ABSTRACT
To survive in most soils in which inorganic phosphate (Pi) levels are limited and constantly changing, plants universally use the vacuoles as cellular Pi “sink” and “source” to maintain Pi homeostasis. However, the transporters that mediate Pi sequestration into the vacuoles remain unknown. Recently, we and other 2 groups independently identified the members of SPS-MSF family as the candidates for tonoplast Pi transporters in Arabidopsis thaliana and Oryza sativa. We and Liu et al. demonstrated that one of SPS-MSF member, VPT1 (Vacuolar Phosphate Transporter 1), also named as PHT5;1 (Phosphate Transporter 5;1), plays a predominant role in Pi sequestration of vacuoles in Arabidopsis. Here we show that vpt1 mutants and VPT1-GFP overexpressing lines displayed sensitive to Pi stress under the hydroponic system containing the medium with low iron, supporting that VPT1 is essential for Arabidopsis to adapt phosphate stress.
KEYWORDS: Iron toxicity, phosphorus nutrition, phosphate stress, SPX-MSF, vacuolar phosphate sequestration
Phosphorus (P) constitutes cellular major metabolites and macromolecules, and its bioavailability is one of the most limiting elements for plant growth and development. Plants absorb most P element from the soil in a readily utilized form, inorganic phosphate (H2PO4− or Pi). Although its abundance and accessibility in the some rhizospheres, soluble Pi in most soils is far below that suitable for plant optimal growth as it easily forms insoluble complexes or precipitates with organic matter or mineral cations.1 To enhance crop yields, Pi fertilizers are widely and excessively casted, and thus cause Pi content largely varied in the soil. Plants apply the vacuole as the major Pi store to maintain Pi homeostasis in the cell to adapt these changes. During most phases of plant development, Pi concentration is much higher in the vacuole than in the cytosol, indicating that the vacuole has transport proteins responsible for the transport of Pi across tonoplast.2
Recently, we,3 Liu et al.,4 and Wang et al.5 independently revealed that SPX-MFS family potentially might function as a new group of vacuolar Pi transporters in plants. SPX-MFS proteins contain an evolutionally conserved SPX domain [named after yeast Syg1 and Pho81 and human xenotropic and polytropic retrovirus receptor 1 (XPR1)] with an MFS (Major Facilitator Superfamily) domain. MFS has multiple trans-membrane domains and transports a diverse range of substrates, such as ions, sugars, nucleosides, amino acids and peptides.6 Rice has 4 putative genes for this family, namely OsSPX-MFS1–SPX-MFS4.5,6 Wang et al. reported that 3 of them, OsSPX-MFS1, OsSPX-MFS2, OsSPX-MFS3, were localized on the tonoplast of rice protoplasts.5 Arabidopsis thaliana has 4 OsSPX-MFS orthologs, namely At1g63010, At4g11810 and At4g22990.6 We found that the transgenic plants expressing At1g63010 with a green fluorescent protein (GFP) fusion displayed the fluorescent signals specifically detected in the tonoplast, and thus named this gene as VPT1 (Vacuolar Phosphate Transporter 1).3 The subcellular location of VPT1 in the tonoplast was further confirmed in an independent study, where it was named as PHT5;1 (PHOSPHATE TRANSPORTER 5;1).4 VPT1 orthologs, At4g11810 and At4g22990 also reside in the tonoplast.4 The observations in rice and Arabidopsis conclude that SPX-MFS family is localized in the tonoplast in plants.
SPX-MFS proteins in plants are closely related to yeast vacuolar phosphate transporters, such as ScVTCs and PHO91,6 suggesting they are qualified candidates for vacuolar Pi transporters. OsSPX-MFS3 facilitated Pi influx or efflux across the plasma membrane of the Xenopus oocytes depending on the external Pi concentrations pH, and caused the Xenopus oocytes to produce Pi outward currents using 2-electrode voltage clamp technique.5 We applied patch-clamp analysis of isolated vacuoles and found that the Pi influx current was severely reduced in vpt1 compared with the wild type Arabidopsis thaliana. When ectopically expressed in the vacuoles of Nicotiana benthamiana mesophyll cells, VPT1 could mediate several anions influx across the tonoplast, including Pi, SO42−, NO3−, Cl−, and malate with Pi as that preferred anion.3 Complementary to our results, 31P-magnetic resonance spectroscopy analysis revealed that loss-of-function of vpt1 mutants accumulated less Pi and exhibited a lower vacuolar-to-cytoplasmic Pi ratio than the wild types.4 Taken together, these results indicate that the members of SPX-MFS family are the candidate transporters conducting Pi across the tonoplast.
The root architecture changes readily occur when plants are subjected to Pi stress, specifically Arabidopsis thaliana accession the Columbia (Col-0).7 We developed a hydroponic system described by Tocquin et al.8 to analyze the potential phenotype of the transgenic Arabidopsis thaliana (Col-0). To avoid the disturbance of agar used as the seed-holder,8 we sowed the seeds directly on an 80-mesh nylon net fixed on a plastic plate floated over the Arabidopsis nutrient solution (ANS). The 2-week-old seedlings after germination were transplanted into a larger floater with 5 mm poles, which were derived from PCR 96-well-plate. The cartoon of the hydroponic system we used5 was shown in Fig. 1. The ANS solution contained NaH2PO4 as the Pi source, and its composition was shown in Table 1. We found that root elongation in the vpt1 seedlings was severely inhibited compared with the wild type under the conditions containing the higher Pi.3 However, Liu et al. reported that they did not observe the dwarf root of this mutant under the conditions using half-strength Hoagland as nutrient medium.4 We speculated that the difference might come from the nutrient medium used in these 2 experimental systems.
Figure 1.
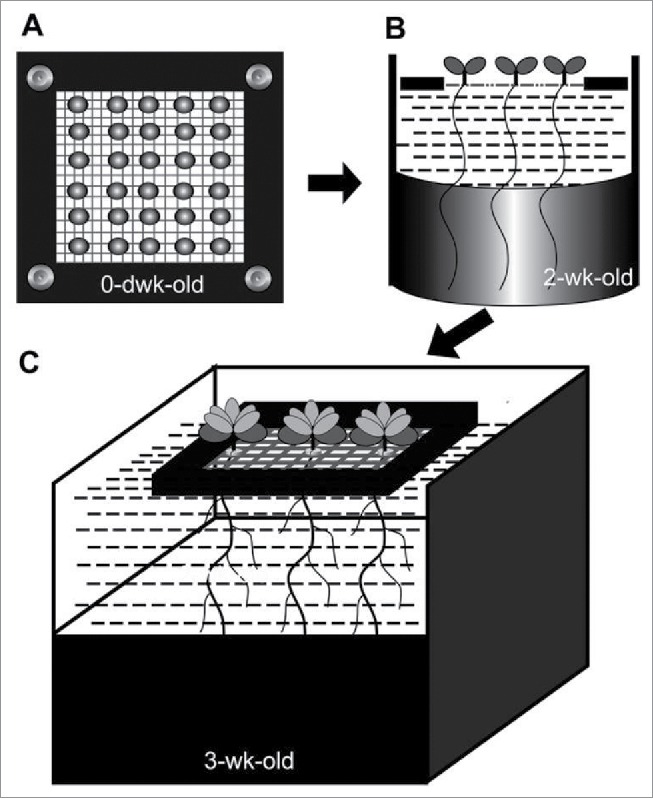
The model of hydroponic system for growing Arabidopsis thaliana plants. (A) The aerial view of the 80-mesh Nylon net fixed in a floater. The single hole of net held a seed with sufficient imbibition and 48h-vernalization. The lower half part was soaked in the nutrient medium. (B) The cartoon of the seedlings with roots at 2 weeks after germination. (C) Two-weak-old seedlings after germination were transferred into a larger floater with 5 mm poles for another one week growth. The 5 mm poles of seedlings holder were derived from PCR 96-well-plate.
Table 1.
The chemical composition of hydroponic nutrient medium.
| Chemical component | ANS medium | 0.5xHoagland | 0.5xModified Hoagland | |
|---|---|---|---|---|
| Macroelements | Ca(N03)2*4H20 | 1.010 mM | 2.015 mM | 2.015 mM |
| NaH2P04 | 0.130 mM | 0.250 mM | 0.250 mM | |
| KN03 | 1.510 mM | 3.020 mM | 3.020 mM | |
| MgSO4·7H2O | 0.498 mM | 0.995 mM | 0.995 mM | |
| NH4NO3 | 0.159 mM | 0.261 mM | 0.261 mM | |
| Microelements | H3BO3 | 9.680 µM | 9.680 µM | 9.680 µM |
| MnCI2-4H20 | 2.030 µM | 2.030 µM | 2.030 µM | |
| ZnS04*7H20 | 0.314 µM | 0.314 µM | 0.314 µM | |
| CuS04*5H20 | 0.210 µM | 0.210 µM | 0.210 µM | |
| Na2Mo04 | 0.139 µM | 0.139 µM | 0.139 µM | |
| Co(N03)2−6H20 | 0.086 µM | 0.086 µM | 0.086 µM | |
| Iron | Na2-EDTA | 22.40 µM | 89.60 µM | 22.40µM |
| FeS04·7H20 | 22.40 µM | 89.60 µM | 22.40 µM |
35S::VPT1-GFP overexpressing transgenic Arabidopsis thaliana (Col-0) lines showed retarded growth under the experimental system used by Liu et al.4 To test whether these transgenic lines have the similar phenotype under our experimental system, we overexpressed 35S::VPT1-GFP in Arabidopsis thaliana (Col-0) and selected transgenic lines using the GFP signals as the selecting marker (Fig. 2A). The VPT1 mRNA in the transgenic lines were further tested by qRT-PCR analysis, and 4 lines with high expression levels, namely OEX-2, OEX-6, OEX-7, and OEX-11, were used for analyzing growth phenotype of plants (Fig. 2B). OEX-7 and OEX-11, whose VPT1 mRNA were dozens of folds of the wild types (Col-0), displayed the stunted growth with curly leaves (Fig. 2C), and had much higher Pi contents in the rosette leaves and roots than that in OEX-2, OEX-6, or the wild type plants under the hydroponic system containing ANS medium (Fig. 2D), consistent with the observations obtained under the experimental system containing half-strength Hoagland medium.4 The mechanisms of this stunted growth of over-transgenic lines inferred by Liu et al. were that the overexpression of PHT5:1 (VPT1) proteins sequestered Pi in the vacuole and thus stopped it from being used in the cytoplasm.4
Figure 2.
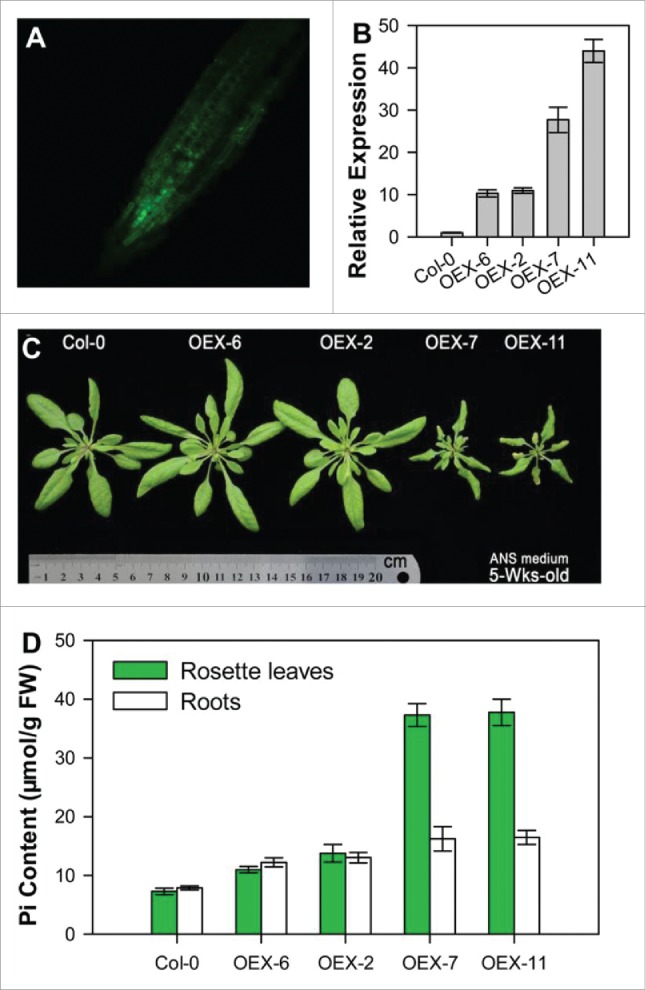
Identification of VPT1-GFP overexpressing transgenic Arabidopsis plants. (A) Selection of VPT1 overexpressing plants with GFP signals detected by confocal microscopy. The fusion gene 35S::VPT-GFP was transformed into the wild type for the construction of VPT1 overexpressing Arabidopsis (OEX). (B) Quantification of VPT1 mRNA by Quantitative real-time PCR (qRT-PCR). (C) The rosette phenotype of 5-week-old seedlings cultured in ANS medium. (D) Pi contents in the leaves of 5-week-old seedlings. Data are mean ±SD of 4 replicate experiments.
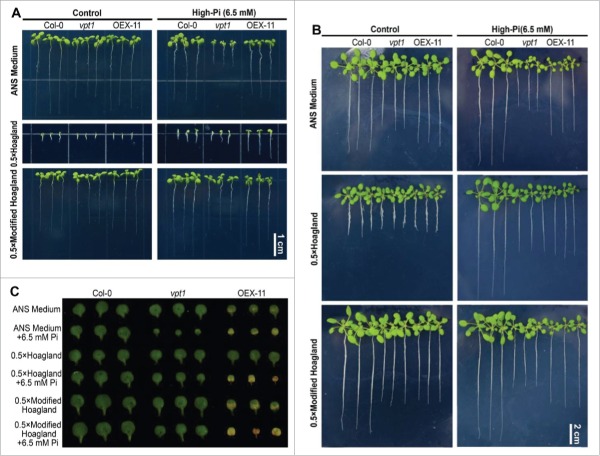
To find out the causes in the different phenotypes between ours3 and the result reported by Liu et al.,4 we planted the wild type Arabidopsis thaliana (Col-0), vpt1 mutant, and transgenic line OEX-11 in the hydroponic system shown in Fig. 1 containing normal ANS or half-strength Hoagland medium (Table 1). All the seedlings grew much weaker in half-strength Hoagland medium compared with those in ANS medium on 2 weeks after germination, indicating half-strength Hoagland medium is not suitably optimal for Arabidopsis to grow. Three-week-old vpt1 mutants and OEX-11 lines displayed the similar phenotype, including the root length, to the wild type in the half-strength Hoagland medium (Fig. 3B), consistent with the results reported by Liu et al.4 However, vpt1 mutant and OEX-11 line showed shorter root compared with the wild type in ANS medium (Fig. 3B), implying the phenotype of vpt1 mutant and OEX-11 line matter the different components in these 2 mediums.
Figure 3.
Phenotypes of wild type, vpt1 mutant, and VPT1-GFP overexpressing plants (OEX11) grown in hydroponic mediums containing different Pi contents. The phenotypes of 2-weak-old (A) and 3-week-old (B) of wild type, vpt1 mutant and OEX-11 plants. (C) The cotyledon phenotypes of the 3-week-old of plants.
We compared the components in ANS and half-strength Hoagland medium, and noted that Fe2+ concentration in half-strength Hoagland medium is 4 times of that in ANS (Table 1). Considering that high Fe2+ is highly toxic to the roots of Arabidopsis thaliana (Col-0) planted in the environments containing low Pi concentration, but this toxicity is relieved when Pi concentration is increased,7 we thus increased Pi concentration to 6.5 mM in half-strength Hoagland medium. Interestingly, both 3-week-old vpt1 mutant and OEX-11 lines showed the shorter root compared with the wild type in the half-strength Hoagland medium containing 6.5 mM Pi, same as those gown in ANS medium containing 6.5 mM Pi (Fig. 3), suggesting that high Fe2+ toxicity causes the non-distinguished phenotype between vpt1 mutant and OEX-11 and the wild type grown in the normal half-strength Hoagland medium.
To further test this hypothesis, we modified Fe2+ concentration in the half-strength Hoagland medium to the concentration of ASN as listed in Table 1, and planted the wild type, vpt1 mutant, and OEX-11 in this modified Hoagland medium. The root length of 3-wk-old vpt1 mutant and OEX-11 was significantly shorter than that of the wild type grown in the normal half-strength modified Hoagland medium, similar to those grown in the normal ANS medium (Fig. 3A, 3B). Furthermore, the rosette leaves of 3-wk-old vpt1 mutant and OEX-11 were smaller than the wild type grown in half-strength modified Hoagland medium or ANS medium, and the leaves of OEX-11 became withered and yellow (Fig. 3B, 3C). These results support the idea that VPT1 is essential for Arabidopsis to adapt phosphate stress.
Despite the importance of vacuolar Pi accumulation in plant Pi nutrition, very little is known about the transporters responsible for Pi translocation into the vacuole to achieve Pi homeostasis in the cell. Our study, as well as Liu et al.4 and Wang et al.5 identified the members of SPS-MFS family as tonoplast Pi transporters functioning as an essential component in Pi accumulation and in plant adaptation to changing Pi status in the environment. However, the Pi mobility and Pi transportation are influenced by other elements, including iron. Here we pointed out an improved hydroponic system containing ANS medium is suitable for plant phosphorus nutrient studies, at least for studies of the vacuolar phosphate transporter VPT1.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Jiangsu Collaborative Innovation Center for Modern Crop Production for technical support.
Funding
This work was supported by funding provided by National Science Foundation Grants MCB-0723931 and ISO-1339239 (to S.L.), National Natural Science Foundation of China Grants 31270303 (to F.Z.) and 31271626 (to W.L.), and Doctoral Fund of Ministry of Education of China (to W.L.).
References
- 1.Raghothama K. Phosphate transport and signaling. Curr Opin Plant Biol 2000; 3:182-187; PMID:10837272; http://dx.doi.org/ 10.1016/S1369-5266(00)00062-5 [DOI] [PubMed] [Google Scholar]
- 2.Pratt J, Boisson AM, Gout E, Bligny R, Douce R, Aubert S. Phosphate (Pi) starvation effect on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: an in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol 2009; 151:1646-57; PMID:19755536; http://dx.doi.org/ 10.1104/pp.109.144626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Yang L, Luan M, Wang Y, Zhang C, Zhang B, Shi J, Zhao FG, Lan W, Luan S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc Natl Acad Sci USA 2015; 112:E6571-78; PMID:26554016; http://dx.doi.org/ 10.1073/pnas.1514598112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu TY, Huang TK, Yang SY, Hong YT, Huang SM, Wang FN, Chiang SF, Tsai SY, Lu WC, Chiou TJ. Identification of plant vacuolar transporters mediating phosphate storage. Nat Commun 2016; 7:11095; PMID:27029856; http://dx.doi.org/ 10.1038/ncomms11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Yue W, Ying Y, Wang S, Secco D, Liu Y, Whelan J, Tyerman SD, Shou H. OsSPX-MFS3, a vacuolar phosphate efflux transporter, is involved in maintaining Pi homeostasis in rice. Plant Physiol 2015; 169:2822-31; PMID:26424157; http://dx.doi.org/ 10.1104/pp.15.01005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secco D, Wang C, Arpat BA, Wang Z, Poirier Y, Tyerman SD, Wu P, Shou H, Whelan J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol 2012; 193:842-51; PMID:22403821; http://dx.doi.org/ 10.1111/j.1469-8137.2011.04002.x [DOI] [PubMed] [Google Scholar]
- 7.Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 2008; 147:1181-91; PMID:18467463; http://dx.doi.org/ 10.1104/pp.108.118562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tocquin P, Corbesier L, Havelange A, Pieltain A, Kurtem E, Bernier G, Périlleux C. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol 2003; 3:2; PMID:12556248; http://dx.doi.org/ 10.1186/1471-2229-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]