated from Duguetia furfuracea. Phytomedicine. 2009;16:1059–1063. doi: 10.1016/j.phymed.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Pereira IO, Marques MJ, Pavan AL, Codonho BS, Barbiéri CL, Beijo LA, et al. Leishmanicidal activity of benzophenones and extracts from Garcinia brasiliensis Mart. fruits. Phytomedicine. 2010;7:339–345. doi: 10.1016/j.phymed.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira ME, De Arias AR, Yaluff G, De Bilbao NV, Nakayama H, Torres S, et al. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine. 2010;17:375–378. doi: 10.1016/j.phymed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Brenzan MA, Santos AO, Nakamura CV, Filho BP, Ueda-Nakamura T, Young MC, et al. Effects of (-) mammea A/BB isolated from Calophyllum brasiliense leaves and derivatives on mitochondrial membrane of Leishmania amazonensis. Phytomedicine. 2012;19:223–230. doi: 10.1016/j.phymed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Morales-Yuste M, Morillas-Márquez F, Martín-Sánchez J, Valero-López A, Navarro-Moll M. Activity of (-) α-bisabolol against Leishmania infantum promastigotes. Phytomedicine. 2010;17:279–281. doi: 10.1016/j.phymed.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Karioti A, Skaltsa H, Kaiser M, Tasdemir D. Trypanocidal, leishmanicidal and cytotoxic effects of anthecotulide-type linear sesquiterpene lactones from Anthemis auriculata. Phytomedicine. 2009;16:783–787. doi: 10.1016/j.phymed.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Tyler VE, Brady LR, Robbers JE. Pharmacognosy. 9th ed. Philadelphia: Lea & Febiger; 1988. p. 75. [Google Scholar]
- 12.Evans WC. Trease and Evans’ Pharmacognosy. 14th ed. London: WB Saunders; 1996. p. 230. [Google Scholar]
- 13.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 14.Kostova I, Raleva S, Genova P, Argirova R. Structure-activity relationships of synthetic coumarins as HIV-1 inhibitors. Bioinorg Chem Appl. 2006:ID68274. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SY, Lee KY, Sung SH, Kim YC. Four new neuroprotective dihydropyranocoumarins from Angelica gigas. J Nat Prod. 2005;68:56–59. doi: 10.1021/np049705v. [DOI] [PubMed] [Google Scholar]
- 16.Iranshahi M, Sahebkar A, Hosseini ST, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of diversin from Ferula diversivittata in vitro and in vivo. Phytomedicine. 2010;17:269–273. doi: 10.1016/j.phymed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Napolitano H, Silva M, Ellena J, Rodrigues B, Almeida A, Vieira P, et al. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz J Med Biol Res. 2004;37:1847–1852. doi: 10.1590/s0100-879x2004001200010. [DOI] [PubMed] [Google Scholar]
- 18.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito Ch, Furukawa H, et al. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med. 2008;74:147–150. doi: 10.1055/s-2008-1034293. [DOI] [PubMed] [Google Scholar]
- 20.Rezaee R, Behravan E, Behravan J, Solati F, Naderi Y, Emami B, et al. Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem Toxicol. 2014;37:144–148. doi: 10.3109/01480545.2013.834352. [DOI] [PubMed] [Google Scholar]
- 21.Casey PJ, Seabra MC. Protein prenyl transferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 22.Rechinger K. Vol. 162. Graz: Akademische Druck-u Verlagsanstalt; 1982. Flora Iranica; p. 433. [Google Scholar]
- 23.Taran M, Ghasempour HR, Shirinpour E. Antimicrobial activity of essential oils of Ferulago angulata subsp. carduchorum. Jundishapour J Microbiol. 2011;3:10–14. [Google Scholar]
- 24.Zargari A. Medicinal Plants. Vol. 2. Tehran: Tehran University Publications; 1988. p. 553. [Google Scholar]
- 25.Sajjadi SE, Zeinvand H, Shokoohinia Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res Pharm Sci. 2009;4:19–23. [Google Scholar]
- 26.Kogure K, Yamauchi I, Tokumura A, Kondou K, Tanaka N, Takaishi Y, et al. Novel antioxidants isolated from plants of the genera Ferula, Inula, Prangos and Rheum collected in Uzbekistan. Phytomedicine. 2004;11:645–651. doi: 10.1016/j.phymed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Tosun A. Occurrence of coumarins in Seseli hartvigii growing in Turkey. Chem Nat Comp. 2006;42:608–609. [Google Scholar]
- 28.Quader MA, El-Turbi JA, Armstrong JA, Gray AI, Waterman PG. Coumarins and their taxonomic value in the genus Phebalium. Phytochemistry. 1992;31:3083–3089. [Google Scholar]
- 29.Mikus J, Harkenthal M, Steverding D, Reichling J. In vitro effect of essential oils and isolated mono-and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000;66:366–368. doi: 10.1055/s-2000-8548. [DOI] [PubMed] [Google Scholar]
- 30.WHO communicable diseases surveillance and response. Geneva: WHO; WHO. World Health Organization, Leishmaniasis: geographical distribution. http://www.who.int/emc/diseases/leish/leisgeo1.html;2003: accessed 15.05.2010 . [Google Scholar]
- 31.Chen YC, Tsai WJ, Wu MH, Lin LC, Kuo YC. Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF‐AT and NFκB. Br J Pharmacol. 2007;150:298–312. doi: 10.1038/sj.bjp.0706987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arango V, Robledo S, Séon-Méniel B, Figadéré B, Cardona W, Sáez J, et al. Coumarins from Galipea panamensis and their activity against Leishmania panamensis. J Nat Prod. 2010;73:1012–1014. doi: 10.1021/np100146y. [DOI] [PubMed] [Google Scholar]
- 33.Luszczki JJ, Andres-Mach M, Cisowski W, Mazol I, Glowniak K, Czuczwar SJ. Osthole suppresses seizures in the mouse maximal electroshock seizure model. Eur J Pharmacol. 2009;607:107–109. doi: 10.1016/j.ejphar.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL, et al. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int. 2010;57:206–215. doi: 10.1016/j.neuint.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Shen X, Wu X, Liu P, Xu W, Li R. Experimental research on anti-tumor activities of natural osthole. Carcinog Teratog Mutagen. 2007;19:119. [Google Scholar]
- 36.Ogawa H, Sasai N, Kamisako T, Baba K. Effects of osthol on blood pressure and lipid metabolism in stroke-prone spontaneously hypertensive rats. J Ethnopharmacol. 2007;112:26–31. doi: 10.1016/j.jep.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Sadraei H, Shokoohinia Y, Sajjadi SE, Ghadirian B. Anti spasmodic effect of osthol from Prangos ferulacea on the rat isolated uterus muscle. Res Pharm Sci. 2012;7:141–149. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhenting Y, Dawei C, Hui X, Pingtian D, Ruhua Z. Studies on effects of enhancers on percutaneous absorption of osthol across excised full thickness rat skin. Chin Pharmaceut J. 2003;38:683–684. [PubMed] [Google Scholar]
- 39.Lacroix D, Prado S, Kamoga D, Kasenene J, Bodo B. Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark. J Nat Prod. 2011;74:2286–2289. doi: 10.1021/np2004825. [DOI] [PubMed] [Google Scholar]
- 40.Trusheva B, Todorov I, Ninova M, Najdenski H, Daneshmand A, Bankova V. Antibacterial mono-and sesquiterpene esters of benzoic acids from Iranian propolis. Chem Cent J. 2010;4 doi: 10.1186/1752-153X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng CM, Li HL, Wu TS, Huang SC, Huang TF. Antiplatelet actions of some coumarin compounds isolated from plant sources. Throm Res. 1992;66:549–557. doi: 10.1016/0049-3848(92)90309-x. [DOI] [PubMed] [Google Scholar]


