ABSTRACT
Evidence indicates that the mechanisms controlling photosynthesis efficiency also regulate plant response to biotic and abiotic stress. Light-induced cell death is genetically maintained for the control of innate immunity. In a recent study we showed that the expression of AtWDR26 was induced by light, multiple plant hormones, and abiotic stress; increased AtWDR26 strongly upregulated gene groups related to chloroplast metabolism, disease resistance, and abiotic stress tolerance. Gain- and loss-of-function analyses in transgenic plants demonstrated the involvement of AtWDR26 in signaling pathways; these controls were osmotic as well as salt stress tolerance. More detailed transcriptome evidence suggested that AtWDR26 was a powerful inducer of gene expression associated with chloroplast metabolism. This included the electron transport chain of the photosystem, carbohydrate synthesis, and enzymatic activity involved in photorespiration. Moreover, genes in auxin synthesis (and perception) constituted a significant portion of those that were upregulated. Gene expression involved in disease resistance, control of cell wall flexibility, Zn uptake, and AP2/ERF transcription factors was also be upregulated. We concluded that AtWDR26 is one component in the regulatory network between light-regulated plant growth and the adaptation response to disease resistance and abiotic stress. Auxin signal acts downstream for AtWDR26 regulation and the adaptation response to biotic and abiotic stress: this occurs through modulating cell wall flexibility, Zn homeostasis, and controlling stress-related transcription factors.
KEYWORDS: AP2/ERF transcription factors, auxin signal transduction, cell wall flexibility, chloroplast metabolism, disease resistance, zinc homeostasis
Light is the most essential environmental factor for plant growth and development. Plant cells have evolved a highly responsive and flexible system to cope with excess excitation energy, generated by high light stress. Efficient acclimation to high light growth conditions is relied on a proper communication between chloroplast signals and nucleus gene expression. Accumulating evidence suggests the involvement of cellular pathways, including redox signals derived from the electron transport chain, carbohydrate metabolism, and phytohormones for chloroplast retrograde signaling.1 In a previous study, we characterized the function of the Arabidopsis WDR gene, AtWDR26.2 The homolog of AtWDR26 plays a role in the cellular pathway of H2O2-induced cell death.3 The AtWDR26 transcript was regulated by light and the phenotypes of transgenic seedling overexpressing AtWDR26 and T-DNA knockout lines exhibited changes in light-regulated seed germination and seedling growth.2 More evidence indicates that the function of WDR family proteins act as scaffolding for diverse cellular pathways.4 The 3 largest groups of upregulated genes in transgenic seedlings overexpressing AtWDR26 are gene groups for metabolism, stress response, and transcription regulation.2 A detailed analysis of the AtWDR26-induced transcriptome revealed a large number of upregulated genes for chloroplast function, including photochemical reaction, carbohydrate synthesis, and photorespiration enzymes; moreover, genes correlated with sugar metabolism constituted the largest portion in this gene group (Fig. 1A). Photochemical reactions and peroxisome-associated reactions play important roles in high light acclimation.5 Sugar metabolism is essential to plant cell acclimation to excess light energy.6 Several phytohormone signals are associated with the regulation of chloroplast retrograde signaling; this includes salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) in the chloroplast retrograde system.7 However, overexpression of AtWDR26 can significantly upregulate genes involved in auxin synthesis and perception (Fig. 1B). It has been reported that carbohydrate metabolism can regulate auxin levels via the PHYTOCHROME-INTERACTING FACTOR (PIF) proteins in Arabidopsis.8 Thus, it is plausible that the increased auxin signal in transgenic plants overexpressing AtWDR26 could be the result of increased carbohydrate metabolism. More evidence for the role of auxin signaling in AtWDR26-regulated transcription showed increased gene expression in transgenic plants. These are genes involved in cell wall flexibility, which constituted a large portion of upregulated genes (Fig. 1C). Auxin is an important hormone in cell growth regulation that works through the modulation of cell wall flexibility.9 Cell wall metabolism is closely correlated with the acclimation response to abiotic stress.10 Overexpression of AtWDR26 also upregulated genes in the control of Zn homeostasis (Fig. 1D), which is an important constituent of superoxide dismutase (SOD) functioning for the removal of oxidative stress derived from the electron transport chain in the chloroplast.11 It is reported that exogenous auxin increases CuZn SOD levels in the tomato.12 Auxin also plays a protective role in photosynthesis by remodeling its apparatus against photooxidative inhibition.13 Hence, transcriptome evidence suggests that AtWDR26 might regulate physiological response to oxidative stress, which occurs through modulation of auxin signaling in plant cells.
Figure 1.
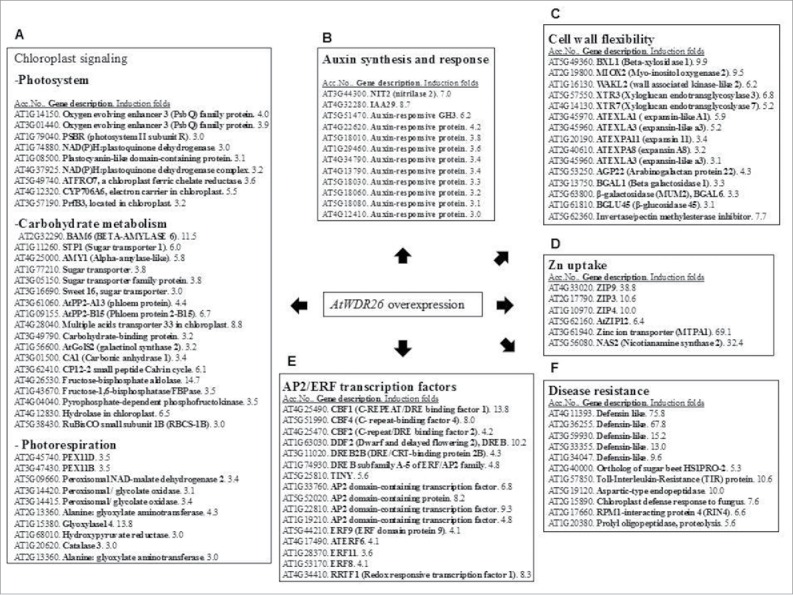
Selective upregulated gene groups in the transcriptome overexpressing AtWDR26. Transcriptome of transgenic Arabidopsis seedlings overexpressing AtWDR26 was analyzed by a DNA microarray. Upregulated genes associated with chloroplast signaling (A), auxin signaling (B), cell wall flexibility (C), Zn uptake (D), transcription factors of AP2/ERF family (E), and disease resistance (upregulation levels > 5-fold) (F).
Overexpression of AtWDR26 upregulated expression of AP2/ERF transcription factors and genes involved in disease resistance (Figs. 1E and F). Among these AP2/ERF transcription factors, the expression of C-repeat binding factor 1 (CBF1) and REDOX RESPONSIVE TRANSCRIPTION FACTOR1 (RRTF1) was induced at 13.8 and 8.3 folds, respectively, in transgenic seedlings, which overexpress AtWDR26.2 CBF1 plays a role in the H2O2-triggered chloroplast retrograde signaling in response to stress.14 The RRTF1 transcript has a rapidly-induced switch from low light to high light conditions.15 Ethylene (ET) and JA are 2 crucial hormones in plant cells that adapt to biotic and abiotic stress through regulation of AP2/ERF family transcription factors.16 Transgenic seedlings overexpressing AtWDR26 exhibited altered sensitivity to ET and JA, and up-regulated gene expression involved in ET and JA synthesis.2 The stimulating role of auxin on ethylene synthesis has been systematically studied (Abel et al., 1995). A positive relationship between the auxin and JA signal was also demonstrated, in which auxin-responsive factors like ARF6 and ARF8, and a member of the AUX/IAA family like IAA8 regulate maturation of floral organs by controlling JA synthesis.17,18 It is plausible that increased auxin, signaling by the overexpression of AtWDR26, can lead to an increased defense response by the ET/JA-mediated signaling pathway. We propose that AtWDR26 is a regulator in controlling plant growth in its response to light quality and quantity. Auxin signaling acts downstream to AtWDR26, and controls cellular pathways for oxidative stress protection and tolerance acquisition for biotic and abiotic stress (Fig. 2).
Figure 2.
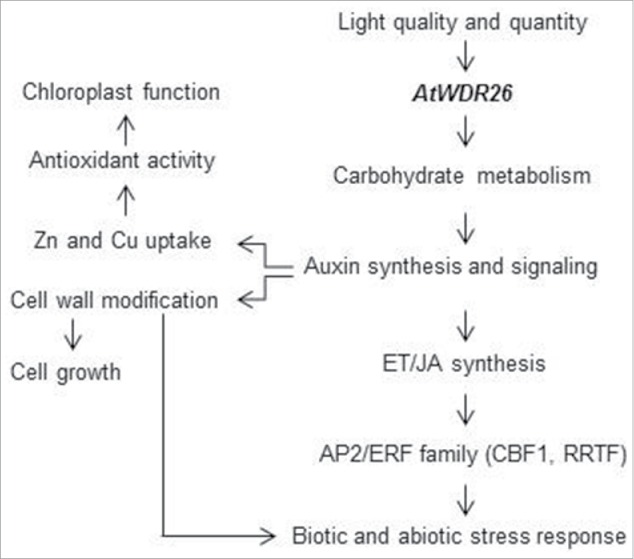
Model for AtWDR26s role in plant growth and defense response. AtWDR26 is one component in the signaling pathway controlling light-regulated growth and development. AtWDR26 could modulate auxin signaling through chloroplast function modification. Auxin signal can act downstream to regulate the adaptation response to light stress, as it modulates cellular pathways in cell wall flexibility and Zn homeostasis. Auxin also controls the ET/JA-mediated transcription regulation, which in turn governed by AP2/ERF transcription factors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Hausler RE, Heinrichs L, Schmitz J, Flugge UI. How sugars might coordinate chloroplast and nuclear gene expression during acclimation to high light intensities. Molecular Cell 2014; 7:1121-37; PMID:25006007; http://dx.doi.org/ 10.1093/mp/ssu064 [DOI] [PubMed] [Google Scholar]
- 2.Chuang HW, Feng JH, Feng YL, Wei MJ. An Arabidopsis WDR protein coordinates cellular networks involved in light, stress response and hormone signals. Plant Sci 2015; 24:23-31; PMID:26706055; http://dx.doi.org/ 10.1016/j.plantsci.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Liu Y, Wei X, Yuan C, Yuan X, Xiao X. A novel WD-40 repeat protein WDR26 suppresses H2O2-induced cell death in neural cells. Neurosci Lett 2009; 460:66-71; PMID:19446606; http://dx.doi.org/ 10.1016/j.neulet.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 4.van Nocker S, Ludwig P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics 2003; 4:50; PMID:14672542; http://dx.doi.org/ 10.1186/1471-2164-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kangasjärvi S, Neukermans J, Li S, Aro EM, Noctor G. Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot 2012; 63:1619-36; PMID:22282535; http://dx.doi.org/ 10.1093/jxb/err402 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Heinrichs L, Scossa F, Fernie AR, Oelze M L, Dietz KJ, Rothbart M, Grimm B, Flügge UI, Häusler RE. The essential role of sugar metabolism in the acclimation response of Arabidopsis thaliana to high light intensities. J Exp Bot 2014; 65:1619-36; PMID:24523502; http://dx.doi.org/ 10.1093/jxb/eru027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobik K, Burch-Smith TM. Chloroplast signaling within, between and beyond cells. Front Plant Sci 2015; 6:781; PMID:26500659; http://dx.doi.org/ 10.3389/fpls.2015.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sairanen I, Novák O, Pěnčík A, Ikeda Y, Jones B, Sandberg G, Ljung K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012; 24:4907-16; PMID:23209113; http://dx.doi.org/ 10.1105/tpc.112.104794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrot-Rechenmann C. Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol 2010; 2:a001446; PMID:20452959; http://dx.doi.org/ 10.1101/cshperspect.a001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenhaken R. Cell wall remodeling under abiotic stress. Front Plant Sci 2015; 5:71; PMID:25709610; http://dx.doi.org/ 10.3389/fpls.2014.00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 2002; 53:1331-41; PMID:11997379; http://dx.doi.org/ 10.1093/jexbot/53.372.1331 [DOI] [PubMed] [Google Scholar]
- 12.Tyburski J, Dunajska K, Mazurek P, Piotrowska B, Tretyn A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol Plant 2009; 31:249-60; http://dx.doi.org/ 10.1007/s11738-008-0225-8 [DOI] [Google Scholar]
- 13.Tognetti VB, Mühlenbock P, Van Breusegem F. Stress homeostasis - the redox and auxin perspective. Plant Cell Environ 2012; 35:321-33; PMID:21443606; http://dx.doi.org/ 10.1111/j.1365-3040.2011.02324.x [DOI] [PubMed] [Google Scholar]
- 14.Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem 2012; 287:11717-29; PMID:22334687; http://dx.doi.org/ 10.1074/jbc.M111.292847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel MO, Moore M, König K, Pecher P, Alsharafa K, Lee J, Dietz KJ. Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 2014; 26:1151-65; PMID:24668746; http://dx.doi.org/ 10.1105/tpc.113.121061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutterson N, Reuber TL. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 2004; 7:465-71; PMID:15231271; http://dx.doi.org/ 10.1016/j.pbi.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, et al.. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 2005; 132:4107-18; PMID:16107481; http://dx.doi.org/ 10.1242/dev.01955 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yan DW, Yuan TT, Gao X, Lu YT. A gain-of-function mutation in IAA8 alters Arabidopsis floral organ development by change of jasmonic acid level. Plant Mol Biol 2013; 82:71-83; PMID:23483289; http://dx.doi.org/ 10.1007/s11103-013-0039-y [DOI] [PubMed] [Google Scholar]


