Abstract
Cardiac myocyte-fibroblast electrotonic coupling is a well-established fact in vitro. Indirect evidence of its presence in vivo exists, but few functional studies have been published. This review describes the current knowledge of fibroblast-myocyte electrical signalling in the heart. Further research is needed to understand the frequency and extent of heterocellular interactions in vivo in order to gain a better understanding of their relevance in healthy and diseased myocardium. It is hoped that associated insight into myocyte-fibroblast coupling in the heart may lead to the discovery of novel therapeutic targets and the development of agents for improving outcomes of myocardial scarring and fibrosis.
This article is part of a special issue entitled “Exploring Fibrosis as the Next Target for Myocardial Remodeling.”
Keywords: scar, gap junction, cardiac electrophysiology
1. Introduction
Although cardiac function is governed by the contractile ability of cardiac myocytes, fibroblasts are one of the more numerous cell populations in the heart. While previously thought to be electrical insulators without exception, we now know that fibroblasts may be electrically connected among themselves, and with myocytes.
Fibroblasts are key players in myocardial remodeling in response to disease and injury, proliferating (or being recruited from progenitors within and outside the heart) and differentiating into an activated phenotype that produces excess extracellular matrix (ECM) proteins, thereby helping to protect the heart from overdistension or rupture. While potentially of mechanical benefit, altered/increased fibroblast activity may contribute to cardiac arrhythmias, and may thus be a critical factor in disease progression. The purpose of this review is to examine the current knowledge on myocyte-fibroblast biophysical coupling in the heart, to sum up how this coupling changes in disease, to identify the steps needed toward a deeper understanding of these interactions, and to suggest how one might try to target these interactions for therapeutic benefit.
2. What occurs in native tissue
2.1. Cardiac composition and cellular function
While myocytes account for approximately 65–75% of cardiac volume, non-myocytes, such as fibroblasts or endothelial cells, constitute more numerous cell populations in the heart. Fibroblasts contribute, by some accounts, up to two thirds of total heart cells in rats [1] and humans [2,3], although mouse models may yield lower counts (20–30%) [4,5]. It is not currently known whether these variations reflect species differences (do large hearts, such as those of human [2,3], have higher fibroblast fractions?) or methodological challenges (counting approaches based on prior cell isolation [1,4,5] require identical ‘relative survivals rates’ of the different cell types – a precondition that is difficult to prove, while morphometric approaches are limited by their partial tissue ‘snapshot’ nature and the difficulty of extrapolating from two-dimensional [2D] section data to three-dimensional [3D] volumes, as cells with extended processes may traverse a given histological sectioning plane multiple times). Fibroblasts were initially thought to serve as a structural support system only, generating ECM into which cardiac cells are embedded, and thus providing the deformable skeleton of the heart. We now know that fibroblasts are of critical importance for a variety of cardiac functions, and that they are extensively involved in signaling in normal myocardium, and in remodeling/fibrosis during cardiovascular diseases. Of note, ‘fibrosis’ is a term that should not be confused with fibroblast density, as it is assessed by, and indicative of, elevated ECM levels, specifically of collagen.
In adult heart, myocytes are ‘brick-shaped’, with lengths in the order of 10−4 m and width/depth in the 10−5 m domain. Ventricular myocytes are organized into laterally reinforced laminae, 3–5 cells thick, that allow sliding of muscle layers (‘sheetlets’) during ventricular deformation. Long axis cell orientation (often, if incorrectly, referred to as ‘fibre’ orientation) varies from roughly −45° (relative to the horizontal plane of the ventricles and ‘viewed’ from the outside) in the epicardium, to +45° in the endocardium. Sheetlet structure is less easily described, and – depending on cutting angle of the virtual imaging or mechanical sectioning plane – could range from a transmural swirl (short-axis planes) to a fishbone pattern (long-axis planes) [6,7]. This fish-bone pattern is important for wall thickening, which is associated with a more horizontal alignment of sheetlets during contraction [7].
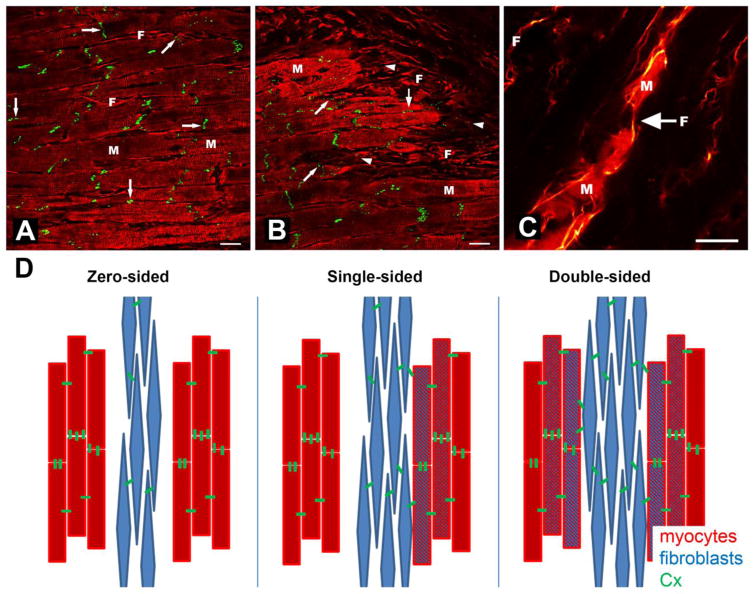
Myocytes within sheetlets are connected in the axial direction at intercalated discs where the mechanical and most of the electrical junctions are found (Fig. 1A). Gap junctions, traditionally viewed as ‘the’ substrate for transmission of electrical signals between cardiac cells, are highly concentrated at intercalated discs, though they are also, if to a lesser extent, present at lateral cell contacts (Fig. 1B) [8,9]. In keeping with this, there is anisotropy in the speed of cardiac action potential (AP) propagation, being fastest along the main orientation of cardiomyocytes, slower in the fibre-normal in-sheetlet direction, and slowest normal to the plane of sheetlets.
Figure 1.
Heterotypic intercellular connections in cardiac tissue. A) Cx43 (green) localized primarily at intercalated discs of myocytes in normal sheep myocardium (right-arrows), but also between lateral myocyte (M) contacts (down-arrows) and, occasionally, at points of heterotypic cell contact with fibroblasts (F; slanted arrow). B) Cardiac scar border zone, showing disturbed Cx43 distribution along M (increased lateralization; down-arrows) and abundant punctate presence in F (left-arrowheads), including at F-M junctions (slanted arrow). C) Confocal image of dye coupling by fibroblasts between groups of myocytes in rabbit right atrial tissue. D) Conceptual representation of myocyte-fibroblast electrotonic interaction scenarios via Cx proteins; for detail see section 2.3. Scale bars = 20 μm. From [17] in A and B with permission from the European Society of Cardiology, and [15] in C with permission from the American Heart Association.
Gap junctions consist of connexin (Cx) proteins. While connexin43 (Cx43) is the most abundant gap junctional protein in the working myocardium, other connexins including Cx40 and Cx45 are found in parts of the atria and in the pacemaker and conduction system [10–14]. All three connexin sub-types have been reported in cardiac fibroblasts [15–17].
Individual fibroblasts in the heart also form sheet-like structures [18], which explains why fibroblasts in 2D tissue sections almost invariably appear to have extended ‘spindle-like’ processes. They are interspersed between cardiomyocytes throughout the myocardium, and themselves interconnected by punctate connexin junctions [15]. Fibroblasts in situ have a surprisingly large surface area. One fibroblast, reconstructed in an electron microscopy (EM) study of the rabbit sinoatrial node, was measured to have 720 μm2 of membrane surface area adjacent to a cluster of myocytes [18]. This only included one face of the cell membrane, and did not account the surface area of membrane folds or cell extensions not in direct apposition with the neighbouring myocytes. At the very least, the total surface area of this fibroblast would have been double the area it shared with the myocyte, i.e. in excess of 1,440 μm2 [18]. This large membrane surface area highlights the ability of individual cardiac fibroblasts to interact, communicate, and signal with multiple homo- and heterotypic cells (the larger the membrane surface area, the larger the number of possible reaction and interaction sites), and to do so over significant distances. Such interactions may involve paracrine signalling, via release of growth factors and cytokines (reviewed in [19–21]), or occur via direct biophysical interactions through mechanical or electrical junctions (e.g. adhesion molecules, connexins, etc.), facilitating electro-mechanical transduction.
Defining ‘the’ cardiac fibroblast has proven difficult, as will be a common theme throughout this focused issue of JMCC. There is considerable variability in the developmental origin of cardiac mesenchymal cells [22]. The majority derive from the pro-epicardium [23]. Fibroblasts also originate from the epicardium by epithelial-to-mesenchymal transformation, followed by migration into atrial and ventricular walls where they differentiate into cardiac fibroblasts [24,25]. Additionally cardiac fibroblasts can originate from the cardiac endothelium, undergoing endothelial to mesenchymal transformation [26–28]. Although perhaps not frequent in normal homeostasis, other contributor pools to the cardiac fibroblast population include bone marrow-derived cells [8,9,10], hematopoietic cells [32], and mesangioblasts [33] (for review, see [34]).
This heterogeneity in cardiac fibroblast origins contributes to the ongoing challenge of finding suitable cardiac fibroblast markers that are specific and inclusive. In order to distinguish fibroblasts from other cardiac cell types, vimentin [35–37], discoidin domain receptor 2 (DDR-2) [38,39], transcription factor-21 (Tcf-21) [40,41], and fibroblast activation protein (FAP) [42–44] have been used – however, none are strictly specific, and each labels only a sub-population of the overall cardiac fibroblast population(s) [44–47].
2.2. Electrophysiological properties of cardiac fibroblasts
While the electrical integration of cardiomyocytes is associated with prominent clusters of connexin proteins – in particular Cx43 – at intercalated discs, fibroblasts in the normal myocardium of murine and rabbit heart express much smaller amounts of Cx43, Cx45, and Cx40 [48,49]. These proteins form punctate coupling sites between fibroblasts, whose functional relevance is ill-explored.
In the sinoatrial node of rabbit heart, functional heterotypic cell coupling of fibroblasts and cardiomyocytes has been illustrated by dye transfer studies (Fig. 1C) [15]. Apart from this, no firm functional data on heterotypic cell coupling in healthy heart has been reported. Structural coupling, for example, as indicated by co-localization of Cx proteins with membranes of heterotypic cells in the heart, is more common than was initially appreciated. This underappreciation may have been caused by the gap junction size, as this is near or below the detection limit of the early fluorescence techniques that gave rise to the text-book notion of cardiac Cx distribution and function [16].
Connective tissue, and by implication the fibroblast itself, was historically thought to be an electrical insulator. More recent studies have shown that this is not the only possible function of these tissues and cells. Patch clamp experiments have shown that the resting membrane potential of electrically discrete fibroblasts is between −10 and −50 mV in situ [50–52]. Fibroblasts in tissue have a high input resistance, with values in the GΩ region [50,51,53]. Since fibroblasts can be electrically interconnected to other fibroblasts in vivo, their membrane capacitance is difficult to quantify accurately. In vitro, values of 6–11 pF have been recorded [54,55], though freshly isolated fibroblasts are about an order of magnitude smaller than what has been documented in vivo (see above). In any case, their relatively low membrane capacitance, combined with a high membrane resistance, could make cardiac fibroblasts excellent long-distance electrical signal conductors.
Fibroblasts, while not an electrically excitable cell type, are able to mimic the AP of electrotonically coupled myocytes, as shown in dual-cell patch clamp experiments [50,52]. In this setting, cardiac myocyte AP generation drives membrane polarization in the passive fibroblast follower (helped by high resistance and limited capacity of the latter cell type). Fibroblasts further experience changes to membrane resistance and potential through mechanical perturbation, thus potentially assisting mechano-electric feedback in the heart [56].
2.3. Myocyte-fibroblast coupling
Exhaustive EM studies of gap junctions in the rabbit sinoatrial node have found extensive coupling between myocytes, small gap junction plaques between fibroblasts, but only a single gap junction-like connection between a myocyte and fibroblast [18]. When fibroblast gap junctions are found in tissue, either by EM or by immunolabeling studies, they are small and punctate, making them difficult to find, and easy to mistake for background noise or artifact. Nonetheless, punctate Cx immunostaining is frequently found in myocardium between fibroblasts and at myocyte-fibroblast heterocellular connections [16]. These junctions may or may not be functional, as the presence of protein does not confirm function.
Tunneling nanotubes, which may involve (but do not require) connexins at their point of contact, can provide an alternative direct link for electrotonic coupling between myocytes and fibroblasts [57]. These thin (20–50 nm wide) membranous tubes can stretch between dendritic corneal cells as far apart as 300 μm in vivo [58], and they can propagate AP from cell to cell [59]. While evidence of myocyte-fibroblast coupling via nanotubes has been shown in vivo and in vitro [57], the electrophysiological function of nanotubes in the myocardium in health or disease has yet to be ascertained [34].
Ephaptic coupling – a non-electrotonic form of coupling – must also be considered as a possible mechanism of heterocellular electrical communication. Computer models have shown that ephaptic effects may be present in all areas of extracellular space, not just at the intercellular clefts of intercalated discs between myocytes [60]. More recent experimental evidence demonstrates this phenomenon in cardiac tissue [61], though there is still a need to understand how fibroblasts may be involved, if they are engaged at all [62].
Based on the arrangement of fibroblasts and myocytes in the heart, there are at least three conceptual modes in which these heterotypic cells could interact electrically. These modes may exist in healthy myocardium, as well as in various disease states.
First, in ‘zero-sided coupling’, fibroblasts are electrically not connected to myocytes, and instead are separating layers of functionally-connected myocytes, serving as an electrical insulator (Fig. 1D). This is presumably the most common form of fibroblast electrophysiological integration in native myocardium.
Second, ‘single-sided coupling’ may connect fibroblasts to an electrophysiologically homogeneous group of myocytes (Fig. 1D). In this case, fibroblasts would serve as a passive load, which could affect myocyte electrophysiological properties. Computer simulations over the past few decades have shown that functional myocyte-fibroblast coupling – by as few as 10–13 gap junctional channels [50] – could give rise to electrical source-sink relations, where fibroblasts may slightly depolarize the myocyte resting membrane potential, potentially accelerating pacemaker rate [50,63] or even having arrhythmogenic consequences [64].
Third, in ‘double-sided coupling’, fibroblasts would link myocytes that are structurally separated, and could serve as short (between muscle layers, Fig. 1D) or long range (in the case of multiple interconnected fibroblasts) conductors of electrical excitation. In vitro, this has been confirmed, as fibroblasts are able to electrotonically connect myocytes that are otherwise separated [65], passively bridging AP conduction over gaps of up to 300 μm [66]. If present at tissue and organ levels, this would have important effects on electrophysiological behavior [67,68]. However, it is challenging to devise a conclusive experiment to test this possibility in native tissue, using traditional electrophysiological techniques. This is because the high membrane resistance of fibroblasts makes them excellent passive followers of an electrical source signal. While this is good for passive AP conduction, it is bad for experimental identification in situ – as well-coupled fibroblasts will be ‘voltage-clamped by coupled myocytes’ to passively display a myocyte-like AP.
3. What is different in a dish
Some of the above already points to differences between the in vivo and in vitro settings, such as increased electrical coupling of cells in a dish, compared to native myocardium. There is more.
Freshly isolated fibroblasts are small and rounded, without the membrane extensions and processes they possess while in situ (by the way: this reinforces the concern about cell quantification approaches that are based on prior isolation of cardiac tissue components). Their diameters are in the 7–9 μm range [54,69,70], and their membrane resistances are an order of magnitude higher than in fibroblasts assessed in situ [52,55].
Isolated atrial and ventricular fibroblasts ‘carry through’ to the dish some of their differences in morphology, with atrial fibroblasts being more elongated than ventricular fibroblasts [71]. When cultured, fibroblasts undergo a phenotype transition to myofibroblasts, induced, in part, by the large increase in substrate stiffness (from 10–15 kPa in native myocardium to GPa levels in plastic tissue culture dishes) [72–74]. This phenotype change involves increased expression of myofibroblast markers [75], including α-smooth muscle actin (α-SMA) [76], increased proliferation and migration, production of (and response to) transforming growth factor-beta (TGF-β) [77] and – increased Cx expression [78].
As a result of the Cx overexpression, electrical coupling of myocytes and fibroblasts via gap junctions in vitro is well-established. Indeed, synchronized contraction of distant cardiomyocytes, interconnected by fibroblasts only, has been observed in vitro in some of the earliest cell culture studies published [65,79,80].
Fibroblasts in culture have been found to express a number of ion channels and several currents have been recorded in patch clamp experiments in vitro. These include voltage gated K+ channels with delayed rectifier currents and transient potassium currents in cultured rat and human cells [55,81–83]. Additionally, it is likely that Kir is a primary determinant of resting membrane potential in both fibroblasts and myofibroblasts in culture, with changes in potassium concentration affecting not only membrane polarization, but also fibroblast proliferation and contraction [55]. Cardiac fibroblasts contain stretch activated non-selective cation channels [84–87]. In a rare report, Nav1.5 expression was seen after long-term culture of human atrial fibroblasts [88], though this may have been a result of cell fusion [89] or exosome transfer [90], as no fast Na+ currents are seen in freshly isolated fibroblasts – defined as a non-excitable cell population.
Genetically altering the expression of Cx and ion channels in fibroblasts in vitro has provided vivid illustrations of plausible effects of fibroblast coupling to myocytes [91]. Cx-overexpression in fibroblasts has shown that coupling between myocytes and fibroblasts is not necessarily disruptive to conduction, and additional engineered ion channel expression in non-excitable cells can lead to conduction velocities close to those seen in pure myocyte cultures [92]. Interestingly, these genetic tools have shown potential as a therapy in post-myocardial infarction scars, as discussed below (section 5).
Fibroblasts cannot generate AP, but their membrane potential is responsive to mechanical stimuli. They may respond differentially to compression and stretch [93,94]. In cultures of rat ventricular fibroblasts and myocytes, increased heterocellular mechanical interactions via adhesion proteins caused conduction slowing in cardiomyocytes [95]. This suggests mechanical interactions may impair conduction as a result of increased mechanosensitive channel activation. Thus mechanical, in addition to electrical, coupling of myocytes and fibroblasts may affect cardiac electrophysiology.
4. What occurs in disease
Regardless of origin, if activated by injury, fibroblasts proliferate and increase production of ECM – one of the mechanisms believed to contribute to the domestication of atrial fibrillation [96]. Atrial and ventricular fibroblasts respond differently to disease, with atrial fibroblasts tending to generate larger amounts of ECM [71].
In the pressure-overloaded heart, resident cardiac fibroblasts of epicardial origin contribute to fibrosis [97], with a minor contribution from neural crest cells [98]. In contrast, studies of myocardial infarction (MI) suggest that, in addition to epicardially derived resident fibroblasts, bone-marrow derived cells contribute up to 60% of fibroblasts in the scar [29,32,99,100]. While TGFβ is an important regulator of cardiac fibrosis post-MI, fibroblasts are also activated by interleukin-1α (IL-1α), released by necrotic myocytes, which may be a critical contributor to the recruitment of extra-cardiac cells to the site of injury [101], compared to pressure-overload models.
In the ventricles, the increase in fibroblast number and ECM deposition after MI is thought to help prevent wall rupture. However, the changes that occur in the injury border zone–separation of myocytes by fibroblasts and ECM deposition, and lateralization of Cx43 (Fig. 1B) – affect AP propagation detrimentally [102–104]. Fibroblasts also remodel electrophysiologically post-MI, and show more hyperpolarized membrane potentials, increased outward current densities [105], and increased membrane resistance compared to fibroblasts isolated from normal hearts [53]. Further, the alteration in connexin profiles post-infarction can lead to increased fibroblast Cx43 expression [17,106,107]. Interestingly, decreased Cx43 expression in Cx43 knockout mice led to cardiac fibroblast activation and increased fibrosis in hearts, with a subsequent increase in arrhythmias [108].
The scar, as a whole, is formed largely by fibroblasts and ECM and is consequently considered to be electrically non-conducting. However, scars are more dynamic than this. Heterotypic fibroblast-myocyte coupling via Cx is often seen in the infarct border zone (Fig. 1B). Indeed, optical mapping of AP spread showed wave-front propagation into post-MI scar tissue, even after chemical ablation of any surviving sub-endocardial myocyte layers, indicating a possible role for non-myocytes in passively conducting electrical signals [68]. The AP measured within the scar showed a reduced upstroke velocity and reduced amplitude – characteristics previously identified in vitro for cardiac fibroblasts coupled to myocytes [50,52] and compatible with computer modeling predictions [105,109]. Additional optical mapping studies identified a lack of calcium transients (a characteristic signature activity of myocytes) in the infarct scar [67]. This suggests that fibroblasts may serve as passive conveyors of AP waveforms in scar tissue. This could potentially explain trans-scar conduction recovery across atrial ablation lines seen in a majority of patients [110], and electrical coupling of recipient and donor tissue seen in about 20% of heart transplant patients [111].
That said, the above observations provide circumstantial evidence only, and functional heterocellular coupling in diseased heart has not been demonstrated with certainty. In the normal heart, functional in vivo data confirming heterocellular coupling has been shown by dye coupling in rabbit sinoatrial node [15]. Understanding the presence, extent, and regulation of myocyte-fibroblast functional coupling in vivo, how this coupling varies during development, by region, in various disease conditions, and whether it responds to medical interventions, is critical for development of fibroblast-targeting therapeutics. This is an area where 3D image reconstruction of scars may be incorporated into detailed computer models of conduction in disease [112,113], to assess plausibility of various concepts, and to devise testable hypotheses for experimental validation [114].
Beyond cellular resolution data, super-resolution microscopy may provide new insight into molecular and structural interactions important for heterocellular interactions within the heart. To date, super-resolution imaging has been applied to the structure of the intercalated disc and perinexus [61,115], the calcium handling machinery [116,117], and the Z-disc [118], some of which may be relevant for ephaptic coupling [61]. Super-resolution studies of cardiac fibroblast surface topography are a critical step towards better understanding of myocyte-fibroblast interactions in the heart.
Finally, while voltage-sensitive fluorescent dyes have allowed research into ‘bulk behaviour’ at the organ level, this approach does not allow attribution of observed behaviour to specific cell types. Such distinction could be achieved, however, by genetic targeting of reporter (or actuator) proteins to a specific cell population (optogenetics; see [119–121]). Recently, use of a voltage reporting protein, expressed under the α-myosin heavy chain (αMHC) promoter to target myocytes, or the Wilm’s Tumor 1 (WT1) promoter to target non-myocytes in the heart, has been reported [122]. This study provided first evidence of AP conduction via non-myocytes within the scar border tissue.
5. Myocyte-fibroblast coupling as a therapeutic target?
Connective tissue remodeling is understood to contribute to arrhythmogenesis. Traditionally, this has ignored altered fibroblast electrophysiology, including potential electrical coupling to other cells including cardiomyocytes.
In vitro, fibroblasts are already well-coupled to co-cultured myocytes, and adeno-viral transduction to express additional ion channels and Cx43 in cultured fibroblasts may not necessarily give rise to drastic changes in electrophysiological behaviour of co-cultures [92]. However, the situation in situ appears different. Work from the Fleischmann group has shown that engraftment of Cx-expressing non-myocytes into post-MI ventricular scars of mice prevents arrhythmogenesis, and that this anti-arrhythmic action depends on the presence of Cx43 [123]. Additionally, injection of (inherently Cx43-expressing) non-myocytes that were transfected to over-express K+ currents reduced automaticity and prolonged refractoriness in rat and pig hearts [124].
Another role of homo- and heterocellular Cx-coupling may be related to spread of injury across the cardiac syncytium. Thus, Cx43 mimetics – peptides that alter Cx function – have been found to improve outcomes after experimental MI. Examples include the peptides Gap26, Gap27 and Gap19, as well as αCT1 – a Cx43 c-terminal mimetic that interrupts Cx43-ZO-1 interaction. These peptides have been shown to reduce Cx hemi-channel function [125–127]. In experimental models of MI, Gap19 and Gap26 were shown to significantly decrease infarct size [128,129], and αCT1 was shown to reduce left ventricular dilation, maintain Cx43 at intercalated discs in the border zone, and decrease the number of inducible arrhythmias [130,131]. Though there is some understanding of the mechanistic effects of these peptides on gap junction intercellular communication, this has yet to be examined in the context of heterocellular coupling.
Delivery of bone marrow cells directly to infarcted myocardium as a therapeutic strategy has resulted in modest improvement of cardiac function at best. Further studies revealed that positive effects of cell injections on tissue repair were likely to be caused by paracrine mechanisms [132]. A different approach could exploit the fact that some non-myocytes are recruited to a healing infarct directly from the bone marrow [29,99,100]. These cells could provide a vehicle for delivering therapeutic ‘payloads’ to post-MI tissue [133]. Intrafemoral injection of lentivirus and adenovirus expressing GFP in mice has shown that cells within the bone marrow cavity can be efficiently transduced in situ [134,135]. Therapies of this type may be challenged by the fact that many more bone marrow cells would be transfected than those that home in to sites of cardiac injury [136]. This could be addressed, however, using ‘auto-terminating’ transfection strategies, or by the use of heterospecific antibodies, which have been shown to increase engraftment of intravascularly injected cells in the heart [137]. These antibodies are engineered to be specific for the antigens of two cell types. In this case, they first bind to the target cardiac cells, and then to specific antigens on the circulating cells. This strategy holds promise both with injected or bone marrow derived cells, as well as those recruited to the site of injury from inside the myocardium.
Interestingly, exosomes appear to account for some of the beneficial effects conferred by intracardiac cell injection [138,139]. In fact, exosomes administered intravenously are able to ameliorate cardiac function in a model of dilated cardiomyopathy [140], and a better understanding of exosome contents and their therapeutic potential is certainly high on the list of research priorities. As an exciting example, exosome cell surface proteins have been genetically engineered to display homing peptides, and this conferred targeting capability to cardiac myocytes and ischemic myocardium [141,142]. Of note, Cx43 is involved in the communication between extracellular vesicles and mammalian cells [90], so exploration of exosome targeting to cardiac non-myocytes is a realistic prospect.
Clearly, there is a critical need for a better understanding of exactly how we should alter electrophysiology of scar tissue in the heart. Depending on purpose, answers may be at opposite ends of the spectrum. In the case of electrical connectivity, for example, ablation lines would ideally be made solidly insulating, while (at least small) cardiac infarcts might become less problematic if they did pass the wave of electrical excitation with minimal delay. So, in one scenario, one might wish to down-regulate nonmyocyte Cx expression, and in the other – up. We further need to understand how to improve the overall electrophysiological function of scar tissue without jeopardizing scar mechanics [143], how to deliver interventions in a spatially and temporally controlled manner, and how to grade them to achieve specific effects. All this depends, in part, on further insight into presence, distribution, functionality, and regulation of homo- and heterotypic coupling of cells in the heart.
6. Conclusions
Myocytes and fibroblasts interact in multiple ways, over a range of space and time scales, and in both normal and diseased heart. Molecular interaction substrates include electrical and mechanical junctions, and biochemical signaling makes significant contributions to myocyte-fibroblast cross-talk, including initiation of cellular phenotype changes in disease.
Presently, there is limited evidence of functional electrical coupling of myocytes and fibroblasts in the heart in vivo. Considering the heterogeneity of resident cardiac fibroblast populations, and the variability in cell types recruited to injured tissue, there is a need for detailed examination of the integrated myocardium in various disease states and regions in the heart, compared to steady state homeostasis. Clinical reports and experimental studies have provided evidence that scars may passively conduct electrical signals. Passive conduction is possible over fairly short distances only (signal amplitude attenuation), unless a supra-threshold stimulus reaches a ‘repeater station’, e.g. an island of surviving myocytes in post-MI scar tissue. Such active/passive/active chains of excitable and non-excitable cells have been engineered to successfully conduct excitation long-range, from atrium to ventricles [144,145]. The full extent of myocyte-fibroblast electrical coupling in the heart, the mechanisms of its regulation, and its importance in health and disease remain to be explored in detail. The growing number of experimental tools available to conduct cell-specific research raise the hope that we may be able to first, understand, and second, tweak scar properties to improve outcomes for patients with cardiac disease.
Highlights.
Fibroblasts (F) matter for cardiac structural integrity
Furthermore, they are important biochemical and biophysical signaling hubs
Biophysical signaling includes electric coupling of F to other F or to myocytes (M)
F-M coupling may affect M excitability, refractoriness, and conduction
The therapeutic potential of tuning F-M interactions requires further investigation
Acknowledgments
ELO acknowledges support from NIH fellowship (F31DE22224). PK is a Senior Fellow of the British Heart Foundation and acknowledges support via the European Research Council CardioNECT Advanced Grant. We thank Dr. Eva Rog-Zielinska for critical review of this manuscript.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- αMHC
α-Myosin Heavy Chain
- αSMA
α-Smooth Muscle Actin
- AP
Action Potential
- Cx
Connexin
- Cx43
Connexin43
- ECM
Extracellular Matrix
- EM
Electron Microscopy
- DDR2
Discoidin domain receptor 2
- Tcf21
Transcription Factor-21
- FAP
Fibroblast Activation Protein
- IL-1α
Interleukin 1α
- MI
Myocardial Infarction
- WT1
Wilm’s Tumor 1
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 2.Vliegen HW, van der Laarse a, Cornelisse CJ, Eulderink F. Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur Heart J. 1991;12:488–94. doi: 10.1093/oxfordjournals.eurheartj.a059928. [DOI] [PubMed] [Google Scholar]
- 3.Adler C, Ringlage W, Böhm N. DNA content and cell number in heart and liver of children. Comparable biochemical, cytophotometric and histological investigations (author’s transl) Pathol Res Pr. 1981;172:25–41. [PubMed] [Google Scholar]
- 4.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol - Hear Circ Physiol. 2007;293:1883–91. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 5.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni M, Debuque RJ, et al. Revisiting Cardiac Cellular Composition. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeGrice I, Pope A, Smaill B. The architecture of the heart: myocyte organization and the cardiac extracellular matrix. In: Villarreal FJ, editor. Interstitial Fibros Hear Fail V 253 Dev Cardiovasc Med. Springer; New York: 2005. pp. 3–21. [DOI] [Google Scholar]
- 7.Hales PW, Schneider JE, Burton RA, Wright BJ, Bollensdorff C, Kohl P. Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI. Prog Biophys Mol Biol. 2012;110:319–30. doi: 10.1016/j.pbiomolbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burt JM, Frank JS, Berns MW. Permeability and structural studies of heart cell gap junctions under normal and altered ionic conditions. J Membr Biol. 1982;68:227–38. doi: 10.1007/BF01872267. [DOI] [PubMed] [Google Scholar]
- 9.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–36. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyett M, Inada S, Yoo S, Li J, Liu J, Tellez J, et al. Connexins in the sinoatrial and atrioventricular nodes. Adv Cardiol. 2006;42:175–97. doi: 10.1159/000092569. [DOI] [PubMed] [Google Scholar]
- 11.van Kempen MJ, Fromaget C, Gros D, Moorman AF, Lamers WH. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ Res. 1991;68:1638–51. doi: 10.1161/01.RES.68.6.1638. [DOI] [PubMed] [Google Scholar]
- 12.Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci. 1993;105:985–91. doi: 10.1242/jcs.105.4.985. [DOI] [PubMed] [Google Scholar]
- 13.Coppen SR, Severs NJ, Gourdie RG. Connexin45 (α6) expression delineates an extended conduction system in the embryonic and mature rodent heart. Dev Genet. 1999;24:82–90. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<82::AID-DVG9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Gourdie RG, Green CR, Severs NJ, Thompson RP. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat Embryol. 1992;185:363–78. doi: 10.1007/BF00188548. [DOI] [PubMed] [Google Scholar]
- 15.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: Structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–35. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 16.Kohl P, Camelliti P. Fibroblast-myocyte connections in the heart. Hear Rhythm. 2012;9:461–4. doi: 10.1016/j.hrthm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–25. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 18.De Maziere A, Vanginneken A, Wilders R, Jongsma H, Bouman L. Spatial and functional-relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J Mol Cell Cardiol. 1992;24:567–78. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 19.Kakkar R, Lee RT. Intramyocardial fibroblast-myocyte communication. Circ Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ottaviano FG, Yee KO. Communication signals between cardiac fibroblasts and cardiac myocytes. J Cardiovasc Pharmacol. 2011;57:513–21. doi: 10.1097/FJC.0b013e31821209ee. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ Res. 2012;110:1023–34. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore-Morris T, Cattaneo P, Puceat M, Evans SM. Origins of cardiac fibroblasts. J Mol Cell Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–32. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.RES.77.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Gittenberger-de Groot AC, Vrancken Peeters MPFM, Mentink MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–52. doi: 10.1161/01.RES.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 26.De Lange FJ, Moorman AFM, Anderson RH, Männer J, Soufan AT, De Gier-De Vries C, et al. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–54. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–70. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell Signal. 2012;24:1031–6. doi: 10.1016/j.cellsig.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Möllmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–71. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–65. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visconti RP, Markwald RR. Recruitment of new cells into the postnatal heart: potential modification of phenotype by periostin. Ann N Y Acad Sci. 2006;1080:19–33. doi: 10.1196/annals.1380.003. [DOI] [PubMed] [Google Scholar]
- 33.Cossu G, Bianco P. Mesoangioblasts - vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–42. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The living scar–cardiac fibroblasts and the injured heart. Trends Mol Med. 2016 doi: 10.1016/j.molmed.2015.12.006. In press http://dx.doi.org/10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed]
- 35.Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, et al. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116:1176–84. doi: 10.1161/CIRCULATIONAHA.106.650903. [DOI] [PubMed] [Google Scholar]
- 36.Grigore A, Arsene D, Filipoiu F, Cionca F, Enache S, Ceauşu M, et al. Cellular immunophenotypes in human embryonic, fetal and adult heart. Rom J Morphol Embryol. 2012;53:299–311. [PubMed] [Google Scholar]
- 37.Evans RM. Vimentin: The conundrum of the intermediate filament gene family. BioEssays. 1998;20:79–86. doi: 10.1002/(SICI)1521-1878(199801)20:1<79::AID-BIES11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, et al. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–94. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 39.Morales MO, Price RL, Goldsmith EC. Expression of Discoidin Domain Receptor 2 (DDR2) in the developing heart. Microsc Microanal. 2005;11:260–7. doi: 10.1017/S1431927605050518. [DOI] [PubMed] [Google Scholar]
- 40.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–49. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tandon P, Miteva YV, Kuchenbrod LM, Cristea IM, Conlon FL. Tcf21 regulates the specification and maturation of proepicardial cells. Development. 2013;140:2409–21. doi: 10.1242/dev.093385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–9. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JE, Lenter MC, Zimmerman RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast Activation Protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. 1999;274:36505–12. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 44.Acharya PS, Zukas A, Chandan V, Katzenstein A, Puré E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol. 2006;37:352–60. doi: 10.1016/j.humpath.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Bhadriraju K, Chung KH, Spurlin Ta, Haynes RJ, Elliott JT, Plant AL. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials. 2009;30:6687–94. doi: 10.1016/j.biomaterials.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 46.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Muller E, et al. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–78. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 47.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, et al. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, et al. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Louault C, Benamer N, Faivre JF, Potreau D, Bescond J. Implication of connexins 40 and 43 in functional coupling between mouse cardiac fibroblasts in primary culture. Biochim Biophys Acta - Biomembr. 2008;1778:2097–104. doi: 10.1016/j.bbamem.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: Interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–56. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 51.Kamkin A, Kiseleva I, Wagner K-D, Pylaev A, Leiterer KP, Theres H, et al. A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol Heart Circ Physiol. 2002;282:H842–9. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- 52.Rook MB, van Ginneken ACG, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol - Cell Physiol. 1992;263:C959–77. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- 53.Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, et al. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J Mol Cell Cardiol. 1998;30:1083–93. doi: 10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 54.Dawson K, Wu CT, Qi XY, Nattel S. Congestive heart failure effects on atrial fibroblast phenotype: differences between freshly-isolated and cultured cells. PLoS One. 2012;7:e52032. doi: 10.1371/journal.pone.0052032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H2931–9. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 56.Kohl P, Kamkin a G, Kiseleva IS, Streubel T. Mechanosensitive cells in the atrium of frog heart. Exp Physiol. 1992;77:213–6. doi: 10.1113/expphysiol.1992.sp003576. [DOI] [PubMed] [Google Scholar]
- 57.He K, Shi X, Zhang X, Dang S, Ma X, Liu F, et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92:39–47. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 58.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–83. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Gerdes HH. Long-distance electrical coupling via tunneling nanotubes. Biochim Biophys Acta - Biomembr. 2012;1818:2082–6. doi: 10.1016/j.bbamem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Keener JP. Ephaptic coupling in cardiac myocytes. IEEE Trans Biomed Eng. 2013;60:576–82. doi: 10.1109/TBME.2012.2226720. [DOI] [PubMed] [Google Scholar]
- 61.Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflügers Arch - Eur J Physiol. 2015:2093–105. doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeigler AC, Richardson WJ, Holmes JW, Saucerman JJ. Computational modeling of cardiac fibroblasts and fibrosis. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquemet V. Pacemaker activity resulting from the coupling with nonexcitable cells. Phys Rev E, Stat Nonlinear, Soft Matter Physics. 2006:74. doi: 10.1103/PhysRevE.74.011908. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen TP, Xie Y, Garfinkel A, Qu Z, Weiss JN. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc Res. 2012;93:242–51. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goshima K. Formation of nexuses and electrotonic transmission between myocardial and FL cells in monolayer culture. Exp Cell Res. 1970;63:124–30. doi: 10.1016/0014-4827(70)90339-3. [DOI] [PubMed] [Google Scholar]
- 66.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–8. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 67.Saba S, Mathier Ma, Mehdi H, Liu T, Choi BR, London B, et al. Dual-dye optical mapping after myocardial infarction: does the site of ventricular stimulation alter the properties of electrical propagation? J Cardiovasc Electrophysiol. 2008;19:197–202. doi: 10.1111/j.1540-8167.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 68.Walker NL, Burton FL, Kettlewell S, Smith GL, Cobbe SM. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction: Response to atrial, endocardial and epicardial pacing. J Cardiovasc Electrophysiol. 2007;18:862–8. doi: 10.1111/j.1540-8167.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 69.Rook MB, Jongsma HJ, van Ginneken ACG. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol. 1988;255:H770–82. doi: 10.1152/ajpheart.1988.255.4.H770. [DOI] [PubMed] [Google Scholar]
- 70.Nikolskaya A, Sharma V. Cell culture models and methods. In: Sigg D, Iaizzo P, Xiao Y-F, He B, editors. Card Electrophysiol Methods Model. 2010. pp. 213–35. [Google Scholar]
- 71.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–41. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 72.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 73.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed]
- 75.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–84. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 76.Rohr S. Myofibroblasts in diseased hearts: New players in cardiac arrhythmias? Hear Rhythm. 2009;6:848–56. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 77.Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–23. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asazuma-Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF-β signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009;315:1190–9. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 79.Mark GE, Strasser FF. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966;44:217–33. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- 80.Hyde A, Blondel B, Matter A, Cheneval JP, Filloux B, Girardier L. Homo- and heterocellular junctions in cell cultures: An electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- 81.Shibukawa Y, Chilton EL, MacCannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J. 2005;88:3924–35. doi: 10.1529/biophysj.104.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walsh KB, Zhang J. Neonatal rat cardiac fibroblasts express three types of voltage-gated K+ channels: regulation of a transient outward current by protein kinase C. AJP Hear Circ Physiol. 2008;294:H1010–7. doi: 10.1152/ajpheart.01195.2007. [DOI] [PubMed] [Google Scholar]
- 83.Li G-R, Sun H-Y, Chen J-B, Zhou Y, Tse H-F, Lau C-P. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One. 2009;4:e7307. doi: 10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res. 2003;57:793–803. doi: 10.1016/S0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 85.Kamkin A, Kiseleva I, Isenberg G, Wagner KD, Günther J, Theres H, et al. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog Biophys Mol Biol. 2003;82:111–20. doi: 10.1016/S0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 86.Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol. 2007;580:255–74. doi: 10.1113/jphysiol.2006.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4α-phorbol 12,13-didecanoate. Life Sci. 2009;85:808–14. doi: 10.1016/j.lfs.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Chatelier A, Mercier A, Tremblier B, Thériault O, Moubarak M, Benamer N, et al. A distinct de novo expression of Nav1. 5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol. 2012;590:4307–19. doi: 10.1113/jphysiol.2012.233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Driesen RB, Dispersyn GD, Verheyen FK, Van Den Eijnde SM, Hofstra L, Thoné F, et al. Partial cell fusion: A newly recognized type of communication between dedifferentiating cardiomyocytes and fibroblasts. Cardiovasc Res. 2005;68:37–46. doi: 10.1016/j.cardiores.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 90.Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:13243. doi: 10.1038/srep13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Askar SF, Bingen BO, Swildens J, Ypey DL, Van Der Laarse A, Atsma DE, et al. Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: Role of maximal diastolic potential. Cardiovasc Res. 2012;93:434–44. doi: 10.1093/cvr/cvr351. [DOI] [PubMed] [Google Scholar]
- 92.Hou L, Hu B, Jalife J. Genetically engineered excitable cardiac myofibroblasts coupled to cardiomyocytes rescue normal propagation and reduce arrhythmia complexity in heterocellular monolayers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiseleva I, Kamkin A, Kohl P, Lab MJ. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc Res. 1996;32:98–111. doi: 10.1016/S0008-6363(96)00047-8. [DOI] [PubMed] [Google Scholar]
- 94.Kamkin A, Kiseleva I, Wagner KD, Lammerich A, Bohm J, Persson PB, et al. Mechanically induced potentials in fibroblasts from human right atrium. Exp Physiol. 1999;84:347–56. [PubMed] [Google Scholar]
- 95.Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation. 2011;123:2083–93. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, et al. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol. 2014;115:173–85. doi: 10.1016/j.pbiomolbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–34. doi: 10.1172/JCI74783.types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–35. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 99.Ruiz-Villalba A, Simón AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–66. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 100.van Amerongen M, Bou-Gharis G, Popa E, van Ark J, Petersen A, van Dam G, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–86. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 101.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol. 2014;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985;56:436–51. doi: 10.1161/01.RES.56.3.436. [DOI] [PubMed] [Google Scholar]
- 103.El-Sherif N, Mehra R, Gough WB, Zeiler RH. Ventricular activation patterns of spontaneous and induced ventricular rhythms in canine one-day-old myocardial infarction. Evidence for focal and reentrant mechanisms. Circ Res. 1982;51:152–66. doi: 10.1161/01.RES.51.2.152. [DOI] [PubMed] [Google Scholar]
- 104.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 2010;107:1011–20. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol. 2011;57:380–8. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol. 2010;19:e233–40. doi: 10.1016/j.carpath.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jansen JA, van Veen TAB, de Jong S, van der Nagel R, van Stuijvenberg L, Driessen H, et al. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythmia Electrophysiol. 2012;5:380–90. doi: 10.1161/CIRCEP.111.966580. [DOI] [PubMed] [Google Scholar]
- 109.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng. 2008;36:41–56. doi: 10.1007/s10439-007-9405-8. [DOI] [PubMed] [Google Scholar]
- 110.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: Is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 111.Lefroy DC, Fang JC, Stevenson LW, Hartley LH, Friedman PL, Stevenson WG. Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: Surface electrocardiographic features and estimated prevalence. Am J Cardiol. 1998;82:444–50. doi: 10.1016/S0002-9149(98)00359-2. [DOI] [PubMed] [Google Scholar]
- 112.Vadakkumpadan F, Arevalo H, Prassl AJ, Chen J, Kickinger F, Kohl P, et al. Image-based models of cardiac structure in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2010;2:489–506. doi: 10.1002/wsbm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pop M, Sermesant M, Liu G, Relan J, Mansi T, Soong A, et al. Construction of 3D MR image-based computer models of pathologic hearts, augmented with histology and optical fluorescence imaging to characterize action potential propagation. Med Image Anal. 2012;16:505–23. doi: 10.1016/j.media.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Quinn TA, Kohl P. Combining wet and dry research: experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc Res. 2013;97:601–11. doi: 10.1093/cvr/cvt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agullo-Pascual E, Reid DA, Keegan S, Sidhu M, Fenyö D, Rothenberg E, et al. Super-resolution fluorescence microscopy of the cardiac connexome reveals plakophilin-2 inside the connexin43 plaque. Cardiovasc Res. 2013;100:231–40. doi: 10.1093/cvr/cvt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–80. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kohl T, Westphal V, Hell SW, Lehnart SE. Superresolution microscopy in heart - Cardiac nanoscopy. J Mol Cell Cardiol. 2013;58:13–21. doi: 10.1016/j.yjmcc.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 118.Hou Y, Crossman DJ, Rajagopal V, Baddeley D, Jayasinghe I, Soeller C. Super-resolution fluorescence imaging to study cardiac biophysics: α-actinin distribution and Z-disk topologies in optically thick cardiac tissue slices. Prog Biophys Mol Biol. 2014;115:328–39. doi: 10.1016/j.pbiomolbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang H-Z, et al. Stimulating cardiac muscle by light: Cardiac optogenetics by cell delivery. Circ Arrhythmia Electrophysiol. 2011;4:753–60. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hegemann P, Nagel G. From channelrhodopsins to optogenetics. EMBO Mol Med. 2013;5:173–6. doi: 10.1002/emmm.201202387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Entcheva E. Cardiac optogenetics. Am J Physiol - Hear Circ Physiol. 2013;304:H1179–91. doi: 10.1152/ajpheart.00432.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quinn TA, Camelliti P, Siedlecka U, Poggioli T, Loew LM, Knopfel T, et al. Abstract 11749: Cell-specific expression of voltage-sensitive protein confirms cardiac myocyte to non-myocyte electrotonic coupling in healed murine infarct border tissue. Circulation. 2014;130:A11749. [Google Scholar]
- 123.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–24. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 124.Yankelson L, Feld Y, Bressler-Stramer T, Itzhaki I, Huber I, Gepstein A, et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–31. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 125.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Vriese AS, Van de Voorde J, Lameire NH. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61:177–85. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 127.Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–52. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 128.Hawat G, Hélie P, Baroudi G. Single intravenous low-dose injections of connexin 43 mimetic peptides protect ischemic heart in vivo against myocardial infarction. J Mol Cell Cardiol. 2012;53:559–66. doi: 10.1016/j.yjmcc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 129.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ongstad EL, O’Quinn MP, Ghatnekar GS, Yost MJ, Gourdie RG. A connexin43 mimetic peptide promotes regenerative healing and improves mechanical properties in skin and heart. Adv Wound Care. 2013;2:55–62. doi: 10.1089/wound.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 133.Gourdie RG, Dimmeler S, Kohl P. Novel Therapeutic Strategies Targeting Fibroblasts and Fibrosis in Heart Disease. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.89. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang M, Baranov E, Moossa AR, Penman S, Hoffman RM. Visualizing gene expression by whole-body fluorescence imaging. Proc Natl Acad Sci. 2000;97:12278–82. doi: 10.1073/pnas.97.22.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther. 2006;14:514–24. doi: 10.1016/j.ymthe.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2005;27:1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 137.Malecki M, Putzer E, Sabo C, Foorohar A, Quach C, Stampe C, et al. Directed cardiomyogenesis of autologous human induced pluripotent stem cells recruited to infarcted myocardium with bioengineered antibodies. Mol Cell Ther. 2014;2:13. doi: 10.1186/2052-8426-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vandergriff AC, de Andrade JBM, De Tang J, Hensley MT, Piedrahita JA, Caranasos TG, et al. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015;2015:1–8. doi: 10.1155/2015/960926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol. 2011;50:841–8. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Won Y-W, McGinn AN, Lee M, Bull Da, Kim SW. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Mol Pharm. 2013;10:378–85. doi: 10.1021/mp300500y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clarke SA, Richardson WJ, Holmes JW. Modifying the mechanics of healing infarcts: Is better the enemy of good? J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kohl P. Structural and functional recoupling of atrial and ventricular myocardium. J Am Coll Cardiol. 2014;64:2586–8. doi: 10.1016/j.jacc.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 145.Cingolani E, Ionta V, Cheng K, Giacomello A, Cho HC, Marbán E. Engineered electrical conduction tract restores conduction in complete heart block. J Am Coll Cardiol. 2014;64:2575–85. doi: 10.1016/j.jacc.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]