Abstract
Herein, we report a novel design and the antimicrobial efficacy of a flexible nitric oxide (NO) releasing patch for potential wound healing applications. The compact sized polydimethylsiloxane (PDMS) planar patch generates NO via electrochemical reduction of nitrite ions mediated by a copper(II)-ligand catalyst using a portable power system and an internal gold coated stainless steel mesh working electrode. Patches are fabricated via soft lithography and 3-D printing. The devices can continuously release NO over 4 days and exhibit potent bactericidal effects on both Escherichia coli and Staphylococcus aureus. The device may provide an effective, safe, and less costly alternative for treating chronic wounds.
Keywords: nitric oxide release, antimicrobial patch, electrochemical devices, portable planar NO generation, 3D printing
The normal wound healing process involves inflammatory, proliferative, and remodeling phases under the regulation of immune cells. However, chronic wounds fail to progress in the normal time frame, and therefore enter a phase of prolonged pathologic conditions, including chronic infection. Although the treatment for chronic wounds often employs topical antimicrobials as well as systemic antibiotics, the high incidence of drug-resistant strains of bacteria and reduced regional circulation limits the therapeutic effect of these conventional approaches to treating chronic wounds.1−3
In vivo, endogenous production of nitric oxide (NO) plays a dual role as both a key agent in signaling and as a cytotoxic antimicrobial agent.4−6 Indeed, NO serves as a regulatory molecule for numerous processes in the immune, nervous and cardiovascular systems. Nitric oxide stimulates pro-inflammatory cytokines, keratinocytes, and fibroblasts, and controls angiogenesis, collagen deposition and re-epithelialization. In addition, NO has been shown to be an important component of the host response during infection at a wound site.7,8 The antimicrobial properties of NO include serving as a signaling molecule capable of dispersing microbial biofilm9 on surfaces (including medical devices such as catheters10) and possessing bactericidal activity against most strains of bacteria,11 both Gram-positive and Gram-negative.12 Lower than normal levels of endogenous NO are also associated with impaired wound healing which can lead to chronic wounds.13 Accordingly, the addition of exogenous topical NO has the potential to enhance the wound healing process, serving as both an antimicrobial agent and also exhibiting significant pro-angiogenic properties (i.e., promoting formation of new blood vessels).14−16
A variety of NO donors have been studied to create NO-releasing polymers/materials for potential use in wound healing applications.17 Nitric oxide donor molecules, such as diazeniumdiolates and S-nitrosothiols, have been incorporated into various polymers or covalently linked to polymeric chains to enable continuous secretion of NO in the presence of heat, light or metal ions.18 These new polymeric materials have been utilized to reduce thrombosis (clotting) on blood contacting biomedical devices, and microbial biofilm/infection on intravascular and urinary catheters.19,20 However, the limited NO reservoir from NO donors and their instability at elevated temperatures or upon exposure to moisture or light during storage results in a high cost for any commercial product based on existing technology in this area.
Recently, Ren et al. developed an electrochemically modulated NO releasing catheter and successfully demonstrated that a significant reduction in the bacterial biofilm formation occurs on the surface in a drip-flow bioreactor inoculated with E. coli.10 Within the lumen of the catheter, a copper(II)-tri(2-pyridylmethyl)amine (CuTPMA) complex was used as a mediator for the electrochemical reduction of nitrite to produce NO at modest cathodic voltages (e.g., −0.3 to −0.5 V vs Ag/AgCl). The NO produced within the catheter lumen is capable of diffusing through the wall of the catheter to produce fluxes of NO that rival those found physiologically from the surfaces of endothelial and other cells in our bodies.
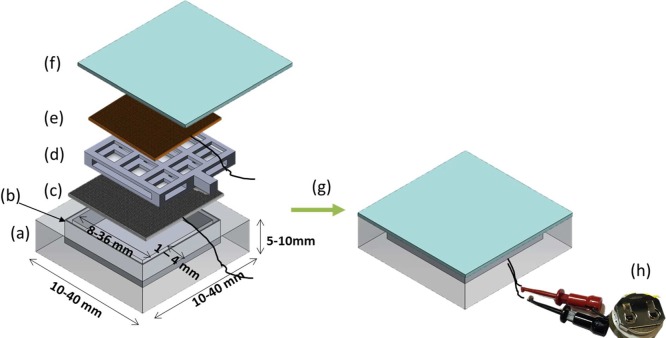
In this work, we describe the adaptation of this electrochemical NO release chemistry to create a new type of NO emitting device that could be employed as a wound-healing patch. A polydimethylsiloxane (PDMS) housing is fabricated using a 3D printing soft lithography method, allowing facile design of different configurations of patches. PDMS was chosen to add more flexibility to the device so that it can be readily applied onto the surface of the skin (see Figure S1 for the picture illustrating flexibility). A thin aluminum layer was embedded in the middle of one external wall so that NO release can only be emitted through the outer silicone layer/wall on one side of the device (i.e., the side that eventually would be applied to the exterior of the wound) (see Figure 1 for device configuration).
Figure 1.
Design of NO-releasing antimicrobial patch containing (a) PDMS, (b) aluminum layer in the middle of base wall (18 μm), (c) silver/silver chloride mesh as a reference/counter electrode, (d) PDMS spacer, (e) gold sputtered stainless steel mesh as a working electrode, (f) silicone rubber as a membrane, (g) sealed with silicone adhesive, and (h) connected with portable voltage supplier.
A 3D printer using polylactic acid (PLA) as the polymer cartridge agent was employed to create a mold and offers considerable design freedom with resolution of 500 μm dimensions via the use of the 3D design software Solidworks. Using the printed PLA devices as the mold, the PDMS patches can be created in various dimensions from 10 to 45 mm in width and 1 to 10 mm in thickness (see experimental details in the Supporting Information). In the following experiments, most of the patches are 40 × 40 × 6 mm in size, unless otherwise specified. A gold-coated stainless mesh and a silver/silver chloride (Ag/AgCl) mesh were incorporated into the device, serving as the working electrode and the reference/counter electrode, respectively. A porous PDMS spacer (also created using a 3D printed mold) was placed in between the two meshes to avoid direct electric contacts between the electrodes. The inner chamber of the device was then filled with a solution containing 4 mM CuTPMA, 0.4 M sodium nitrite, 0.2 M NaCl, and 0.5 M HEPES buffer (pH 7.2).
The portable voltage supplier is battery operated and consists of a microcontroller (that incorporates an analog-to-digital converter), a low-pass filter, and an operational amplifier. The microcontroller generates a pulse-width modulated digital signal that is passed through the low-pass filter, converting the digital signal into an analog signal with a constant voltage. The analog output voltage can be programmed to any level within the range of ±1 V. The low-pass filter is followed by an operational amplifier in a unity-gain configuration, which forms an output stage capable of supplying electrical currents to support the electrochemical reduction of nitrite. The system operates in a closed loop, with the output voltage continuously monitored and adjusted to be at the preprogrammed level.
The amount of released NO in the wound patch system was analyzed using various applied potentials between the Au mesh electrode and the Ag/AgCl electrode. As shown in Figure 2, the amount of NO flux coming from the surface of the device is in the range of 0.7 to 8.4 × 10–10 mol min–1 cm–2 at applied voltages in the range of −0.2 to −0.5 V. The NO flux increases as the potential increases from −0.1 V to −0.4 V. At even more negative potentials, we observed a decrease in the NO flux, which can be attributed to the oxidation of NO on the counter electrode. For most experiments, a voltage of −0.44 V was employed, and this voltage can be readily applied using a portable battery system to yield an NO surface flux >80% of the maximum flux with more tolerance of voltage drifting. It was also found that >85% of the NO flux is released from the top side (no aluminum foil, Figure 1f) of the device, whereas <5% of the NO is released from the back side of the patch (aluminum foil incorporated, Figure 1a) demonstrating that aluminum layer indeed blocks the NO diffusion effectively as designed (see Figure S2).
Figure 2.
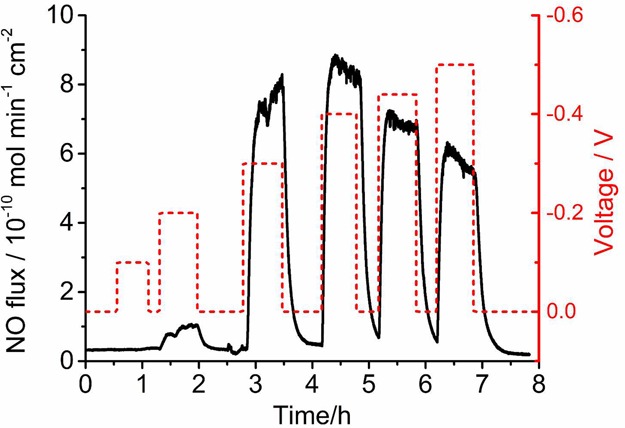
Control of NO release fluxes (black line) from a PDMS patch with 0.4 M NaNO2, 0.2 M NaCl, 4 mM Cu(II)TPMA, 0.5 M HEPES buffer under various cathodic voltages (red line) at room temperature.
The lifetime of NO release for the new patch system was tested continuously for 4 d. As shown in Figure 3, the PDMS patch exhibited NO fluxes of 8–10 × 10–10 mol cm2-min–1 during the first 2 days as measured by a chemiluminescence NO analyzer.21 However, the signal dropped to half of these values by day 3 and day 4. This drop is not due to the loss of battery power as the value of NO obtained using a potentiostat showed the same level of decrease on days 3 and 4. A change in the composition of the inner filling solution, including a reduction in nitrite levels, or increase in the pH that slows the NO generation reaction is likely to occur. Nonetheless, using a syringe with a small needle, it is viable to replace the inner solution with fresh CuTPMA and nitrite solution as the device’s PDMS walls would be self-sealing after removal of the needle. For the purpose of demonstration, we replaced the inner filling solution on day 4 with a fresh solution using a syringe (see Movie S1) and restored the flux (Figure 3). In practice, small Luer Lock ports for removing and refilling the solution can be designed onto the patch, which will greatly facilitate solution changing.
Figure 3.
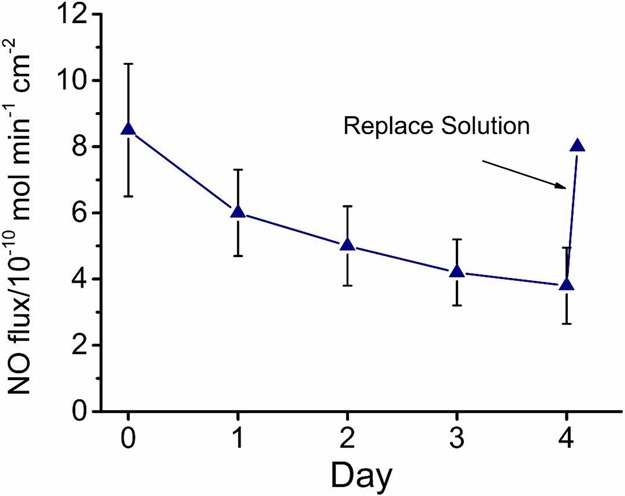
Continuous NO release profile from wound healing patches at −0.4 V over 4 days (n = 3 patches, error bars indicate standard deviation). The internal solution is replaced by a fresh nitrite/copper complex solution on day 4 using a syringe to restore the flux as indicated by the arrow.
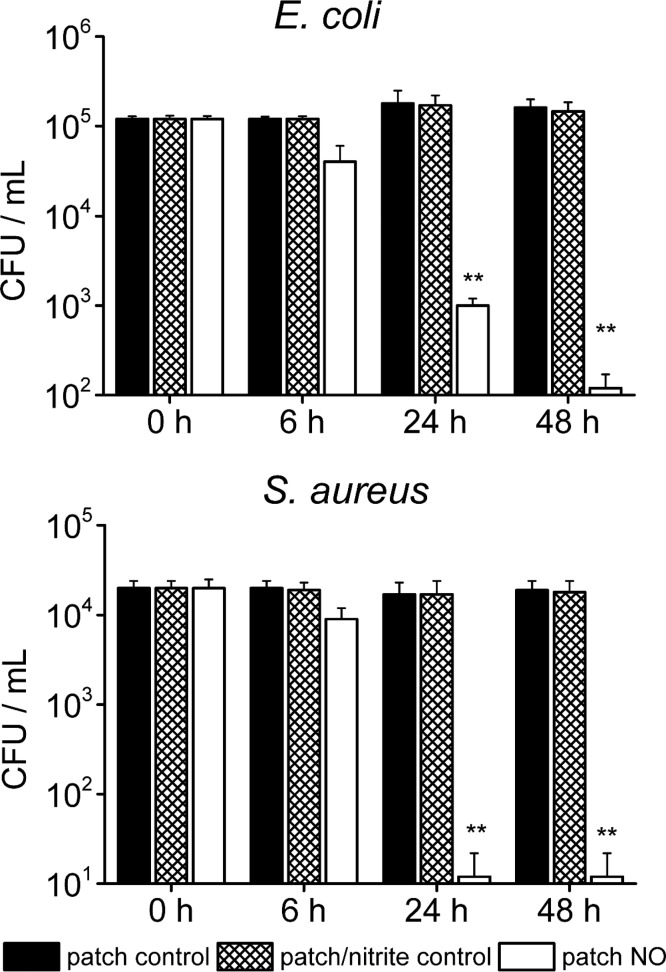
To investigate the effectiveness of the electrochemical NO releasing patch to kill bacteria via released NO, we selected E. coli and S. aureus as microbial strains for in vitro studies. Both of these bacteria are common pathogens associated with skin and soft tissue infections.22 First, the efficacy of the NO releasing patch was tested in liquid media because the cell counting method in the liquid phase can provide an exact change in the cell number. After 6 h of NO release to a solution of E. coli or S. aureus (with original cell counts of 1 × 105 CFU/mL), 35 and 20% of these two organisms were killed, respectively. After 24 h, 99% of the E. coli was removed and none of S. aureus survived (see Figure 4). At 48 h, 99.9% of the E. coli was removed. Note that the detection limit for cell counting was 20 CFU/mL due to the serial dilution.
Figure 4.
Plate counting of viable E. coli and S. aureus on the surface of patches with NO turned on for 6, 24, and 48 h (n = 3 for each strain. *p < 0.01; S. aureus: n = 3, **p < 0.05). Patch control is a plain patch without inner filling solution. Patch/nitrite control is the patch with inner nitrite solution. Patch NO is the patch where NO release is turned “on”.
A solid phase antimicrobial test approach was also examined since the patch ultimately will be directly employed on the surface of skin. In this configuration, PDMS patches equipped with the battery power circuit were applied onto plated blood agar gels that were inoculated with E. coli and S. aureus cultures. A PDMS patch without applying voltages to the working Au mesh electrode was also tested on the same inoculated agar plates as a control. After 2 days of culturing, no E. coli or S. aureus bacteria were found in the regions where the NO releasing patches were applied, while significant cell growth pertained in the areas under the control patch regions (see Figure 5). This confirms that electrochemically released NO from the proposed PDMS patches significantly decrease the viability of the bacteria. We did not observe an inhibition zone, perhaps because of the relatively low NO flux compared to most studies where an inhibition zone is observed.11,12 It should be noted that NO fluxes in our study cause no toxicity to mammalian cells.11
Figure 5.
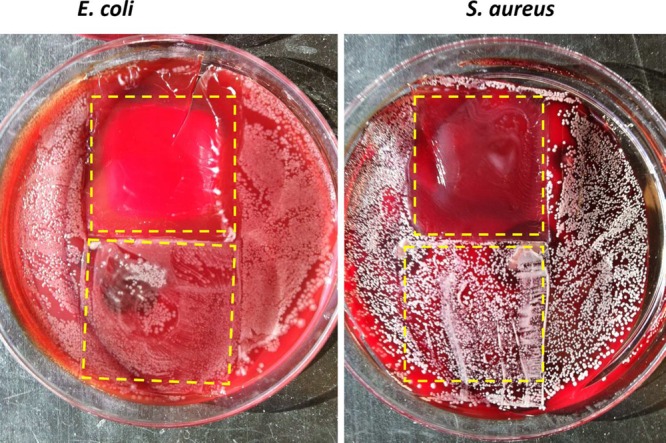
Antimicrobial test of the electrochemical NO releasing patches on blood agars with E. coli (left) and S. aureus (right). E. coli and S. aureus were grown for 48 h, and NO was turned on for the PDMS patches for 24 h. The patches have been removed to better show the bacteria under the patches, with dashed lines indicating the location of the patches during the experiment.
In addition to the bactericidal effect, NO has also shown several other beneficial effects in different processes of wound healing, including angiogenesis, inflammation, cell proliferation, matrix deposition, and remodeling. The electrochemical NO releasing patch reported here would provide a good tool in studying these effects during different stages of the wound healing, as the temporal profile of NO fluxes can readily be controlled electrochemically (see Figure 2). This is in contrast to most of the chemical NO release methods, for which the release is passive and cannot be modulated easily.
In summary, we have demonstrated that a new, low cost, and simple-to-fabricate electrochemically modulated NO generation patch can be prepared that releases NO fluxes that are effective in killing/inhibiting Gram-positive and Gram-negative bacteria that are commonly associated with chronic wound infections (S. aureus and E. coli). The level of NO release from the surface of the patch that would contact the skin can be controlled by the magnitude of the applied voltage between the Au-coated stainless steel mesh working electrode and the Ag/AgCl reference mesh electrode. For long-term applications, the inner nitrite/CuTMPA solution will likely need to be changed every 2–3 days to maintain the desired levels of NO flux from the surface of the device. Of course, in practice, use of modulated release (e.g., turned “on” periodically for several 2–3 h periods per day) may be all that is needed and this can extend the lifetime for a given load of inner solution. The next phase of research will involve testing this patch configuration in animal models of chronic wounds to prove the effectiveness of the system in enhancing the wound healing process. Prior studies with NO release patches made with chemical release of NO have already demonstrated efficacy in such animal studies.23
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.6b00360.
Author Contributions
† W.L. and H.R. contributed equally to this work.
This research was supported by the National Institutes of Health (Grant HL119403).
The authors declare no competing financial interest.
Supplementary Material
References
- Fischbach M. A.; Walsh C. T. Antibiotics for emerging pathogens. Science 2009, 325 (5944), 1089–1093. 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospenthal D. R.; Murray C. K.; Andersen R. C.; Blice J. P.; Calhoun J. H.; Cancio L. C.; Chung K. K.; Conger N. G.; Crouch H. K.; Laurie C. Guidelines for the prevention of infection after combat-related injuries. J. Trauma Acute Care Surg. 2008, 64 (3), S211–S220. 10.1097/TA.0b013e318163c421. [DOI] [PubMed] [Google Scholar]
- Taubes G. The bacteria fight back. Science 2008, 321 (321), 356–361. 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L.; Lowenstein C.; Snyder S. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ. Res. 1993, 73 (2), 217–222. 10.1161/01.RES.73.2.217. [DOI] [PubMed] [Google Scholar]
- Kerwin J. F. Jr; Lancaster J. R.; Feldman P. L. Nitric oxide: a new paradigm for second messengers. J. Med. Chem. 1995, 38 (22), 4343–4362. 10.1021/jm00022a001. [DOI] [PubMed] [Google Scholar]
- Moncada S.; Palmer R.; Higgs E. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43 (2), 109–142. [PubMed] [Google Scholar]
- Jones M. L.; Ganopolsky J. G.; Labbé A.; Wahl C.; Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88 (2), 401–407. 10.1007/s00253-010-2733-x. [DOI] [PubMed] [Google Scholar]
- Schairer D. O.; Chouake J. S.; Nosanchuk J. D.; Friedman A. J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3 (3), 271–279. 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N.; Hassett D. J.; Hwang S.-H.; Rice S. A.; Kjelleberg S.; Webb J. S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188 (21), 7344–7353. 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H.; Colletta A.; Koley D.; Wu J.; Xi C.; Major T. C.; Bartlett R. H.; Meyerhoff M. E. Thromboresistant/anti-biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry 2015, 104, 10–16. 10.1016/j.bioelechem.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbois E. J.; Bayliss J.; Wu J.; Major T. C.; Xi C.; Wang S. C.; Bartlett R. H.; Handa H.; Meyerhoff M. E. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomater. 2014, 10 (10), 4136–4142. 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulli R.; McElhaney-Feser G.; Hrabie J.; Cihlar R. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Rec. Res. Devel. Microbiol. 2002, 6, 177–183. 10.4161/viru.20328. [DOI] [Google Scholar]
- Rizk M.; Witte M. B.; Barbul A. Nitric oxide and wound healing. World J. Surg. 2004, 28 (3), 301–306. 10.1007/s00268-003-7396-7. [DOI] [PubMed] [Google Scholar]
- Wold K. A.; Damodaran V. B.; Suazo L. A.; Bowen R. A.; Reynolds M. M. Fabrication of biodegradable polymeric nanofibers with covalently attached NO donors. ACS Appl. Mater. Interfaces 2012, 4 (6), 3022–3030. 10.1021/am300383w. [DOI] [PubMed] [Google Scholar]
- Jen M. C.; Serrano M. C.; van Lith R.; Ameer G. A. Polymer-based nitric oxide therapies: recent insights for biomedical applications. Adv. Funct. Mater. 2012, 22 (2), 239–260. 10.1002/adfm.201101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W.; Wu J.; Xi C.; Ashe A. J.; Meyerhoff M. E. Carboxyl-ebselen-based layer-by-layer films as potential antithrombotic and antimicrobial coatings. Biomaterials 2011, 32 (31), 7774–7784. 10.1016/j.biomaterials.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. W.; Schoenfisch M. H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41 (10), 3742–3752. 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.; Megson I. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151 (3), 305–321. 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoako K. A.; Archangeli C.; Handa H.; Major T.; Meyerhoff M. E.; Annich G. M.; Bartlett R. H. Thromboresistance characterization of extruded nitric oxide-releasing silicone catheters. ASAIO J. 2012, 58 (3), 238–246. 10.1097/MAT.0b013e31824abed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum A. R.; Kappler M. P.; Wu J.; Xi C.; Meyerhoff M. E. The preparation and characterization of nitric oxide releasing silicone rubber materials impregnated with S-nitroso-tert-dodecyl mercaptan. J. Mater. Chem. B 2016, 4 (3), 422–430. 10.1039/C5TB01664A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Annich G. M.; Miskulin J.; Osterholzer K.; Merz S. I.; Bartlett R. H.; Meyerhoff M. E. Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials 2002, 23 (6), 1485–1494. 10.1016/S0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- Dryden M. S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65 (suppl 3), iii35–iii44. 10.1093/jac/dkq302. [DOI] [PubMed] [Google Scholar]
- Bokhari A. R.; Murrell G. A. The role of nitric oxide in tendon healing. J. Shoulder Elbow Surg. 2012, 21 (2), 238–244. 10.1016/j.jse.2011.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.