Abstract
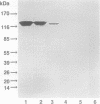
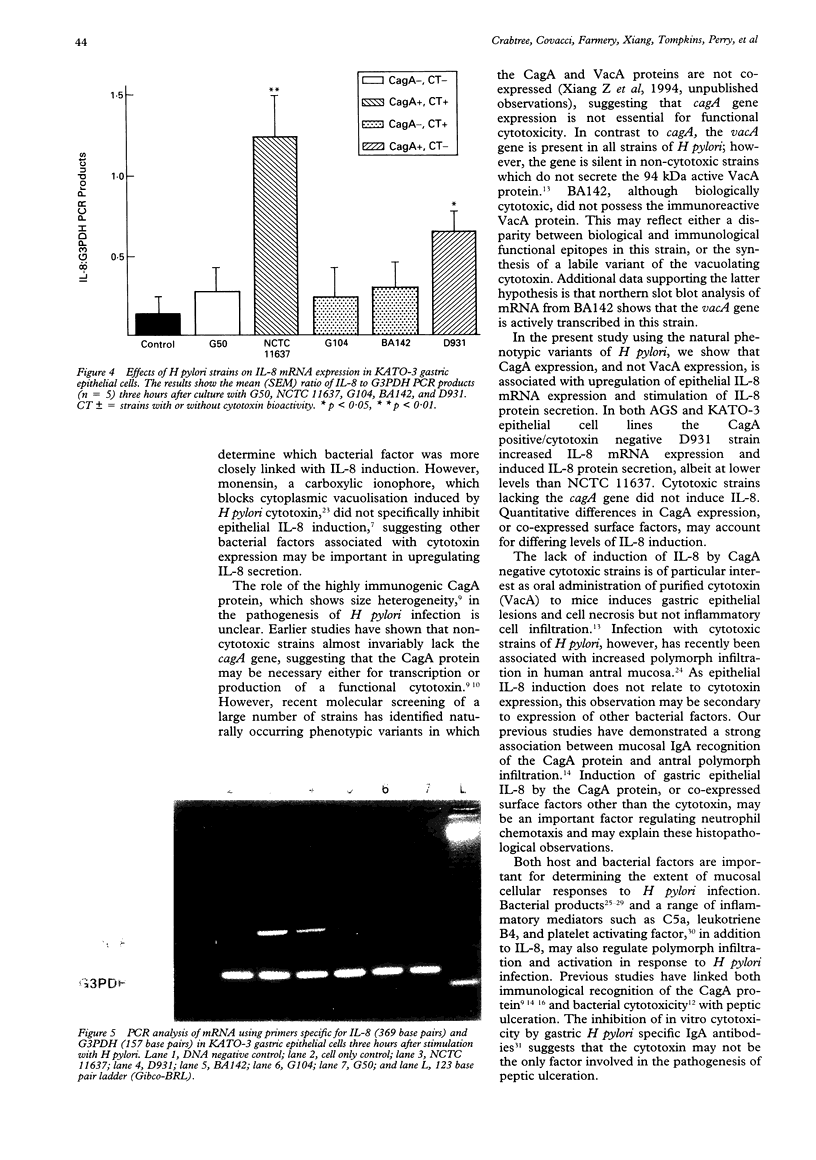
AIMS--To use a range of natural phenotypically variant strains of Helicobacter pylori with disparate CagA and VacA (vacuolating cytotoxin) expression to determine which bacterial factors are more closely associated with epithelial interleukin-8 (IL-8) induction. METHODS--Gastric epithelial cells (AGS and KATO-3) were co-cultured with five H pylori strains which were variously shown to express the cagA gene/CagA protein, VacA and/or to exhibit biological cytotoxicity. Secreted IL-8 was assayed by enzyme leaked immunosorbent assay (ELISA) and IL-8 messenger RNA (mRNA) was assayed using a reverse transcription polymerase chain reaction based technique (RT-PCR). RESULTS--Strains expressing CagA, including a variant strain (D931) which is non-cytotoxic and does not express the VacA protein, were found to upregulate epithelial IL-8 secretion and gene expression. In contrast, strains with no CagA expression, even in the presence of VacA and/or biological cytotoxicity, (G104, BA142), failed to induce IL-8 protein or mRNA above control values. CONCLUSIONS--These results strongly support a role for H pylori CagA or coexpressed factors other than the cytotoxin in upregulation of gastric epithelial IL-8. Increased epithelial IL-8 secretion and concomitant neutrophil chemotaxis and activation in addition to direct cytotoxicity may be an important factor in tissue damage and ulceration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agace W. W., Hedges S. R., Ceska M., Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993 Aug;92(2):780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace W., Hedges S., Andersson U., Andersson J., Ceska M., Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993 Feb;61(2):602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Clayton C. L., Kleanthous H., Coates P. J., Morgan D. D., Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Cao P., Lind C. D., Tham K. T., Blaser M. J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993 Dec;61(12):5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Vaughn S. G., Cao P., Blaser M. J. Potentiation of Helicobacter pylori vacuolating toxin activity by nicotine and other weak bases. J Infect Dis. 1992 Nov;166(5):1073–1078. doi: 10.1093/infdis/166.5.1073. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Figura N., Taylor J. D., Bugnoli M., Armellini D., Tompkins D. S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992 Aug;45(8):733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Peichl P., Wyatt J. I., Stachl U., Lindley I. J. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993 Jan;37(1):65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Wyatt J. I., Trejdosiewicz L. K., Peichl P., Nichols P. H., Ramsay N., Primrose J. N., Lindley I. J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994 Jan;47(1):61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P. M., Territo M. C., Karnes W. E., Walsh J. H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992 Aug;33(8):1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M. F., Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993 Nov;61(11):4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Armellini D., Bugnoli M., Bayeli P. F., Gennari C., Crabtree J. E. Activity of omeprazole on Helicobacter pylori and relation to toxicity of strains. J Clin Pathol. 1994 May;47(5):440–442. doi: 10.1136/jcp.47.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert M. Q., Herrmann R., Schaller H. Surface proteins of Mycoplasma hyopneumoniae identified from an Escherichia coli expression plasmid library. Infect Immun. 1985 Aug;49(2):329–335. doi: 10.1128/iai.49.2.329-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. H pylori-initiated ulcerogenesis: look to the host. Lancet. 1993 Jan 30;341(8840):280–281. doi: 10.1016/0140-6736(93)92624-3. [DOI] [PubMed] [Google Scholar]
- Lindley I., Aschauer H., Seifert J. M., Lam C., Brunowsky W., Kownatzki E., Thelen M., Peveri P., Dewald B., von Tscharner V. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: biological equivalence between natural and recombinant neutrophil-activating factor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Allen J. B., Wahl S. M., Blaser M. J., Smith P. D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992 Feb 1;175(2):517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992 Dec;103(6):1747–1753. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992 Jun;33(6):738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Blomberg B., Fredlund H., Järnerot G., Danielsson D. Incidence of Helicobacter pylori strains activating neutrophils in patients with peptic ulcer disease. Gut. 1993 May;34(5):599–603. doi: 10.1136/gut.34.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E. Genetics of Campylobacter and Helicobacter. Annu Rev Microbiol. 1992;46:35–64. doi: 10.1146/annurev.mi.46.100192.000343. [DOI] [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]