ABSTRACT
C. elegans spe-9 class genes are male germline-enriched in their expression and indispensable during sperm-oocyte fusion. Identification of mammalian orthologs that exhibit similar functions to these C. elegans genes has been a challenge. The mouse Izumo1 gene encodes a sperm-specific, immunoglobulin (Ig)-like transmembrane (TM) protein that is required for gamete fusion. We recently identified the C. elegans spe-45 gene, which shows male germline-enriched expression and encodes an Ig-like TM protein. spe-45 mutant worms produced otherwise normal spermatozoa that cannot fuse with oocytes, causing essentially the same phenotype as that seen in the Izumo1-knockout mice. By counting the number of self-sperm in the spermatheca of spe-45 hermaphrodites, it was found that this gene might be involved in sperm guidance from the uterus into the spermatheca, as well as gamete fusion. Moreover, we discovered that SPE-45 and IZUMO1 share certain functions for gamete fusion, which are presumably related to binding with cis- and/or trans-partners. Intriguingly, various organisms have Ig-like TM proteins that act during gamete interactions, indicating the wide-spread utility of Ig-like domains during fertilization.
KEYWORDS: C. elegans, evolution, fertilization, immunoglobulin-like domain, IZUMO1, male germline, mouse, SPE-45, SPE-9 class protein, sperm, transmembrane protein
Introduction
Fertilization is a key feature of sexual reproduction in eukaryotes. In mammals, such as the mouse, fertilization consists of multiple sperm interactions with the cumulus cell layer, zona pellucida and oocyte plasma membrane (Fig. 1, left).1 The proteins thought to be important for these interactions evolve rapidly, likely in order to prevent the production of cross-species hybrids.2-4 Thus, even if there is a conserved mechanism(s) among evolutionary distant species that are required for sperm-oocyte interactions during fertilization, it might be difficult to identify it by homology-based searches.
Figure 1.
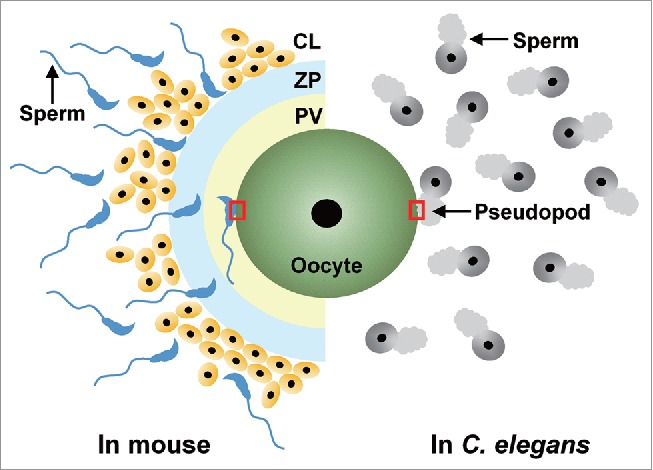
Schematic comparison of fertilization in the mouse and C. elegans. (Left) In the mouse, a sperm must pass through the cumulus cell layer (CL, cells are yellow with a black nucleus) and the zona pellucida (ZP, pale blue) for entry into the perivilelline space (PV, light yellow), where the oocyte plasma membrane is exposed. The sperm becomes acrosome-reacted prior to contacting with the ZP,34 and gamete fusion occurs on the equatorial segment of the acrosome-reacted sperm1 (shown by a red square). (Right) Since a C. elegans oocyte lacks any types of accessory cells and egg coats such as the CL and ZP, respectively, a sperm directly binds to and fuses with the oocyte plasma membrane through the pseudopod5,6 (red square).
Caenorhabditis elegans has several advantages as a model organism for studying sperm-oocyte fusion.5,6 For example, a C. elegans oocyte lacks accessory cells and a thick egg coat, unlike mammalian oocytes (Fig. 1, right), so that the fusogenic reaction in C. elegans can be examined more simply than those in mammals. Also, four spe-9 class genes (spe-9,7-9 spe-38,10,11 spe-41/trp-311,12 and spe-4213,14) are expressed specifically or predominantly in the C. elegans male germline and required during fertilization (in other words, “during gamete fusion”). Human and/or mouse homologs of these C. elegans genes can be predicted according to the domain architectures of each SPE-9 class protein (Table 1), but it is unknown whether those mammalian homologs are functionally related to the C. elegans spe-9 class genes.
Table 1.
C. elegans spe-9 class genes.
| Gene | TM1 | Domain2/Feature | Localization | Function | Ortholog3 | Reference |
|---|---|---|---|---|---|---|
| spe-9 | 1 | EGF (10) | PM of spermatid | Involvement in later step | DLL1 (h) | 7-9 |
| Pseudopod of sperm | post gamete binding | DLL3 (h) | ||||
| DLL4 (h) | ||||||
| spe-38 | 4 | No clear domain | MOs of spermatid | Regulation of SPE-41/TRP-3 relocation during spermiogenesis | Not found in mammals | 10,11 |
| MOs and PM of sperm | ||||||
| spe-41/trp-3 | 6 | TRPC family | MOs of spermatid | Regulation of Ca2+-influx during fertilization | TRPC6 (h) | 11,12 |
| PM of sperm | Trpc6 (m) | |||||
| spe-42 | 6 | DC-STAMP (1) | Not determined | Involvement of gamete binding | DCTS2 (h) | 13,14 |
| RING finger (1) | Dcts1 (m) | |||||
| spe-45 | 1 | Ig (1) | Not determined | Involvement in later step post gamete binding | Izumo1 (m) | 15.17 |
Notes. Abbreviations used are as follows: TM, transmembrane; EGF, epidermal growth factor; TRPC, transient receptor potential-canonical; DC-STAMP, dendritic cell-specific transmembrane protein; Ig, immunoglobulin; PM, plasma membrane; MO, membranous organelles that are analogous to the acrosome;35 DLL, Delta-like; DCST, DC-STAMP domain containing.
The number of TM domains in each predicted protein.
Protein domains that are present in each predicted protein besides the TM domain. The number of those domains is shown in parentheses.
Human (h) and/or mouse (m) orthologous genes were predicted by WormBase (www.wormbase.org), except for Izumo1, on the basis of their nucleotide and/or predicted protein sequences.
By a reverse genetic approach, we identified C. elegans spe-45, which has expression confined to the male germline and encodes a transmembrane (TM) protein with a single immunoglobulin (Ig)-like domain (Fig. 2).15 Our study showed that spe-45 is a functional ortholog of Izumo1, a mouse gene encoding a sperm Ig-like TM protein required during gamete fusion.16 Singaravelu et al. also identified the spe-45 gene in a forward genetic screen for spe-9 class mutants.17
Figure 2.
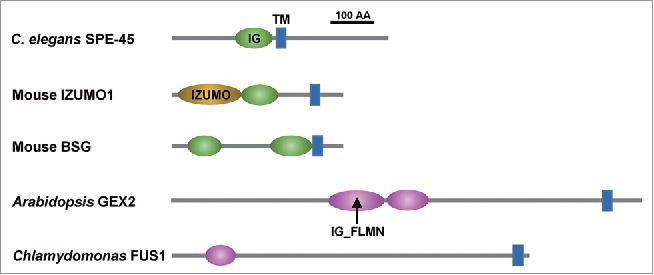
Domain architectures of immunoglobulin-like transmembrane proteins that are involved during gamete interactions in diverged organisms.Transmembrane (TM, blue box) proteins with the immunoglobulin (IG)-like (green) or IG-like folded filamin (IG_FLMN, purple) domains from a variety of species, which play important roles in gamete interactions. A black bar represents a 100-amino acid (AA) stretch. IZUMO, IZUMO domain.
In this commentary, we review these data and discuss the conserved roles of TM proteins containing Ig-like domains during gamete interactions in diverse organisms.
spe-45 is a novel spe-9 class gene
Our work is guided by the hypothesis that, despite obvious differences in sperm and egg appearance (compare the left and right halves of Figure 1), gamete fusion during fertilization in mammals will have conserved features shared with C. elegans. If any mammalian orthologs of the C. elegans spe-9 class genes act during gamete fusion, we can predict that an ortholog of the mouse Izumo1–like gene will play a key role during C. elegans fertilization. This hypothesis triggered our recent work.15 Mouse IZUMO1 contains one Ig-like and one TM domain (Fig. 2)16 and, guided by these structural features, we identified C. elegans F28D1.8 as a candidate gene that showed male germline-enriched expression.15
The tm3715 deletion mutation of F28D1.8 caused hermaphrodites to be self-sterile because of a defect(s) in either or some of male germline functions (therefore, F28D1.8 was renamed spe-45).15,17 However, spe-45(tm3715) mutant males produced spermatids with wild-type-like cytology, number and activation rate into spermatozoa.15,17 A wild-type (N2) adult hermaphrodite has ∼300 self-sperm right after the L4 stage and nearly all are sequentially consumed by fertilization, resulting in disappearance of sperm from the spermatheca by 72 h post L4 stage. In contrast, spe-9 class mutant hermaphrodites that were at 72 h post L4 stage retained ∼52% (spe-9), ∼100% (spe-42) or ∼63% (spe-45) of the spermatozoa observed right after the L4 stage.15 Like the other spe-9 class mutants spe-97,9 and spe-42,13,14 spe-45(tm3715) laid very few fertilized eggs,15,17 consistent with the failure of mutant self-sperm in the spermatheca to participate in fertilization.
These data suggest that the following distinctive defects characterize spe-9 class mutants: spe-9 and spe-45 sperm can bind to oocytes, but fail to undergo later steps during gamete fusion in the spermatheca. These unfertilized oocytes, with sperm bound to their surface, are squeezed through the spermathecal valve and ejected into the uterus. In some cases, the mutant sperm remain bound to oocytes when they enter the uterus. However, ejection into the uterus involves the spermathecal valve being swept over the oocyte surface and this frequently dislodges bound sperm. These now-free sperm are attracted to and subsequently crawl back into the spermatheca where they await the next oocyte. In contrast, spe-42 sperm seem to be incapable of binding to the oocyte plasma membrane. Izumo1-deficient mouse spermatozoa cannot fuse with oocytes despite successful sperm binding to the oocyte plasma membrane,16 which is essentially the same phenotype as C. elegans spermatozoa that lack SPE-45.
Another possibility is that spe-9 and/or spe-45 might be required for sperm guidance, in addition to their roles in gamete fusion. F-series prostaglandins that are synthesized from yolk lipoprotein complex-derived unsaturated fatty acids act as sperm guidance factors. Therefore, when genes involved in the synthesis are mutated, spermatozoa become partly defective in their relocation from the uterus into the spermatheca.18-20 These phenotypes seem similar to those of spe-9 and spe-45 mutants, resulting in the incomplete sperm recruitment into the spermatheca. Currently the sperm prostaglandin-receptor(s) is not known, but SPE-9 and/or SPE-45 could function in the prostaglandin binding to spermatozoa and/or subsequent sperm reaction(s).
SPE-45 and IZUMO1 share a common function through their Ig-like domains
To examine if SPE-45 and IZUMO1 are functionally related to each other, we constructed a transgene encoding chimeric SPE-45, where the native Ig-like domain was removed and replaced by the mouse IZUMO1 Ig-like domain. When the transgene was expressed in spe-45(tm3715) hermaphrodites, the self-sterility of the mutant was rescued to ∼77% of the level observed for the wild-type transgene.15 To our knowledge, this finding was the first to show that two genes involved in fertilization are functionally orthologous to each other, even though they are evolutionally distant (∼900 million years)21 and show a low level of amino acid identity (8.7%).15
The precise roles played by these Ig-like domains in IZUMO1 and SPE-45, however, are currently unknown. Sperm IZUMO1 is known to bind to egg JUNO, a glycosylphosphatidylinositol (GPI)-anchored folate receptor-like protein.22-24 This binding is indispensable for sperm-oocyte fusion in mice, and the IZUMO domain of IZUMO1 (Fig. 2) is required for the gamete interaction.22-24 Moreover, Inoue et al. suggested the presence of a secondary IZUMO1 receptor on the oocyte surface that possibly acts after IZUMO1 binding to JUNO.24 Therefore, SPE-45's Ig-like domain might indirectly act in binding to an oocyte SPE-45 receptor or participate in a later step(s) after the primary ligand-receptor binding during gamete fusion.
It seems probable that the SPE-45, like IZUMO1, interacts with a receptor on the oocyte surface and, perhaps, the Ig-like domain plays a role. Since C. elegans seems to have no genes encoding mouse JUNO-like proteins,25 GPI-anchored proteins or folate-related proteins such as FOLT (folate transporter family) proteins, which contain multiple TM domains, might be alternative candidates of the oocyte-resident receptor.
Ig-like domains play key roles during gamete interactions in various organisms
Besides C. elegans SPE-45 and mouse IZUMO1, other Ig-like TM proteins from a variety of organisms have been found to participate in gamete interactions (Fig. 2). The mutant mouse line BART97b, where the Spaca6 gene encoding an Ig-like TM protein is deleted, produces spermatozoa that appear to be incapable of fusing with the oocyte plasma membrane.26 Another mouse example is the Bsg gene that encodes a single-pass TM protein containing two Ig-like domains. Both male and female Bsg-knockout mice are sterile and, in males, spermatogenesis is arrested.27 Later work revealed that the BSG protein also acts during sperm interactions with the cumulus cells and the zona pellucida.28 Arabidopsis and Chlamydomonas have GEX229,30 and FUS1,31,32 respectively, both of which are single-pass TM proteins with Ig-like filamin repeat domains. These two proteins are involved in gamete fusion or attachment.
As in the case of SPE-45 and IZUMO1, it remains unclear how Ig-like domains found in the mouse, plant and algal proteins are involved in gamete interactions. However, the Hydractinia (a cnidarian) Alr1 and Alr2 genes might provide clues to the functions of Ig-like domains during fertilization. These two genes encode TM proteins with multiple Ig-like domains that play critical roles in recognition of self- versus non-self (allo-recognition) through trans-homotypic interactions of Alr proteins.33 Perhaps the other Ig-like TM proteins shown in Figure 2, like the Alr proteins, associate with the same and/or other TM proteins containing Ig-like domains to form cis- and/or trans-protein complexes that are important for fertilization. For example, on the mouse sperm surface, IZUMO1 and SPACA6 might interact with each other, although any functional relationship between them is presently unclear. Since the C. elegans genome has both male and female germline-specifically or predominantly expressed genes encoding Ig-like TM proteins besides spe-45,15 there might be a SPE-45 partner(s) with such the domain architecture on the surface of spermatozoa and/or oocytes.
Abbreviations
- TM
transmembrane
- Ig
immunoglobulin
- GPI
glycosylphosphatidylinositol.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by MEXT KAKENHI Grant Number 24112716 and JSPS KAKENHI Grant Numbers 23870029 and 24570241 to H.N., Japan. We were also supported by grants from the NSF IOB-0544180, NIH GM082932 and NIH HD066577 and by funds from Emory College to S.W.L., USA.
References
- [1].Florman HM, Fissore RA. Knobil and Neill's Physiology of Reproduction. 4th ed. Amsterdam, Netherlands: Elsevier Academic Press, c2014. Chapter 4, Fertilization in mammals; 149-96. [Google Scholar]
- [2].Wyckoff GJ, Wang W, Wu CI. Rapid evolution of male reproductive genes in the descent of man. Nature 2007; 403:304-9. [DOI] [PubMed] [Google Scholar]
- [3].Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet 2002; 3:137-44; PMID:11836507; http://dx.doi.org/ 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- [4].Haerty W, Jagadeeshan H, Kulathinal RJ, Wong A, Ravi Ram K, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A, Singh RS. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 2007; 177:1321-35; PMID:18039869; http://dx.doi.org/ 10.1534/genetics.107.078865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nishimura H, L'Hernault SW. Spermatogenesis-defective (spe) mutants of the nematode Caenorhabditis elegans provide clues to solve the puzzle of male germline functions during reproduction. Dev Dyn 2010; 239:1502-14; PMID:20419782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol 2013; 757:321-50; PMID:22872482; http://dx.doi.org/ 10.1007/978-1-4614-4015-4_11 [DOI] [PubMed] [Google Scholar]
- [7].Singson A, Mercer KB, L'Hernault SW. The C. elegans spe-9 gene encodes a sperm transmembrane protein that contains EGF-like repeats and is required for fertilization. Cell 1998; 93:71-9; PMID:9546393; http://dx.doi.org/ 10.1016/S0092-8674(00)81147-2 [DOI] [PubMed] [Google Scholar]
- [8].Zannoni S, L'Hernault SW, Singson AW. Dynamic localization of SPE-9 in sperm: a protein required for sperm-oocyte interactions in Caenorhabditis elegans. BMC Dev Biol 2003; 3:10; PMID:14653860; http://dx.doi.org/ 10.1186/1471-213X-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Putiri E, Zannoni S, Kadandale P, Singson A. Functional domains and temperature-sensitive mutations in SPE-9, an EGF repeat-containing protein required for fertility in Caenorhabditis elegans. Dev Biol 2004; 272:448-59; PMID:15282160; http://dx.doi.org/ 10.1016/j.ydbio.2004.05.014 [DOI] [PubMed] [Google Scholar]
- [10].Chatterjee I, Richmond A, Putiri E, Shakes DC, Singson A. The Caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development 2005; 132:2795-808; PMID:15930110; http://dx.doi.org/ 10.1242/dev.01868 [DOI] [PubMed] [Google Scholar]
- [11].Singaravelu G, Chatterjee I, Rahimi S, Druzhinina MK, Kang L, Xu XZ, Singson A. The sperm surface localization of the TRP-3/SPE-41 Ca2+-permeable channel depends on SPE-38 function in Caenorhabditis elegans. Dev Biol 2012; 365:376-83; PMID:22425620; http://dx.doi.org/ 10.1016/j.ydbio.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu XZ, Sternberg PW. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell 2003; 114:285-97; PMID:12914694; http://dx.doi.org/ 10.1016/S0092-8674(03)00565-8 [DOI] [PubMed] [Google Scholar]
- [13].Kroft TL, Gleason EJ, L'Hernault SW. The spe-42 gene is required for sperm-egg interactions during C. elegans fertilization and encodes a sperm-specific transmembrane protein. Dev Biol 2005; 286:169-81; PMID:16120437; http://dx.doi.org/ 10.1016/j.ydbio.2005.07.020 [DOI] [PubMed] [Google Scholar]
- [14].Wilson LD, Sackett JM, Mieczkowski BD, Richie AL, Thoemke K, Rumbley JN, Kroft TL. Fertilization in C. elegans requires an intact C-terminal RING finger in sperm protein SPE-42. BMC Dev Biol 2011; 11:10; PMID:21345212; http://dx.doi.org/ 10.1186/1471-213X-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nishimura H, Tajima T, Comstra HS, Gleason EJ, L'Hernault SW. The immunoglobulin-like gene spe-45 acts during fertilization in Caenorhabditis elegans like the mouse Izumo1 gene. Curr Biol 2015; 25:3225-31; PMID:26671669; http://dx.doi.org/ 10.1016/j.cub.2015.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434:234-8; PMID:15759005; http://dx.doi.org/ 10.1038/nature03362 [DOI] [PubMed] [Google Scholar]
- [17].Singaravelu G, Rahimi S, Krauchunas A, Rizvi A, Dharia S, Shakes D, Smith H, Golden A, Singson A. Forward genetics identifies a requirement for the Izumo-like immunoglobulin superfamily spe-45 gene in Caenorhabditis elegans fertilization. Curr Biol 2015; 25:3220-4; PMID:26671668; http://dx.doi.org/ 10.1016/j.cub.2015.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol 2006; 8:1143-8; PMID:16998478; http://dx.doi.org/ 10.1038/ncb1476 [DOI] [PubMed] [Google Scholar]
- [19].Hoang HD, Prasain JK, Dorand D, Miller MA. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet 2013; 9:e1003271; PMID:23382703; http://dx.doi.org/ 10.1371/journal.pgen.1003271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McKnight K, Hoang HD, Prasain JK, Brown N, Vibbert J, Hollister KA, Moore R, Ragains JR, Reese J, Miller MA. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science 2014; 344:754-7; PMID:24833393; http://dx.doi.org/ 10.1126/science.1250598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blaxter M. The timetree of life. New York: Oxford University Press, c2009. Nematodes (nematoda); 247-50. See also www.timetree.org [Google Scholar]
- [22].Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014; 508:483-7; PMID:24739963; http://dx.doi.org/ 10.1038/nature13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Inoue N, Hamada D, Kamikubo H, Hirata K, Kataoka M, Yamamoto M, Ikawa M, Okabe M, Hagihara Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013; 140:3221-9; PMID:23824580; http://dx.doi.org/ 10.1242/dev.094854 [DOI] [PubMed] [Google Scholar]
- [24].Inoue N, Hagihara Y, Wright D, Suzuki T, Wada I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat Commun 2015; 6:8858; PMID:26568141; http://dx.doi.org/ 10.1038/ncomms9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grayson P. Izumo1 and Juno: the evolutionary origins and coevolution of essential sperm-egg binding partners. R Soc Open Sci 2015; 2:150296; PMID:27019721; http://dx.doi.org/ 10.1098/rsos.150296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lorenzetti D, Poirier C, Zhao M, Overbeek PA, Harrison W, Bishop CE. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm-egg fusion. Mamm Genome 2014; 25:141-8; PMID:24275887; http://dx.doi.org/ 10.1007/s00335-013-9491-x [DOI] [PubMed] [Google Scholar]
- [27].Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 1998; 194:152-65; PMID:9501026; http://dx.doi.org/ 10.1006/dbio.1997.8819 [DOI] [PubMed] [Google Scholar]
- [28].Saxena DK, Oh-Oka T, Kadomatsu K, Muramatsu T, Toshimori K. Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction 2002; 123:435-44; PMID:11882021; http://dx.doi.org/ 10.1530/rep.0.1230435 [DOI] [PubMed] [Google Scholar]
- [29].Mori T, Igawa T, Tamiya G, Miyagishima SY, Berger F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr Biol 2014; 24:170-5; PMID:24388850; http://dx.doi.org/ 10.1016/j.cub.2013.11.030 [DOI] [PubMed] [Google Scholar]
- [30].Mori T, Igawa T. Gamete attachment process revealed in flowering plant fertilization. Plant Signal Behav 2014; 9:e977715; PMID:25517689; http://dx.doi.org/ 10.4161/15592324.2014.977715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ferris PJ, Woessner JP, Goodenough UW. A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell 1996; 7:1235-48; PMID:8856667; http://dx.doi.org/ 10.1091/mbc.7.8.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Misamore MJ, Gupta S, Snell WJ. The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol Biol Cell 2003; 14:2530-42; PMID:12808049; http://dx.doi.org/ 10.1091/mbc.E02-12-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Karadge UB, Gosto M, Nicotra ML. Allorecognition proteins in an invertebrate exhibit homophilic interactions. Curr Biol 2015; 25:2845-50; PMID:26455308; http://dx.doi.org/ 10.1016/j.cub.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A 2011; 108:4892-6; PMID:21383182; http://dx.doi.org/ 10.1073/pnas.1018202108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gleason EJ, Hartley PD, Henderson M, Hill-Harfe KL, Price PW, Weimer RM, Kroft TL, Zhu GD, Cordovado S, L'Hernault SW. Developmental genetics of secretory vesicle acidification during Caenorhabditis elegans spermatogenesis. Genetics 2012; 191:477-91; PMID:22446317; http://dx.doi.org/ 10.1534/genetics.112.139618 [DOI] [PMC free article] [PubMed] [Google Scholar]


