Abstract
2,3-Butanediol (2,3-BD) is a valuable bulk chemical owing to its extensive application in chemical and pharmaceutical industry with diverse applications in drug, cosmetics and food products. In the present study, the biotransformation of acetoin to 2,3-BD by five plant species (Brassica oleracea, Brassica rapa, Daucuscarota, Pastinaca sativa, and Raphnussativus) and five microorganisms (Aspergillusfoetidus, Penicillumcitrinum, Saccharomyces carlbergensis, Pichiafermentans, and Rhodotrulaglutinis) was investigated as a method for the production of 2,3-BD, which can serve as an alternative to the common pentoses and hexoses fermentation by microorganisms. The produced 2,3-BD stereoisomers were characterized and their total conversion yields were determined. The results showed that the examined plants can be used as a green factory for the production of all 2,3-BD stereoisomers, except B. rapa. In microorganisms, P. fermentans and S. carlbergensis produced (–)-2R,3R and mesobutanediol, while P. citrinum produced (+)-2S,3S and mesobutanediol. R. glutinis and A. foetidus produced all three isomers. In conclusion, efficient whole-cell biocatalysts from plants and microorganisms were determined in the bioconversion of acetoin to 2,3-BD. The profile of produced stereoisomers demonstrated that microorganisms produce more specific stereoisomers.
Keywords: 2,3-Butanediol; Acetoin; Plants; Microorganisms; Biotransformation
INTRODUCTION
2,3-Butanediol (2,3-BD) is known as an important precursor in the production of various chemical feed stocks and liquid fuels (1). For example, 2,3-BD has potential applications in the manufacture of printing inks, perfumes, fumigants, moistening and softening agents, explosives, plasticizers, food, and pharmaceuticals (1,2). Currently, it is evident that the bioproduction of a substance is more legitimatethan conventional chemical synthesis due to its renewable feed stocks and lesser pollution to the environment. Chemical processes for the production of 2,3-BD are unambiguously more costly than the microbial routes and therefore, the commercial production of this compound would be limited to the fermentation of pentoses and hexoses bymicroorganisms (3). Although fermentation by microorganisms is a cost-effective and green procedure, only a few do so in what might be considered significant quantities (4). In any given process, the optical isomer and the amount of 2,3-BD produced is dependent upon the particular microorganism employed (Fig. 1). For example in glucose fermentation by most klebsiella species, only (2S,3S)-2,3-BD and meso-2,3-BD were produced (5,6,7,8), whereas Bacillus subtilis produced (2R,3R)-2,3-BD and meso-2,3-BD (9). In this study, an attempt was made to investigate the possibility of producing 2,3-BD by acetoin reduction in some plants including Brassica oleracea, Brassica rapa, Daucuscarota, Pastinaca sativa, Raphnussativus and microorganisms encompasses of Aspergillusfoetidus, Penicillumcitrinum, Saccharomyces carlbergensis, Pichiafermentans, Rhodotrulaglutinis as an alternative to the common fermentation routes.
Fig. 1.
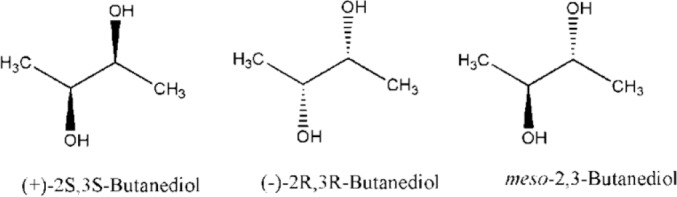
Stereoisomers of 2,3-butanediol.
These species have shown high stereoselectivity and yield toward a wide range of substrates and were the most reported species for biotransformation of various substrates in literature (10,11,12,13,14), however, to the best of our knowledge, they are yet to be used for the bioreduction of acetoin to 2,3-BD.
In order to elucidate the stereospecificity of the biotransformation, the characterization of different produced stereoisomers was further investigated. A literature survey revealed that no microorganism has been identified as a biocatalyst source for the production of different 2,3-BD stereoisomers (4).
MATERIALS AND METHODS
Materials and instruments
(+)-2S,3S-Butandiol, (–)-2R,3R-butanediol, 2,3-butanediol (mixture of meso- D- and L-form), (-)-R-acetoin, and (+)-S-acetoin were obtained from commercial sources (Merck, Darmstadt, Germany). Freeze dried A. foetidus (PTCC 5099), P. citrinum (PTCC 5304), S. carlbergensis (PTCC 5051), P. fermentans (PTCC 5296), and R. glutinis (PTCC 5256) was purchased from the Persian Type Culture Collection (Iranian Research Organization for Science and Technology). Sabouraud-2%-dextrose broth and 4%-dextrose agar were purchased from Merck (Darmstadt, Germany). Biotransformation reactions were monitored using an Agilent technologies 7890A Gas Chromatograph/Agilent technologies mass spectrometer 5975C(GC/MS) (USA) equipped with anHP-5 capillary column (30 m × 0.25 mm; 0.25 μm film thicknesses). Enantiomeric composition analyses were performed using an Agilent technologies 6890N instrument (USA) equipped with a CP-Chirasil-DEX CB Varian GC column.
Biotransformation with plant root
Fresh plants of D. carota, B. oleracea, R. sativus, and P. sativa were obtained from a local market. For sterile biotransformation condition, the roots were washed thoroughly with water and surface disinfected with EtOH 70% and an UVlamp. The external layer of the roots was removed and the left-over was carefully minced into small thin pieces. A certain amount of acetoin (50 mg) was added to a suspension of fresh cut plant tissue (100 g) in distilled water and incubated at 25°C in a rotary shaker at 150 rpm. The reaction mixtures were stirred for the time necessary to obtain the appropriate conversion (48–96 h). Thereafter, the suspension was filtered and the reaction mixture was saturated with NaCl and extracted with ethyl acetate (3 × 40mL). The organic solution dried over anhydrous Na2SO4 and was then evaporated in vacuo. The samples were collected and labeled.
Microbial biotransformation
The stock cultures were maintained on Sabouraud dextrose agar medium at 4°C and were freshly sub-cultured before being considered for the transformation experiments. Erlenmeyer flasks, each containing 100 mL of Sabouraud broth medium, were inoculated with freshly obtained cells and incubated at 25°C in a rotary shaker at 150 rpm. The cultures were allowed to reach a constant cell density (detected by optical density or dry cell weight). Acetoin (50 mg) was added to each Erlenmeyer flask. The reaction mixtures were stirred for the time necessary to obtain the appropriate conversion (48–96 h). After 2 and 4 days of incubation, biomass was separated from the media by centrifugation. The extraction method was the same as biotransformation with plant root.
Conversion rate analysis and stereoisomeric characterization
After extraction and concentrating the samples were injected and chromatographed on GC-MS and gas chromatography and flame ionization detector(GC-FID) apparatus. The course of the reactions was analyzed by GC-MS and the stereoisomeric characterization of the product was done by GC/FID. The methods were established prior to sample analysis by use of standard samples.
Conversion rate and the course of the reaction were monitored using GC/MS: column temperature: 40-150°C (10.0°C/min), an injector temperature of 250°C. Enantiomeric composition analysis: GC/FID, column temperature: 95-130°C (1.0°C/min), injector temperature of 145°C. Retention times (min): (–)-R-acetoin, 3.39; (+)-S-acetoin, 3.57; (+)-2S,3S-butanediol, 7.73; (–)-2R,3R-butanediol, 8.03; meso-butanediol, 8.56.
All the reactions were repeated three times and the results shown in this study are the arithmetic mean values.
RESULTS
The determination of produced stereoisomers was carried out using the established GC-FID analysis methods (Fig. 2a). The percentage of each 2,3-BD stereoisomer was defined as:
Fig. 2.
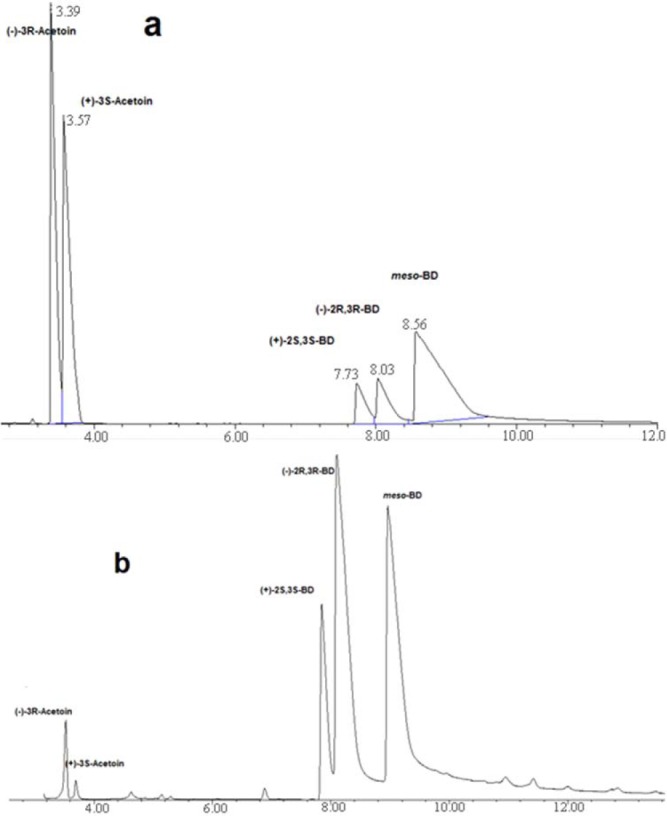
(a) GC analysis of the acetoin and 2,3-BD stereoisomer mixture used as standard for setting up the method. (b) GC analysis of the reaction catalyzed by Daucuscarota (48 h).
For precise determination of the amount of product (2,3-BD), a calibration curve was also established in relation to the area under the peak and concentration of 2,3-BD. It was linear for concentration in the range of 5-50 mg/mL (r = 0.99).
Bioreduction of acetoin using plant biocatalysts
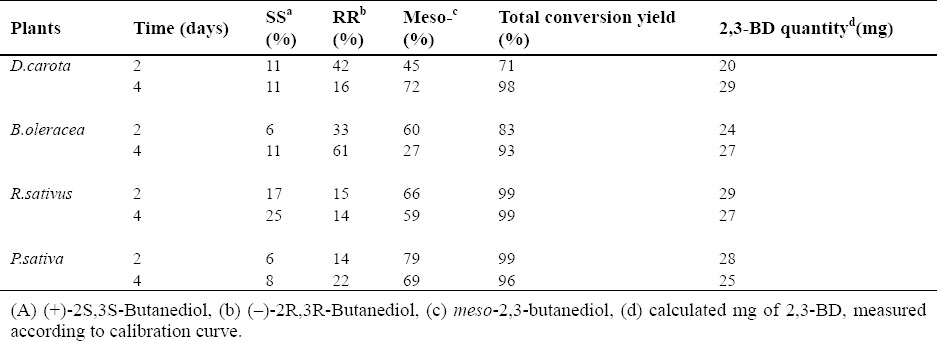
The results are shown in Table 1. The GC analysis chromatogram of D. carota is presented in Fig. 2b. As shown in Table 1, among all plants considered for this study, only B. rapa failed to accomplish the bioreduction of acetoin;bioreduction was achieved in the other four plants. Therefore, high quantity and yield of 2,3-BD could be achieved within a short period of time.
Table 1.
2,3-BD stereoisomers produced by different species of plants; The percentage amount of the isomer is given.
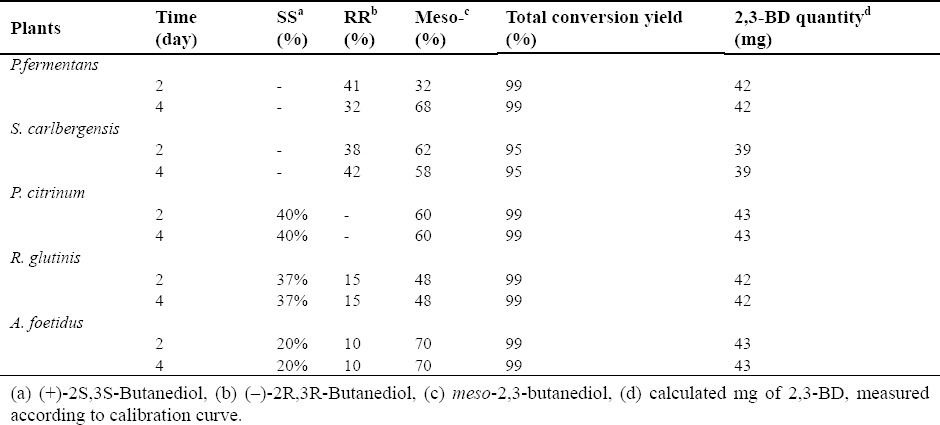
Bioreduction of acetoin using microbial biocatalyst
Furthermore, in this study, bio-transformation was carried out using microorganisms in which some interesting results were achieved. For example, P. fermentans and S. carlbergensis produced (–)-2R,3R and mesobutanediol, while P. citrinum produced (+)-2S,3S and mesobutanediol. R. glutinis and A. foetidus produced all three isomers (Table 2).
Table 2.
2,3-BD stereoisomers produced by different species of microorganisms; percentage amount of the isomer is given.
DISCUSSION
According to these data, if 2,3-BD is considered the favorite product regardless of the isomer produced, plants can be considered cost-effective, easy to manipulate green biocatalysts. However, if a specific stereoisomer of 2,3-BD is the desired product, other ways must be sought to reach a specific enantiomer.
Since the separation of meso and racemic 2,3-butanediol is possible and their resolution in the preparative scale has also been reported (15), the use of P. fermentans and S. carlbergensis for the production of (–)-2R,3R will be useful. On the other hand, if obtaining (+)-2S,3S-butanediol is expected, one can use P. citrinum as whole cell biocatalyst for this purpose. These biotransformations had significant conversion yields. The use of P. fermentans and S. carlbergensis could help achieve up to 40% (+)-2S,3S butanediol and the same amount of (–)-2R,3R-butanediol was achieved when P. citrinum was used (Table 2).
Another issue is that the total conversion of acetoin to 2,3-BD is almost complete in shorter periods of time using microorganisms in comparison with plants. The relative percentage of 2,3-BD produced by plants is more similar to the 2,3-BD commercially available as a mixture of (+)-2S,3S-BD, (–)-2R,3RBD and mesoBD. Nevertheless, the relative percentage of 2,3-BD produced by microorganisms is well differentiated from the commercially available 2,3-BD (Table 2), therefore, good prospects are expected from its future use in the production of a specific 2,3-BD stereoisomer.
CONCLUSION
2,3-BD is a particularly important chemical with a variety of applications and as such, its demand is increasing worldwide. So far, 2,3-BD is mainly produced by microbial route and fermentation of pentoses and hexoses. Acetoin is a precursor of 2,3-BD and can be biotransformed by plants and microorganisms to 2,3-BD stereoisomers. In the present study, efficient whole-cell biocatalysts from plants and microorganisms were determined in the bioconversion of acetoin to 2,3-BD. Also the profile of produced stereoisomers was characterized and some of them could be suggested for the production of a specific stereoisomer in the future.
ACKNOWLEDGEMENTS
The authors like to thank Radan English Edit Center (www.englishedit.ir), a scientific writing company, for their expert review and editing assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Syu MJ. Biological production of 2,3-butanediol. ApplMicrobiolBiotechnol. 2001;55:10–18. doi: 10.1007/s002530000486. [DOI] [PubMed] [Google Scholar]
- 2.Garg S, Jain A. Fermentative production of 2,3-butanediol: a review. Bioresour Technol. 1995;51:103–109. [Google Scholar]
- 3.Xiu ZL, Zeng AP. Present state and perspective of downstream processing of biologically produced 1, 3-propanediol and 2,3-butanediol. ApplMicrobiolBiotechnol. 2008;78:917–926. doi: 10.1007/s00253-008-1387-4. [DOI] [PubMed] [Google Scholar]
- 4.Ji XJ, Huang H, Ouyang PK. Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv. 2011;29:351–364. doi: 10.1016/j.biotechadv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Voloch M, Ladisch M, Rodwell V, Tsao G. Reduction of acetoin to 2,3‐butanediol in Klebsiellapneumoniae: A new model. BiotechnolBioeng. 1983;25:173–183. doi: 10.1002/bit.260250114. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, et al. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. ApplMicrobiolBiotechnol. 2009;82:49–57. doi: 10.1007/s00253-008-1732-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Wang Y, Jiang T, Li L, Ma C, Xu P. Production of 2, 3-butanediol from corncob molasses, a waste by-product in xylitol production. ApplMicrobiolBiotechnol. 2010;87:965–970. doi: 10.1007/s00253-010-2557-8. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Dai J-Y, Xiu ZL. A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2, 3-butanediol by Klebsiellapneumoniae. Bioresour Technol. 2010;101:8342–8347. doi: 10.1016/j.biortech.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Moes J, Griot M, Keller J, Heinzle E, Dunn I, Bourne J. A microbial culture with oxygen‐sensitive product distribution as a potential tool for characterizing bioreactor oxygen transport. BiotechnolBioeng. 1985;27:482–489. doi: 10.1002/bit.260270413. [DOI] [PubMed] [Google Scholar]
- 10.Utsukihara T, Watanabe S, Tomiyama A, Chai W, Horiuchi CA. Stereoselective reduction of ketones by various vegetables. J MolCatal B: Enzym. 2006;41:103–109. [Google Scholar]
- 11.Andrade LH, Utsunomiya RS, Omori AT, Porto ALM, Comasseto JV. Edible catalysts for clean chemical reactions: Bioreduction of aromatic ketones and biooxidation of secondary alcohols using plants. J MolCatal B: Enzym. 2006;38:84–90. [Google Scholar]
- 12.Homann MJ, Vail RB, Previte E, Tamarez M, Morgan B, Dodds DR, et al. Rapid identification of enantioselective ketone reductions using targeted microbial libraries. Tetrahedron. 2004;60:789–797. [Google Scholar]
- 13.Patel RN, Goswami A, Chu L, Donovan MJ, Nanduri V, Goldberg S, et al. Enantioselective microbial reduction of substituted acetophenones. Tetrahedron: Asymmetry. 2004;15:1247–1258. [Google Scholar]
- 14.Chartrain M, Greasham R, Moore J, Reider P, Robinson D, Buckland B. Asymmetric bioreductions: application to the synthesis of pharmaceuticals. J MolCatal B: Enzym. 2001;11:503–512. [Google Scholar]
- 15.Voloch M, Ladisch MR, Rodwell VW, Tsao GT. Separation of meso and racemic 2, 3‐butanediol by aqueous liquid chromatography. BiotechnolBioeng. 1981;23:1289–1296. [Google Scholar]


