Abstract
BACKGROUND:
Recently, total laparoscopic pancreatectomy has been performed at many centres as an alternative to open surgery. In this study, we aimed to present the difficulties that we have encountered in converting from classic open pancreaticoduodenectomy to total laparoscopic pancreatectomy.
MATERIALS AND METHODS:
Between December 2012 and January 2014, we had 100 open pancreaticoduodenectomies. Subsequently, we tried to perform total laparoscopic pancreaticoduodenectomy (TLPD) in 22 patients. In 17 of these 22 patients, we carried out the total laparoscopic procedure. We analysed the difficulties that we encountered converting to TLPD in three parts: Preoperative, operative and postoperative. Preoperative difficulties involved patient selection, preparation of operative instruments, and planning the operation. Operative difficulties involved the position of the trocars, dissection, and reconstruction problems. The postoperative difficulty involved follow-up of the patient.
RESULTS:
According to our experiences, the most important problem is the proper selection of patients. Contrary to our previous thoughts, older patients who were in better condition were comparatively more appropriate candidates than younger patients. This is because the younger patients have generally soft pancreatic texture, which complicates the reconstruction. The main operative problems are trocar positions and maintaining the appropriate position of the camera, which requires continuous changes in its angles during the operation. However, postoperative follow-up is not very different from the classic procedure.
CONCLUSION:
TLPD is a suitable procedure under appropriate conditions.
Keywords: Pancreatic cancer, total laparoscopic pancreaticoduodenectomy, Whipple procedure
INTRODUCTION
In recent years, total laparoscopic pancreaticoduodenectomy (TLPD) has been implemented at the advanced centres on a regular basis.[1,2,3,4] Pancreatic resection is technically challenging due to anatomic factors such as the organ's retroperitoneal location and close proximity to the duodenum and major vasculature.[5,6] As a result, the application of laparoscopy to pancreatectomy has been slower than with other abdominal procedures.[1] The complication rates and oncologic outcomes of this method are similar to those of the open method.[7,8,9,10] I, n addition TLPD has some advantages, such as comparatively less time to return to daily activities, less scar formation and early initiation of adjuvant treatment.[10] This method requires experience not only in pancreatic surgery but also in the field of laparoscopic surgery. Therefore, the number of centres where this technique is regularly performed is limited. In particular, using robotic surgery at the time of reconstruction increases the possibility that the procedure can be completed entirely by the laparoscopic method. We aimed to present the difficulties we experienced in the transition to laparoscopic surgery following 100 classic open Whipple operations.
MATERIALS AND METHODS
Between December 2011 and April 2014, 100 classic open pancreaticoduodenectomies were performed by the same surgeon. The same surgeon began to perform TLPD procedure. During study period, a total of 17 patients underwent TLPD procedure. The difficulties encountered in the transition to laparoscopic surgery are classified as preoperative, intraoperative and postoperative problems. The specific difficulties in these periods and their specific solutions are described separately.
Preoperative period
The most important difficulty encountered during this period was the selection of a suitable patient. In determining the eligibility of patients suitable for laparoscopy, the patients who had tumour size below 2.5 cm or who had intraductal papillary mucinous neoplasm (IPMN) and benign conditions were given priority. Advanced tumours and tumours with vascular invasion were excluded from the study. Before the procedure, all patients were evaluated with triphasic computed tomography (CT) and endoscopic ultrasound for appropriate staging. Of 5 patients who underwent surgery, 2 had pancreatic head tumour, 2 had cystic neoplasms of the pancreas and 1 had a diagnosis of chronic pancreatitis.
Intraoperative period
Surgical procedures were performed by the same surgical team. Five trocars of 10-12 mm were used, and 5-mm Nathanson retractor was used for liver retraction, which was placed in the sub-xiphoid position. The trocar positions are shown in Figure 1. After establishing pneumoperitoneuma, 30-degree telescope was used. In the initial phase of the operation, laparoscopic ultrasound was performed for detecting liver metastases. Later, other trocars were entered. The operation began with dissection of the gastrocolic omentum. Then the Kocher manoeuvre was performed. Subsequently, the superior mesenteric vein were dissected, a tunnel was opened from the posterior of the pancreas and the pancreas was hanged. After this, hilar dissection was performed. The gastroduodenal artery in the liver hilum was found, ligated and divided. Hilar lymph nodes were dissected. The first portion of the duodenum was transected by means of endostapler and the pancreas was transected by means of ultrasonic dissector. The Treitz ligament was dissected and the specimen was taken to the patient's right. The uncinate process of the pancreas and portopancreatic junction was dissected and pancreatic tissue was separated from the portal vein and superior mesenteric artery. During this period, we preferred the second trochar position [Figure 2]. Finally, cholecystectomy was performed; the bile duct was cut and the specimen was put into an endobag. The reconstruction process began with pancreatic anastomosis. An end-to-side pancreaticojejunostomy and duct-to-mucosa anastomosis was performed. Subsequently, an end-to side hepaticojejunostomy and end-to-side duodenojejunostomy were performed. Finally, the infraumblical trocar site was extended 5 cm and the specimen was removed [Figure 3]. Operative drains were placed near the pancreaticojejunostomy and hepaticojejunostomy anostomosis.
Figure 1.
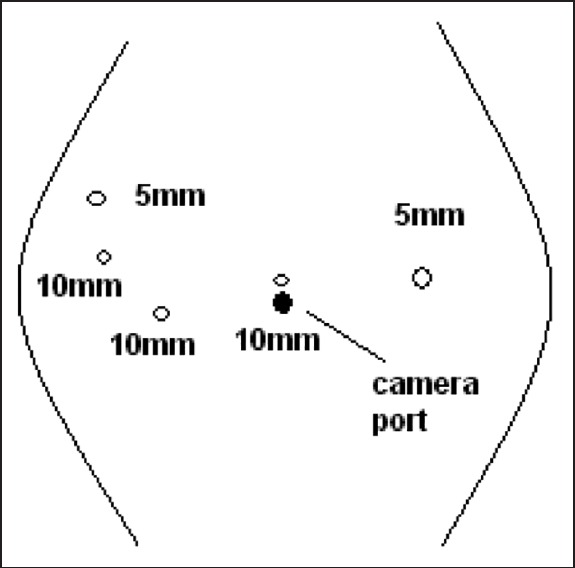
The beginning of the operation period. The surgeon stands between the patient's arms. The camera is in the umblical port. In this period we can prepare gastrocolic ligament dissection, pancreatic head mobilisation, partial Kocher manoeuvre and hilus dissection
Figure 2.
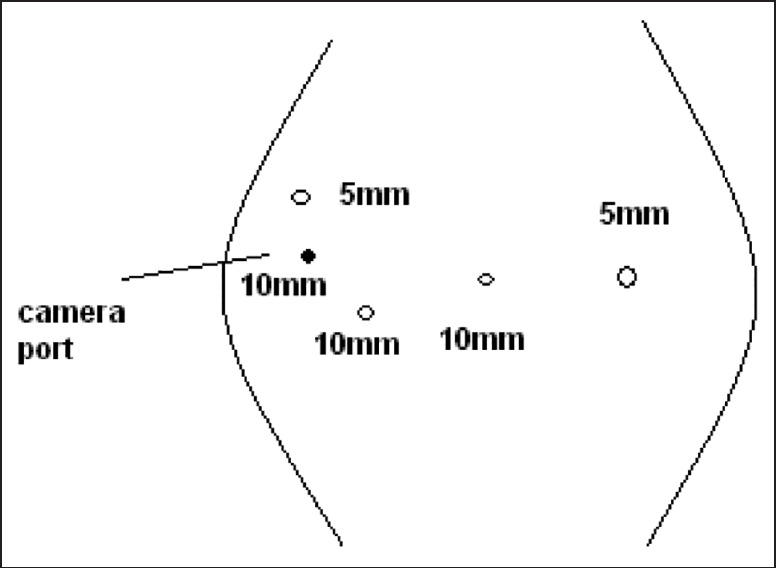
At this position, we prepare extended Kocher manoeuvre and uncinate dissection. Additionally, in the final step, we can do hepaticojejunostomy in this position. The surgeon stand on the right side of the patient
Figure 3.
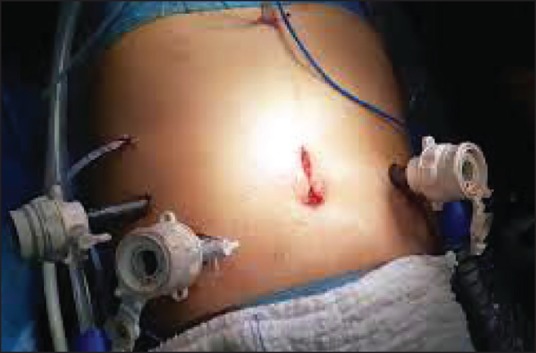
Final step of our operation
Postoperative period
Postoperative early enteral nutrition was started in a manner similar to classic open pancreaticoduodenectomy. The patients’ drain amylase levels were measured on days 4. Patients’ drain with normal amylase levels were removed and full oral diet was begun. Analgesia was provided with a patient-controlled pump for the early postoperative period of 3 days and with oral narcotic analgesics in the following period. The patients were discharged on postoperative day 7 if they had an uneventful postoperative period.
RESULTS
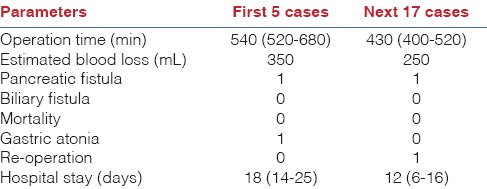
In total there were 22 cases. Seventeen cases were completed laparoscopically and 5 cases were converted to open surgical procedure. Here, we have given some infomation about cases converted to open surgical procedures. Indications of the other 17 cases were head of pancreas cancer, periampullary tumour, IPMN and cancer doubt of chronic pancreatitis. During surgical procedure of the first five patients, even though the operations were started by laparoscopic method, 3 of them at various stages due to bleeding and 2 of them during the reconstruction of the pancreas, which was of bad quality, underwent conversion to open surgery. On the other hand, the next 17 patients had total laparoscopic pancreatectomy. The data collected from the first 5 patients’ operations, which were converted to open surgery, were analysed. Conversion to open surgery was done due to bleeding during dissection of the first branch of the portal vein in 1 patient and due to bleeding during dissection of the uncinate process of the pancreas in 2 patients. In 2 patients, dissection was completed laparoscopically and conversion to open surgery was done due to the soft quality of the pancreas. According to these early results, bleeding seems to be the most frequent cause of conversation to open surgery. Uncinate process dissection is the most troublesome point of dissection. In addition, fragile vessels make it difficult to control bleeding. The bleeding of the first branch of the portal vein due to excessive traction was yet another reason for uncontrollable bleeding. At this stage the most important preventive method is isolating and separating vessels properly and placing the clips if necessary. The average length of hospital stay of the patients who had TLPD was 10 days (6-21). Estimated blood loss was 340 mL (250-440). Mean operative time was 430 min (400-510). One patient was re-operated on postoperative day 6 due to duodenojejunostomy leakage. There were no other complications. We did not discover any surgical side infection or seroma. Therewithal, none of the patients had an anastomotic leakage from pancreaticojejunostomy. We did not encounter any mortality postoperatively [Table 1]. We do not prefer to perform jejunostomy on the first day of surgery; enteral nutrition is started by nasojejunal tube and increased daily. On day 3 or day 4, the nasojejunal tube is removed and full oral diet is started.
Table 1.
TLPD early results
DISCUSSION
In the last 25 years, the most significant advance in pancreatic head tumour surgery has been the decrease of the mortality rate to below 1%.[11] In parallel to developments in technical equipment, the use of minimally invasive surgery in pancreatic cancer is another significant advance over this same period.[12,13] TLPD, which requires high technical skill levels, is one of the most difficult kinds of laparoscopic surgery. Nowadays, although there are many centres dealing with this issue, a large case series has not been made.[14,15] In the initial period of TLPD, besides being a highly skilled pancreatic surgeon, it is necessary to have good skills in laparoscopic surgery. In recent years, studies comparing open and laparoscopic pancreatectomy have begun to be published which have not revealed any superiority of TLPD to the classic open technique.[10,11,12,13,14,15] However, having the same survival advantage compared with open surgery through a smaller incision is likely to be an advantage of this technique. It is a fact that by performing TLPD in early-stage and cystic pancreatic tumours, better cosmetic results can be expected in this group of patients. Moreover, it is known that TLPD can provide an advantage in terms of earlier onset of patients to chemotherapy.
Initially the surgeon has to reach a certain level of experience in pancreatic surgery in order to start performing TLPD[15] because the problems of this technique in the start-up period are similar to the early-stage difficulties of open surgery (more estimated blood loss, more operation time, more fistula, and the mortality rate). Thus, patient selection is very important in the initial phase. For initial cases, those with early stage tumours or tumour-like lesions such as IPMN should be selected. In these patients, although dissection can be performed in an easier way, the pancreaticojejunostomy may become problematic. This is because the quality of the pancreas is softer in early-stage tumours. According to our experience in the older patients who have early-stage tumours and no serious comorbidities, the pancreatic quality is better and this makes comfortable dissection possible.
In TLPD, dissection is different from the classic open technique. Optionally in the conventional method, resection is clockwise:First stomach, then the common bile duct and pancreatic resection is performed whether the retro-pancreatic dissection is done in the last part of the dissection phase. In TLPD the pancreas is dissected after the division of the stomach. Optionally, the posterior pancreas around the superior mesenteric artery is dissected and finally common bile duct and gallbladder dissection is done. One of the biggest advantages of TLPD including artery- first approach is that it allows the surgeon to evaluate the relationship between the tumour and surrounding arterial structures. Thus, determination of resectability of the tumor can be made at early stage of operation. However, in our series, there was no patient with major vessel invasion including portal vein or mesenteric vascular structures due to meticulous selection of initial cases. If there is any doubt about invasion of artery during open procedure, retropancreatic approach is reasonable to determine resectability as it is described in textbooks. Another advantage is that gallbladder can be used for traction of the liver upward.
In general, one of the most difficult parts of TLPD is positioning the trocars. The use of sub-xiphoid automatic retractor facilitates the procedure. In addition, 10-mm trocars are important because their use allows the camera to move, helping the surgeon by working in different angles [Figure 2]. In the reconstruction period, the most difficult anastomosis initially is duodenojejunostomy due to the trocar positions. Thus, after doing the back wall, the anterior wall can be complete from the incision which is done for removing the specimen. This decreases the duration of the surgery.
The most important disadvantages of TLPD are longer operation time and higher cost compared to open procedures. We believe that operation time can reduced by increasing the number of cases.
CONCLUSION
In conclusion, it can be suggested that TLPD is a safer procedure, while having comparable technique and oncologic outcomes to open surgery.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Pisters PW, Lee JE, Vauthey JN, Charnsangavej C, Evans DB. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325–37. doi: 10.1046/j.1365-2168.2001.01695.x. [DOI] [PubMed] [Google Scholar]
- 2.Camacho D, Reichenbach D, Duerr GD, Venema TL, Sweeney JF, Fisher WE. Value of laparoscopy in the staging of pancreatic cancer. JOP. 2005;6:552–61. [PubMed] [Google Scholar]
- 3.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–10. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 4.Gagner M, Palermo M. Laparoscopic Whipple procedure: Review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726–30. doi: 10.1007/s00534-009-0142-2. [DOI] [PubMed] [Google Scholar]
- 5.Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg. 2009;13:1129–37. doi: 10.1007/s11605-008-0797-z. [DOI] [PubMed] [Google Scholar]
- 6.Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: A review of 285 published cases. Ann Surg Oncol. 2011;18:1335–41. doi: 10.1245/s10434-010-1503-4. [DOI] [PubMed] [Google Scholar]
- 7.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051–4. doi: 10.1016/s0039-6060(96)80054-7. [DOI] [PubMed] [Google Scholar]
- 8.Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: A UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084–99. doi: 10.1007/s00464-010-1538-4. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C, O’Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, et al. Laparoscopic distal pancreatectomy: The Brisbane experience of forty-six cases. HPB (Oxford) 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CK, Kooby DA. Laparoscopic surgery for pancreatic tumors. Surg Oncol Clin N Am. 2010;19:311–33. doi: 10.1016/j.soc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642–51. doi: 10.1007/s00464-010-1456-5. [DOI] [PubMed] [Google Scholar]
- 12.Menon KV, Hayden JD, Prasad KR, Verbeke CS. Total laparoscopic pancreaticoduodenectomy and construction for a cholangiocarcinoma of the bile duct. J Laparoendosc Adv Surg Tech A. 2007;17:775–80. doi: 10.1089/lap.2006.0236. [DOI] [PubMed] [Google Scholar]
- 13.Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131–7. doi: 10.1002/rcs.380. [DOI] [PubMed] [Google Scholar]
- 14.Gumbs AA, Gayet B. The laparoscopic duodenopancreatectomy: The posterior approach. Surg Endosc. 2008;22:539–40. doi: 10.1007/s00464-007-9635-8. [DOI] [PubMed] [Google Scholar]
- 15.Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J. 2012;18:571–6. doi: 10.1097/PPO.0b013e31827b8f86. [DOI] [PubMed] [Google Scholar]


