Abstract
Biologically monitoring marijuana exposure from active and passive use requires both a wide linear range and sensitive detection. We have developed and validated a multifunctional method using ultrahigh performance liquid chromatography coupled with tandem mass spectrometry (UHPLC–MS/MS) for analysis of urinary Δ9-tetrahydrocannabinol (THC), cannabidiol and cannabinol, and two major metabolites of THC, 11-nor-9-carboxy-THC and 11-hydroxy-THC, in active users and particularly in people exposed to secondhand marijuana smoke (SHMS). The method used positive electrospray ionization (ESI) mode to reach the sensitivity needed to detect trace SHMS exposure with limits of detection (LOD) ranging from 0.002 to 0.008 nanograms per milliliter (ng/mL) and 0.005 to 0.017 ng/mL for “free” (unconjugated forms) and “total” (unconjugated plus conjugated forms) measurements, respectively. These LODs were approximately 10–100 times more sensitive than those reported in the literature. To reduce or avoid time-consuming repetitive sample preparation and analysis, the method simultaneously monitored multiple reaction monitoring transitions in negative ESI mode to quantify high analyte levels typically found in the urine of active marijuana users (linear dynamic range of 12.5–800 ng/mL). The validation results indicated this method was accurate (average inter/intra-day bias, <10%), precise (inter/intra-day imprecision, <10%), and fast (6 min run time). In addition, sample preparation throughput was greatly improved using an automation liquid-handling system, meeting the needs for potential large-scale population studies.
Graphical Abstract
Increasing use of marijuana both medicinally and recreationally1,2 may lead to increased health risks resulting from exposure to both cannabinoids and the toxic chemicals found in marijuana smoke.3–5 Traditionally, cannabinoids and their metabolites, i.e., Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN), and two major metabolites of THC, 11-nor-9-carboxy-THC (COOH-THC) and 11-hydroxy-THC (OH-THC) (Figure S-1), are measured in the urine of people to assess their exposure to marijuana products and smoke.
In the last decades, a number of analytical methods have been applied to analyze THC, OH-THC, COOH-THC, CBD, and CBN in urine samples, including methods employing liquid or gas chromatography (LC or GC) coupled with either a single quadrupole mass spectrometry (MS) or tandem mass spectrometry (MS/MS).6–10 Reported limits of detection (LOD) for these analytes ranged from 0.2 to 5.0 nanograms per milliliter (ng/mL). Given the relatively high exposure levels resulted from active marijuana smoking, these LODs meet the needs accordingly for desired detection rates. Nevertheless, persons smoking marijuana could also cause passive exposure of other people through inhalation of secondhand marijuana smoke (SHMS), which are often characterized by low biomarker levels although collectively depending on factors including environmental circumstances, smoker density, and exposure duration. While most studies have focused on active use of marijuana, information on SHMS, including exposure characteristics and adverse health consequences, is still limited in the open literature. A sensitive analytical method to quantify trace biomarker levels is therefore indispensable for effectively monitoring and assessing SHMS exposure.
The objective of this study was to develop and analytically validate a sensitive ultrahigh pressure LC (UHPLC)–electrospray ionization (ESI) combined with MS/MS method for quantifying THC, COOH–THC, OH–THC, CBD, and CBN in urine from people exposed to marijuana smoke, particularly to SHMS. To the best of our knowledge, the analyte-specific LODs achieved in this method for urine samples were approximately 10–100 times more sensitive than previous studies. In addition, time-consuming repetitive preparation and analysis of unknown urine samples often become necessary when analyte levels fall out of a method’s linear dynamic ranges. We simultaneously monitored multiple reaction monitoring (MRM) transitions of those five analytes under both positive and detuned negative ESI modes for low-concentration (LODs, 50 ng/mL) and high-concentration (up to 800 ng/mL) samples, respectively. This combined analysis greatly increased the applicable quantitation ranges and facilitated the data acquisition by reducing or avoiding potential repetitive sample preparation and analysis. Finally, we increased sample preparation throughput using an automation liquid-handling system to meet the needs for potential large-scale population studies.
EXPERIMENTAL SECTION
Details on chemicals and materials are provided in the Supporting Information.
Standard Solution Preparation
Blank urine pools, used as a matrix for calibration standard (calibrators) and quality control (QC), were prepared using human urine anonymously collected in compliance with Institutional Review Board protocol. The CDC Human Subjects Review Board determined this activity was not a human subject research. Blank urine samples were combined to form a matrix urine pool, which was kept at 4 °C and stirred overnight to ensure thorough mixing. This urine pool was subsequently spiked with target analytes to form calibrators and QCs.
We prepared working solutions for calibrators and QCs from serial dilutions of primary stock solutions with methanol and water (v/v 60:40) and stored them in Teflon-capped amber glass vials at −24 °C. A volume of 50 µL of each working solution was automatically added to 500 µL of blank urine (see Sample Preparation), creating calibrators at 0.001 to 800 ng/mL, and QC samples at 0.05, 25, and 500 ng/mL. The internal standard spiking solution had concentrations of 0.06 ng/µL for CBD-d3, CBN-d3 and 0.1 ng/µL for OH-THC-d3, COOHTHC-d3, and THC-d3. Details regarding the standard solution preparation are given in the Supporting Information.
Sample Preparation
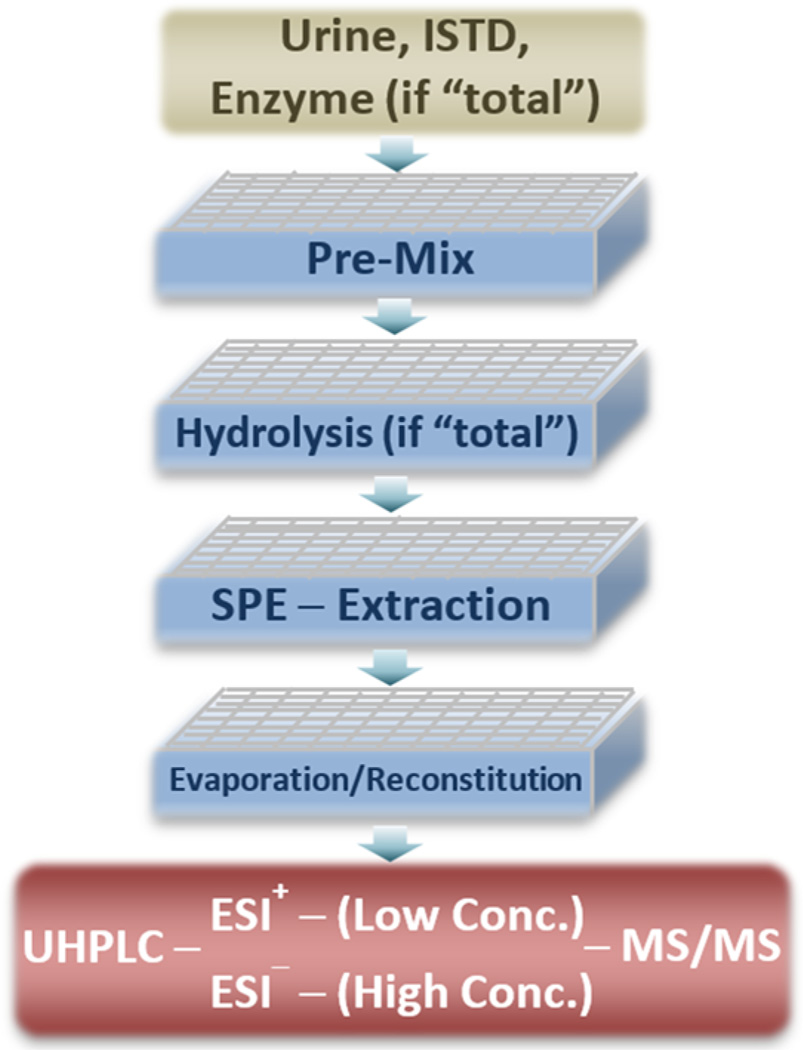
We prepared calibrators, QCs, blanks, and unknown urine samples following same procedures, as depicted in Figure 1. Briefly, urine samples stored at temperatures ≤−65 °C were thawed and vortex-mixed for 10 min at room temperature. To measure “total” concentrations (unconjugated and conjugated forms), 500 µL of each urine sample (blank and unknown) was transferred to each well in the 96-well plate, followed by adding 50 µL of calibrator and QC working solutions to each blank urine sample. Working solutions were replaced with water for unknown samples. Then, 50 µL of internal standard solution and 50 µL of enzyme solution (Escherichia coli, type IX-A, 20 units/µL, 0.5 M ammonium acetate, pH 6.8) were added into all samples. After gentle mixing (“Pre-Mix”), the 96-well plate was incubated at 37 °C for 2 h. Then, 50 µL of 10 N NaOH was added to each well and incubated at 70 °C for 20 min. After cooling the plate to room temperature, 400 µL of formic acid, water, and methanol (v/v/v 12.5:12.5:75) was added to each well. To measure “free” unconjugated concentrations, enzyme and NaOH solutions were replaced with 450 µL of formic acid, water, and methanol (v/v/v 5.5:27.8:66.7) and no incubation was performed. After mixing for 5 min, the plate was centrifuged for 30 min at −5 °C, and then 0.96 mL mixtures from each well was transferred onto the 96-well SPE plate, pre-equilibrated with 1.0 mL of methanol and 1.0 mL of buffer (5 mM ammonium formate, 0.05% formic acid). After soaking for 10 min, the mixtures were pushed through the SPE under approximately 1.0 psi positive pressure. Then, the samples were washed with 1.0 mL of water and 1.0 mL of methanol and water (v/v 60:40). After drying for 15 min with nitrogen (25 psi), the samples were collected in a second 96-well plate by elution with 1.0 mL of methanol and then evaporated to dryness using a TurboVap evaporator (Biotage, Charlotte, NC) at room temperature. The residuals were reconstituted with 50 µL of method and water (v/v 50:50). After 5 min of vortex, 10 µL of each sample was injected into the LC system.
Figure 1.
Sampling preparation scheme. Abbreviations: ISTD, internal standard spiking solution; Conc., concentration. “Pre-Mix” included adding ISTD and enzymatic solution (if measuring “total” concentration) to urine samples followed by gentle mixing. “Hydrolysis” included enzymatic and alkaline procedures in order and was omitted to measure “free” (“unconjugated”) concentrations. “SPE Extraction” included SPE pre-equilibrium sample cleanup and elution.
Instrumentation and Operation
The “Pre-Mix” procedure (Figure 1), also the critical one impacting the accuracy and precision of analytical results, was performed on a Hamilton automated liquid-handling system, Microlab Star (Reno, NV), aimed at improving data reproducibility and sample preparation throughput. On an as-needed basis in future, the throughput can be further expanded using a fully automated system illustrated in our previous study.11 Additional information can be obtained upon request.
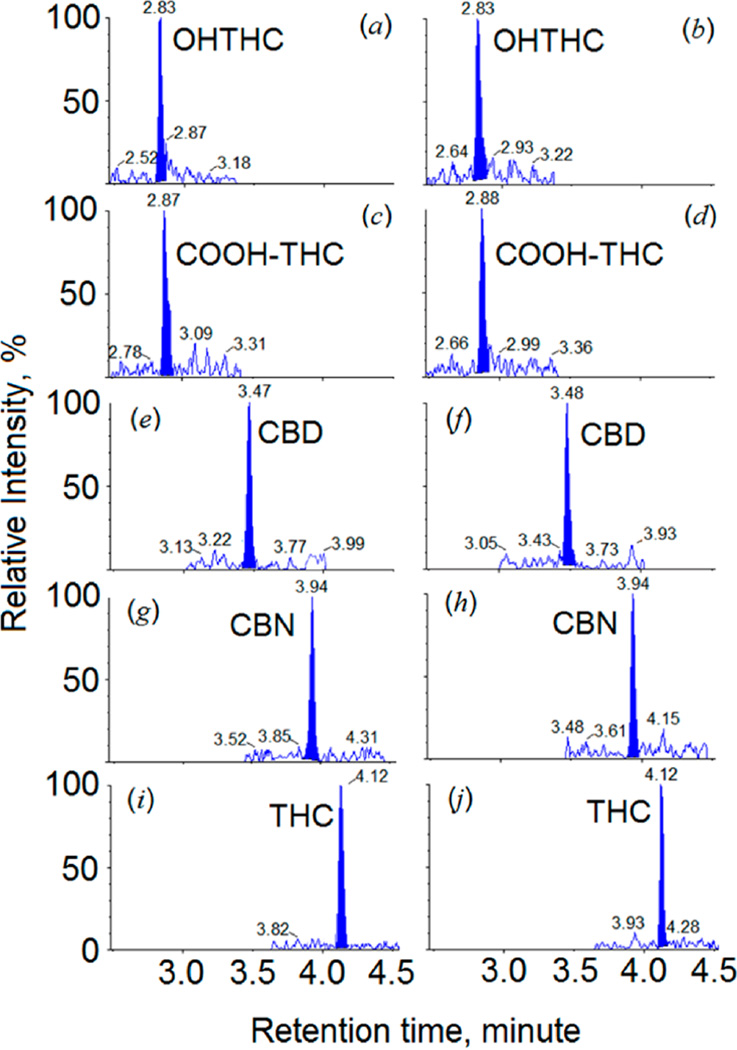
Chromatographic separation was achieved using a Kinetex reversed phase column (100 mm × 2.1 mm, particle size 2.6 µm, C18) (Phenomenex, Torrance, CA) on a Shimadzu UHPLC system (Columbia, MD) at a flow rate of 0.4 mL/min. Column temperature was maintained at 40 °C during the entire analysis. The gradient program contained 5.0 mM of ammonium formate with 0.05% formic acid (solvent A) and acetonitrile (solvent B). After injection, LC flow during the first 2.5 min and the last 1.5 min was directed to a waste container by the switching valve. Only the flow occurring between 2.5 and 4.5 min was directed to the MS. Detailed gradient conditions are provided in Table S-1. Figures 2a,c,e,g,i and 2b,d,f,h,j) depict representative chromatograms for urine samples processed with the “free” and “total” methods, respectively.
Figure 2.
Chromatograms of fortified urine samples analyzed on the Sciex triple quadrupole 6500. Chromatograms illustrated in left column (0.010 ng/mL) and right column (0.025 ng/mL) were for “free” (“unconjugated”) and “total” (“conjugated” + “unconjugated”) concentrations, respectively.
MS/MS analysis was performed on a Sciex triple quadrupole 6500 with a TurboIonSpray source (Foster City, CA). ESI+ and ESI− modes were used to acquire scheduled MRM transition data. Optimum MS source parameters were as follows: source temperature, 600 °C; ionspray voltage (ESI+/ESI−), 5500/–4500 V; ion source gas-1, 80 psi, and gas-2, 90 psi; curtain gas, 35 psi; target scan time (ESI+/ESI−), 0.18/0.041 s. Two MRM precursor/product transitions for each native analyte and one transition for the isotope labeled internal standard were monitored. The collision offset energy (CE) under ESI− mode was “de-tuned” to higher value so as to yield a lower response of the corresponding MRM transition to avoid detector saturation when analzying high concentrations typically found in active smokers’ urine. Detailed MRM transitions and voltage settings are given in Table S-2, and the instrument method for data acquisition is also described in the Supporting Information.
RESULTS AND DISCUSSION
We first evaluated different 96-well plates containing various materials, including C18, mixed-mode cation/anion-exchange polymer, strongly hydrophilic water wettable polymer, and inert diatomaceous earth. Overall, C18 SPE provided the highest sample extraction recoveries. The high lipophilicity of these analytes enabled us to apply high percentages of methanol in washing solvent (50–70%) to purify the urine samples on C18 SPE plates. We found washing solvent with methanol and water (v/v 60:40) had a balanced performance between extraction recoveries and matrix effects.
Because ESI+ analysis yielded higher sensitivity for all analytes, we monitored their MRM transitions using ESI+ for low-concentration samples (LOD–50 ng/mL). To avoid variation caused by MS detector saturation using ESI+ analysis, we simultaneously monitored those five analytes under ESI− mode to quantify any unknown samples whose concentrations were in the range of 12.5–800 ng/mL. Under both ionization modes, we observed excellent calibration linearity with average determination coefficients (R2) ≥ 0.995. We also assessed data consistency between two ionization analyses by comparing fortified samples whose concentrations fell into the overlapping linear dynamic ranges of two modes, and we observed excellent agreement in analytical results (Table 2 and Table S-4). Expectedly, this combined analysis would greatly facilitate data acquisition by increasing the applicable dynamic ranges and reducing the repeating rates of sample preparation and analysis.
Table 2.
Accuracy and Precision of Replicate Analysis of Five Compounds in Fortified Urine Samples by “Free” Method (Without Enzymatic-Alkaline Hydrolysis)a
| within-day | between-day | |||||||
|---|---|---|---|---|---|---|---|---|
| ESI mode | target, ng/mL | measured | error % | RSD % | measured | error % | RSD % | |
| OHTHC | + | 0.025 | 0.0244 | −2.3 | 9.2 | 0.0245 | −1.9 | 5.7 |
| 0.100 | 0.103 | 3.0 | 5.0 | 0.105 | 5.5 | 3.5 | ||
| 1.25 | 1.24 | −0.8 | 2.2 | 1.28 | 2.7 | 3.0 | ||
| 25.0 | 26.3 | 5.1 | 5.3 | 26.9 | 7.6 | 2.1 | ||
| − | 25.0 | 26.4 | 5.4 | 8.1 | 24.6 | −1.6 | 7.6 | |
| 100 | 107 | 7.0 | 4.9 | 107 | 7.0 | 1.0 | ||
| 500 | 480 | −4.1 | 2.2 | 475 | −5.1 | 2.9 | ||
| COOH-THC | + | 0.025 | 0.0245 | −2.2 | 6.5 | 0.0247 | −1.2 | 6.6 |
| 0.100 | 0.105 | 5.4 | 5.7 | 0.104 | 4.0 | 4.2 | ||
| 1.25 | 1.19 | −4.7 | 9.9 | 1.26 | 1.1 | 0.5 | ||
| 25.0 | 26.5 | 6.0 | 3.6 | 26.8 | 7.3 | 1.8 | ||
| − | 25.0 | 26.3 | 5.2 | 4.6 | 26.5 | 5.8 | 2.4 | |
| 100 | 105 | 4.9 | 8.3 | 107 | 7.3 | 4.6 | ||
| 500 | 473 | −5.4 | 3.3 | 457 | −8.6 | 1.8 | ||
| CBD | + | 0.025 | 0.0246 | −1.5 | 6.6 | 0.0244 | −2.6 | 6.5 |
| 0.100 | 0.099 | −0.6 | 4.4 | 0.110 | 9.6 | 8.3 | ||
| 1.25 | 1.15 | −8.4 | 1.5 | 1.26 | 1.1 | 9.8 | ||
| 25.0 | 25.9 | 3.4 | 2.9 | 25.7 | 2.6 | 2.4 | ||
| − | 25.0 | 27.2 | 8.8 | 2.0 | 24.8 | −1.0 | 4.8 | |
| 100 | 107 | 6.7 | 4.9 | 105 | 4.8 | 8.2 | ||
| 500 | 453 | −9.5 | 1.4 | 453 | −9.4 | 3.3 | ||
| CBN | + | 0.025 | 0.0264 | 5.6 | 9.0 | 0.0253 | 1.3 | 5.7 |
| 0.100 | 0.109 | 8.7 | 5.6 | 0.108 | 7.6 | 4.7 | ||
| 1.25 | 1.23 | −1.3 | 3.9 | 1.23 | −1.9 | 4.0 | ||
| 25.0 | 25.8 | 3.1 | 6.1 | 26.8 | 7.0 | 3.8 | ||
| − | 25.0 | 25.4 | 1.6 | 9.7 | 25.7 | 2.9 | 3.4 | |
| 100 | 98.3 | −1.7 | 7.5 | 104 | 3.7 | 3.2 | ||
| 500 | 469 | −6.2 | 3.0 | 464 | −7.3 | 3.1 | ||
| THC | + | 0.025 | 0.0249 | −0.5 | 9.9 | 0.0274 | 9.7 | 3.1 |
| 0.100 | 0.108 | 8.0 | 5.1 | 0.106 | 5.5 | 7.4 | ||
| 1.25 | 1.18 | −5.9 | 4.4 | 1.23 | −1.3 | 5.1 | ||
| 25.0 | 26.7 | 6.7 | 7.7 | 27.3 | 9.1 | 2.7 | ||
| − | 25.0 | 25.3 | 1.3 | 7.8 | 25.8 | 3.2 | 5.2 | |
| 100 | 107 | 7.2 | 4.7 | 106 | 6.0 | 3.6 | ||
| 500 | 476 | −4.8 | 2.6 | 473 | −5.4 | 4.7 | ||
Abbreviations: ESI = electrospray ionization; RSD = relative standard deviation.
One of the challenges in measuring urinary cannabinoids and their metabolites is that the high lipophilicity of these compounds can result in substantial adsorption to materials used for sample preparation, i.e., tips and 96-well plates. To minimize chemical loss during sample preparation, we added 0.3 mL of methanol into all samples before SPE cleanup. After injection, the analytes could also absorb to other instrumental surfaces, i.e., tubing, column, LC injection loop, needle, and port, etc. Over time, amounts accumulated on LC parts can potentially cause carry-over contamination. To avoid this issue, we added the following LC autosampler washing program: before each injection, the autosampler measuring line was washed with 600 µL of acetonitrile and water (v/v 55/45), and both internal and external surfaces of the sampling needle were rinsed with 300 µL of acetonitrile, 2-propanol, and water (v/v/v 45/45/10). We evaluated the effectiveness of this washing program by measuring the carry-over concentrations in the subsequent blank urine samples following the processed highest calibrator (800 ng/mL). The results indicated that no targeted peaks at the retention times of all analytes were detected.
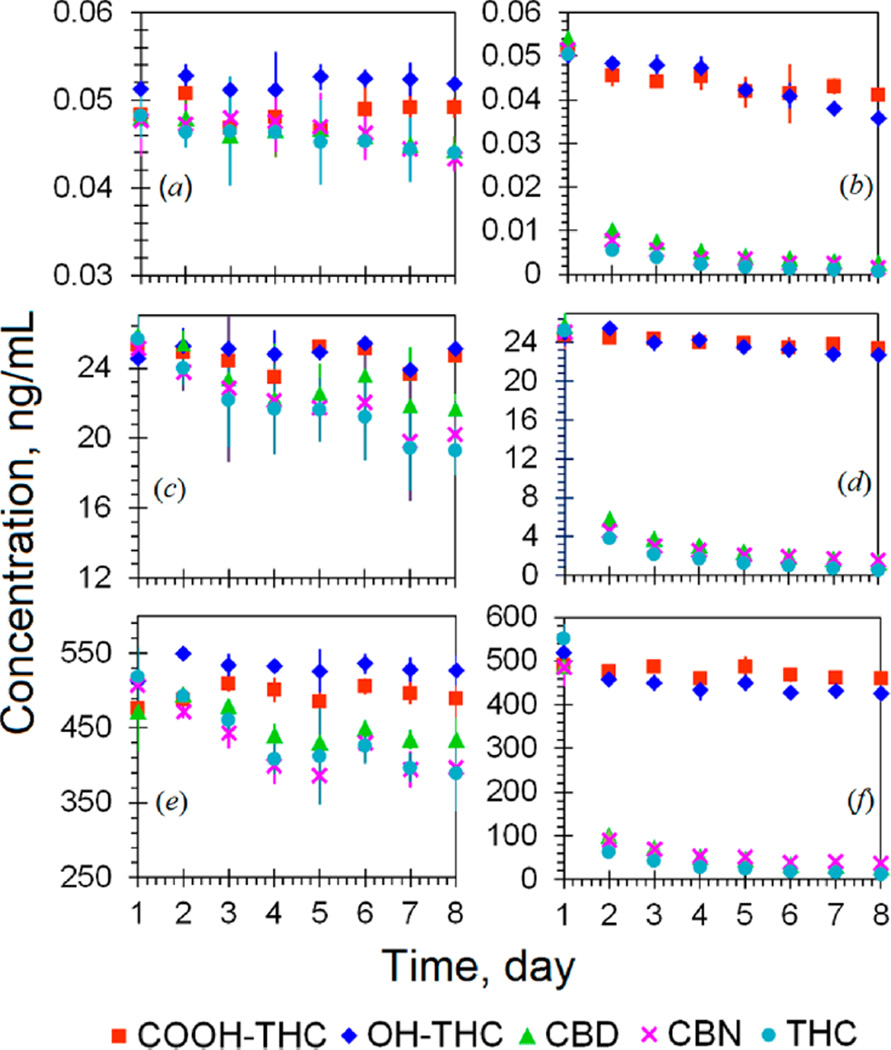
Thermal instability of these analytes in urine was another issue that requires extra caution in the entire processes of sample collection, transport, storage, and analysis. Our stability tests with fortified urine at room temperature (~25 °C) indicated >80% of total CBD, CBN, and THC were lost after 24 h (Figure 3b,d,f). The losses of total OHTHC and COOH-THC at 25 °C were approximately 5 and 12% after 24 h, respectively. At 4 °C, approximately 10–30% of total CBD, THC, and CBN decomposed after 3 days, and no obvious losses of total OHTHC and COOH-THC were observed (Figure 3a,c,e). We did not observe a significant decrease in total analyte concentrations ≤−20 °C during the same study period. These results are consistent with those reported in a recent study by Desrosiers et al.12 Studies regarding long-term stability at temperatures ≤−20 °C and the stability to indoor light are in progress. Available evidence indicated that urine samples should always be kept frozen during storing and shipping. For analysis, samples need to be prepared in a timely manner and analyzed within 1 day. The high-throughput liquid-handling automation system and fast UHPLC–MS/MS analysis (~two 96-well plates/instrument/day) presented in this study would better meet such needs so as to maintain the quality of analytical results.
Figure 3.
Thermal stability of OHTHC, COOH-THC, CBD, CBN, and THC in fortified urine pools. Parts a, c, and e show the results for samples stored at 4 °C and parts b, d, and f display the results for samples stored at 25 °C.
One of the main advantages of this method, aside from the broad applicable quantitation ranges, high-throughput preparation, and fast analysis, is the marked sensitivity that is essential for effectively monitoring and assessing SHMS exposure. We determined the LODs and limits of quantitation (LOQ) by preparing and analyzing four low-concentration urine pools (0.005, 0.010, 0.025, and 0.050 ng/mL) over a 3-month period. We first determined the standard deviation (SD) of each pool’s concentration quantified using first MRM transition with confirmation by the second transition (Table S-2) (More details regarding analytical specificity is described in the Supporting Information), and then we plotted the SD of each pool against the concentration, and finally obtained the S0 given as the Y-intercepts.13–15 LODs and LOQs (Table 1) were calculated as 3S0 and 10S0, respectively. We observed the method for unconjugated form had more sensitive LODs (0.002–0.008 ng/mL or equivalently 0.064–0.242 fmol on-column) than those for “total” form (0.005–0.017 ng/mL or equivalently 0.159–0.514 fmol on-column) (Table S-3). This was most likely the result of the tandem-hydrolysis procedure (enzymatic-alkaline hydrolysis). During hydrolysis incubation at higher temperature, analytes can partially degrade due to their thermal instability; meanwhile, they can also partially bind to added enzyme (E. coli.) because of the high lipophilicity and then precipitate by centrifugation. Despite the mass loss during sample preparation, the detection sensitivity achieved in this method for urine samples were 10–100 times the values (0.2–5.0 ng/mL) reported in the literature.6–9
Table 1.
Limit of Detection (LOD), Limit of Quantitation (LOQ), Average Extraction Efficiency of Sample Preparation and Average Matrix Effect
| LOD, ng/mL | LOQ, ng/mL | extraction efficiency (“free”/”total”), % | matrix effect (“free”/”total”), % | |||||
|---|---|---|---|---|---|---|---|---|
| analyte | “free”/“total”a | “free”/“total” | lowb | mid | high | low | mid | high |
| OHTHC | 0.008/0.017 | 0.028/0.048 | 93/69 | 81/67 | 82/75 | 1.0/13 | −5/1 | −9/−32 |
| COOH-THC | 0.005/0.015 | 0.018/0.045 | 83/58 | 81/60 | 83/59 | 5/9 | −4/−6 | −2/−41 |
| CBD | 0.004/0.009 | 0.012/0.030 | 88/22 | 70/26 | 71/28 | 8/23 | −9/−4 | −5/−9 |
| CBN | 0.002/0.007 | 0.008/0.023 | 76/20 | 64/18 | 71/21 | 2/−6 | −8/−16 | −4/−12 |
| THC | 0.002/0.005 | 0.008/0.015 | 74/18 | 59/19 | 54/22 | 6/32 | −7/−5 | −7/−12 |
“Free” and “total” refer to unconjugated and the sum of conjugated and unconjugated forms, respectively.
Low, mid and high levels refer to 0.025, 25, and 500 ng/mL, respectively.
We used three sets of samples at low, medium, and high concentrations (0.025, 25, and 500 ng/mL, respectively) to determine the optimized extraction recoveries and matrix effects (details in the Supporting Information).11 Average extraction recoveries for all analytes ranged from 54 to 93% and 18 to 75% for “free” and “total” measurements, respectively (Table 1). Ion suppression due to matrix effect varied from −9 to 32 and −41 to 32 for “free” and “total” measurements, respectively.
Calibrators and QCs were always freshly produced using the liquid-handling automation system and then prepared in the same manner along with unknown samples and laboratory blanks. We observed excellent interday and intraday accuracy (90–110%), and imprecision was less than 10% (Table 2 and Table S-4) based on replicate analyses of a serial of fortified urine samples prepared by spiking known amounts of analytes over 3 consecutive months.
Supplementary Material
Acknowledgments
We thank Ernest McGahee and Jason Terranova for maintaining the performance of the automated liquid-handling system. The manuscript has certainly benefited from those constructive suggestions from the reviewers.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Experimental details, LC gradient program, MRM ion transitions, LODs in terms of femtomole on-column, accuracy and precision for “total” method, and molecular structures of five analytes (PDF)
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the U.S. Centers for Disease Control and Prevention.
The authors declare no competing financial interest.
REFERENCES
- 1.Murray RM, Morrison PD, Henquet C, Di Forti M. Nat. Rev. Neurosci. 2007;8(11):885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 2.United Nations Office on Drugs and Crime (UNODC) World Drug Report. 2014
- 3.Hall W, Degenhardt L. Lancet. 2009;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 4.Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. Chem. Res. Toxicol. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Baler RD, Compton WM, Weiss SRN. Engl. J. Med. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, Abusada GA. J. Anal. Toxicol. 1995;19(5):285–291. doi: 10.1093/jat/19.5.285. [DOI] [PubMed] [Google Scholar]
- 7.Grauwiler SB, Scholer A, Drewe JJ. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007;850(1):515–522. doi: 10.1016/j.jchromb.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Fernández MdMR, Wille SM, Samyn N, Wood M, López-Rivadulla M, De Boeck GJ. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2009;877(22):2153–2157. doi: 10.1016/j.jchromb.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Scheidweiler KB, Desrosiers NA, Huestis MA. Clin. Chim. Acta. 2012;413(23):1839–1847. doi: 10.1016/j.cca.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skopp G, Pötsch L. Clin. Chem. 2002;48(2):301–306. [PubMed] [Google Scholar]
- 11.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, Wang L. Clin. Chim. Acta. 2014;436:290–297. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrosiers NA, Lee D, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. Anal. Bioanal. Chem. 2014;406(3):785–792. doi: 10.1007/s00216-013-7524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DNR. [Accessed January 22, 2014];Analytical Detection Limit Guidance & Laboratory Guide for Determining Method Detection Limits. http://dnr.wi.gov/regulations/labcert/documents/guidance/-LODguide.pdf.
- 14.ICH. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology. 1994. [Google Scholar]
- 15.Taylor JK. Quality Assurance of Chemical Measurements. Chelsea, MI: Lewis Publishers; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.