Summary
Human metapneumovirus (hMPV) is a major cause of respiratory tract infections in children, elderly and immunocompromised hosts, for which no vaccine or treatment are currently available. Oxidative stress and inflammatory responses represent important pathogenic mechanism(s) of hMPV infection. Here, we explored the potential protective role of dietary antioxidants in hMPV infection. Treatment of airway epithelial cells with resveratrol and quercetin during hMPV infection significantly reduced cellular oxidative damage, inflammatory mediator secretion and viral replication, without affecting viral gene transcription and protein synthesis, indicating that inhibition of viral replication occurred at the level of viral assembly and/or release. Modulation of proinflammatory mediator expression occurred through the inhibition of transcription factor nuclear factor (NF)-κB and interferon regulatory factor (IRF)-3 binding to their cognate site of endogenous gene promoters. Our results indicate the use of dietary antioxidants as an effective treatment approach for modulating hMPV induced lung oxidative damage and inflammation.
Keywords: Human metapneumovirus, resveratrol, quercetin
Human metapneumovirus (hMPV) is a paramyxovirus identified a little over a decade ago (van den Hoogen et al., 2001), with genome closely resembling that of respiratory syncytial virus (RSV), with few variances in gene order and absence of the non-structural genes NS1 and NS2 (Schildgen et al., 2011). hMPV is a major cause of lower respiratory tract infections, such as bronchiolitis and pneumonia, in children, elderly and immunocompromised hosts, with an economic impact in the pediatric population similar to that of influenza virus (Boivin et al., 2007;Edwards et al., 2013;Jartti et al., 2002).
Several viral infections, including human immunodeficiency virus (HIV), Hepatitis B and C, rhinovirus, influenza and RSV, have been shown to induce reactive oxygen species (ROS) formation in a variety of cell types (Bao et al., 2008b;Casola et al., 2001;Peterhans, 1997). ROS play an important role in cellular signaling, leading to the expression of proinflammatory mediators, and uncontrolled ROS generation is associated with cellular damage, a condition known as oxidative stress (Ciencewicki et al., 2008;Djordjevic, 2004). Many features of influenza- and RSV-mediated lung disease including inflammation, airway hyperreactivity, and epithelial cell damage are due to oxidative stress (Castro et al., 2006;Vlahos et al., 2011). Recently, we have shown that hMPV infection of airway epithelial cells induces proinflammatory gene expression through activation of transcription factors nuclear factor kappa B (NF-κB) p65 subunit and interferon regulatory factor (IRF)-3 (Bao et al., 2007;Bao et al., 2008a;Kolli et al., 2012). hMPV infection also induces oxidative stress by progressive decreasing gene expression and protein levels of antioxidant enzymes (AOEs) such as superoxide dismutase 3, catalase, glutathione S-transferase, both in vitro and in vivo (Bao et al., 2008b;Hosakote et al., 2011). We also found that hMPV infection reduced nuclear translocation of the transcription factor NF-E2-related factor 2 (Nrf2)(Komaravelli & Casola, 2014), a major regulator of AOE gene expression (Jaiswal, 2004).
Dietary supplements like resveratrol and quercetin, which possess anti-oxidant and anti-inflammatory properties, have been beneficial in modulating oxidative stress and inflammation against a variety of diseases (Angeloni et al., 2007; Baur & Sinclair, 2006). Resveratrol is a non-flavonoid polyphenol abundant in skin of red grapes, peanuts and red wine (Pollack & Crandall, 2013). Quercetin is a flavonoid abundant in apple skins, onion peels, peppers, certain berries and green tea (Boyer et al., 2004). Both these dietary supplements possess anti-carcinogenic, anti-inflammatory, antioxidant and antiviral properties (Baur & Sinclair, 2006;Campagna & Rivas, 2010;Chirumbolo, 2010). In respiratory viral infections, resveratrol and quercetin treatment had a protective effect against influenza infection [reviewed in (Uchide & Toyoda, 2011)], while quercetin protected against rhinovirus both in vitro and in vivo (Ganesan et al., 2012). However, the role of these molecules in the context of hMPV infection has never been investigated.
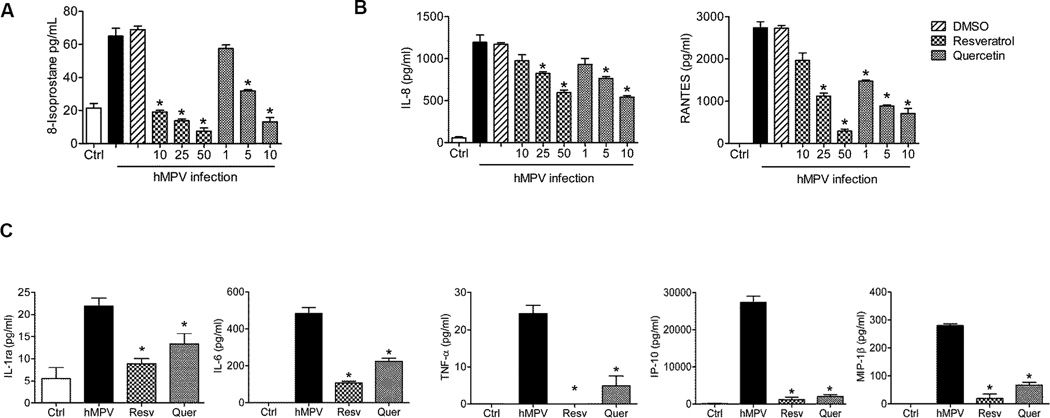
To investigate the effect of resveratrol and quercetin treatment on hMPV-induced cellular oxidative damage and inflammation response, A549 cells (alveolar type II cancerous cell line) were infected with hMPV at multiplicity of infection (MOI) of 1 as previously described (Bao et al., 2008b), in the presence or absence of various doses of resveratrol (10–50µM in DMSO) or quercetin (1–10µM in DMSO). Culture supernatant was harvested at 24h post-infection (p.i.) to measure the oxidative stress damage marker F2 8-isoprostane, using a competitive enzyme immunoassay from Cayman Chemical (Ann Arbor, MI), and proinflammatory markers CXCXL8 (IL-8) and CCL5 (RANTES). We also tested for changes in viability due to antioxidant treatment by Trypan blue exclusion and there was no effect of both compounds on uninfected cells. hMPV infection was associated with significant oxidative damage in A549 cells, as evident by increased release of 8-isoprostane, and with a significant increase in the secretion of the proinflammatory cytokines IL-8 and RANTES. Both resveratrol and quercetin treatment significantly reduced hMPV-induced 8-isoprostane generation, to levels comparable to mock-infected (Ctrl) cells (Fig.1A), as well as chemokines IL-8 and RANTES (Fig. 1B). Mock infected cells represent uninfected cells treated as infected cells, with media contains lower serum concentrations (2%) and an amount of sucrose equivalent to the one present in the viral inoculum (Ueba, 1978). We also assessed other cytokine and chemokine production in the cell supernatant of the highest effective concentrations of resveratrol and quercetin using the Bio-Plex Cytokine Human Multi-Plex Assay (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s instructions. hMPV infection was associated with significant a significant increase in the secretion of the proinflammatory cytokines IL-1α, IL-6 and TNF-α, and the chemokines CXCL10 (IP-10) and CCL4 (MIP-1β). Both resveratrol and quercetin treatment significantly reduced these cytokine and chemokine secretion from infected cells (Fig.1C).
Figure 1. Effect of resveratrol and quercetin on oxidative stress and inflammatory mediator production.
A549 cells were either mock-infected (Ctrl) or infected with hMPV in the presence or absence of DMSO at MOI of 1 for 24h and treated or untreated with resveratrol (10–50 µM in DMSO) or quercetin (1–10 µM in DMSO). Culture supernatants were harvested to measure (A) oxidative stress marker 8-isoprostane; (B) IL-8 and RANTES (C) Since DMSO did not have any significant effect on hMPV infection, we did not include vehicle control in further experiments. Supernatants from A549 cells either uninfected (Ctrl) or infected with hMPV and treated or untreated with resveratrol (50 µM) or quercetin (10 µM) were measured for cytokines and chemokines. Results are expressed as mean ± standard error and are representative of three independent experiments. *P < 0.05 relative to hMPV-infected cells.
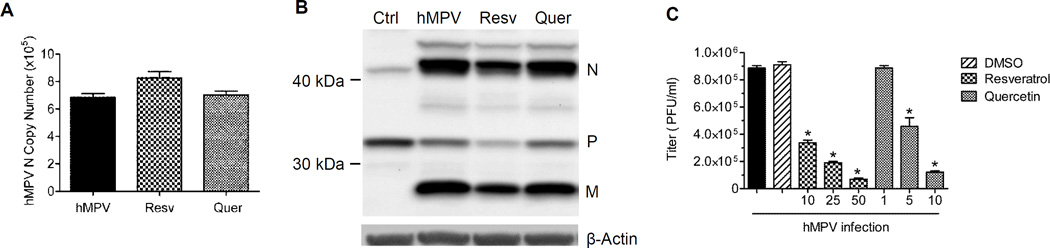
To investigate the effect of these dietary antioxidants on viral replication, A549 cells were infected with hMPV at MOI of 1 in the presence or absence of resveratrol or quercetin and harvested at 24h p.i. to prepare total RNA by ToTALLY RNA kit (cat AM1910, Ambion, Life technologies, Austin TX) and analyze the absolute copy number of the hMPV gene N by quantitative real-time PCR (qRT-PCR). Neither resveratrol nor quercetin treatment had an effect on hMPV N gene transcription (Fig.2A). To investigate their effect on hMPV protein expression, total cell lysates were prepared in RIPA buffer and Western blot analysis was performed using a polyclonal rabbit anti-hMPV antibody, as described in (Bao et al., 2013). Again, there was no significant change in the amount of hMPV total protein expression in resveratrol or quercetin treated compared to untreated, infected cells (Fig.2B). To determine whether dietary antioxidant treatment would affect later steps of viral replication, production of viral infection particles was determined by plaque immunoassay, as previously described (Guerrero-Plata et al., 2005). Resveratrol and quercetin treatment of A549 cells during hMPV infection was associated with a much lower viral titer, compared to untreated, infected cells (Fig.2C), suggest that these antioxidants interferes with viral assembly and formation of mature virus. In resveratrol treated cells, the amount of infectious viral particles reduced from almost a million/ml down to 50,000 and quercetin treatment reduced viral particles to little above 100,000. Resveratrol treatment has been shown to be protective against influenza (Palamara et al., 2005), RSV (Liu et al., 2014;Xie et al., 2012;Zang et al., 2011), herpes simplex virus 1 and 2 (Docherty et al., 1999;Faith et al., 2006), Epstein-Barr virus (De et al., 2011), human cytomegalovirus (Evers et al., 2004), varicella-zoster virus (Docherty et al., 2006) and polyomavirus (Berardi et al., 2009) infections, however it enhanced viral replication of Hepatitis C virus in vitro (Nakamura et al., 2010) and aggravated Theiler's murine encephalomyelitis virus-induced demyelinating disease in mice (Sato et al., 2013). Resveratrol treatment inhibition of influenza A virus replication was due to reduced expression of viral proteins through inhibition of a PKC-dependent pathway (Palamara et al., 2005). Resveratrol treatment has also been shown to suppress RSV replication in airway epithelial cells (Liu et al., 2014;Xie et al., 2012;Zang et al., 2011) and reduce RSV-induced production of cytokines IFN-γ and IL-6 through inhibition of TLR3/TRIF signaling (Liu et al., 2014;Xie et al., 2012;Zang et al., 2011), although no significant effect was observed on the production of chemokines, such as IL-8. Quercetin has been shown to inhibit rhinovirus and influenza replication in vitro and in vivo, by affecting different stages of virus life cycle, and to reduce proinflammatory mediator production (Ganesan et al., 2012;Kim et al., 2010). There is only one study investigating the effect of dietary flavonoids, including quercetin, on the infectivity and replication of RSV, in which quercetin did not affect the ability of the virus to infect the cells or replicate (Kaul et al., 1985). We observed a similar result when we tested quercetin in RSV-infected cells at the same concentration that was effective in reducing hMPV replication (data not shown), suggesting that quercetin antiviral effect is virus-specific.
Figure 2. Effect of resveratrol and quercetin on viral replication.
A549 cells were either mock-infected (Ctrl) or infected with hMPV at MOI of 1 for 24h in the presence or absence of 50 µM resveratrol or 10 µM quercetin, and harvested to prepare (A) total RNA to quantify RSV N gene expression by qRT-PCR; and (B) total cell lysates for western blot analysis for hMPV viral protein expression. For loading controls, membrane was stripped and reprobed with anti β-actin antibody; (C) A549 cells infected with hMPV at MOI of 1 for 24h and treated or untreated with various doses of resveratrol (10 – 50 µM in DMSO) or quercetin (1 – 10 µM in DMSO) or vehicle alone were harvested to measure viral titer by plaque assay. Results are expressed as mean ± standard error and are representative of three independent experiments. *P < 0.05 relative to hMPV-infected cells.
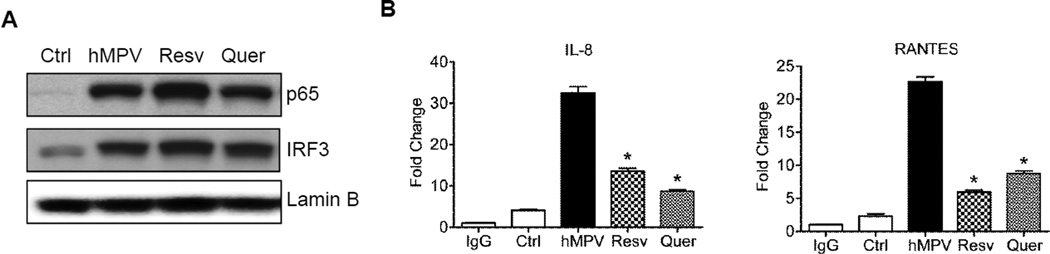
We have previously shown that hMPV activates the RIG-I-MAVS signaling pathway in airway epithelial cells, leading to the activation of NF-κB and IRF-3 and the corresponding expression of proinflammatory and antiviral molecules (Liao et al., 2008). To investigate the mechanism(s) by which resveratrol and quercetin decreased hMPV-induced proinflammatory mediator production, we assessed NF-κB/p65 and IRF-3 activation. A549 cells infected with hMPV at MOI of 1 in the presence or absence of these antioxidants were harvested at 24h p.i. to prepare nuclear protein by hypotonic/nonionic detergent lysis, as previously described (Bao et al., 2008a;Schreiber et al., 1989). NF-κB/p65 and IRF-3 nuclear levels were assessed by western blot analysis using specific antibodies anti-p65 (#4764, Cell Signaling, Boston, MA) and anti-IRF-3 (sc-9082, Santa Cruz Biotechnology, Dallas, TX). hMPV infection resulted in significant p65 and IRF-3 nuclear translocation, compared to mock-infected cells, which was not affected by resveratrol or quercetin treatment (Fig.3A). To determine whether antioxidants influenced occupancy of NF-κB/p65 and IRF-3 of their cognate sites of endogenous gene promoters, we performed chromatin immunoprecipitation (ChIP) and quantitative genomic PCR assays (Q-gPCR) (Tian et al., 2012). For ChIP assays, we used ChIP-IT Express kit from Active Motif (53008 Carlsbad, CA), following manufacturer instruction with some modifications. Briefly, cells infected with hMPV for 24h in the presence or absence of resveratrol or quercetin were fixed with 2mM disuccinimidyl gluterate and formaldehyde at room temperature, nuclei were isolated, sheared by sonication, and 20 µg of sheared chromatin was immunoprecipitated with 5 µg of ChIP grade anti-NF-?B/p65 (ab7970, abcam, Cambridge, MA) or -IRF-3 antibodies (sc-9082x, Santa Cruz Biotechnology, Dallas, TX), and magnetic beads conjugated with protein G at 4°C overnight. Immunoprecipitation with IgG antibody was used as negative control. Chromatin was reverse cross linked, eluted from magnetic beads, and purified. Q-gPCR was done by SyBR green based real-time PCR using the following primers spanning the IL-8 gene NF-?B promoter site: forward-AGGTTTGCCCTGAGGGGATG and reverse- GGAGTGCTCCGGTGGCTTTT, or the RANTES gene ISRE promoter site: forward-AGCGGCTTCCTGCTCTCTGA and reverse- CAGCTCAGGCTGGCCCTTTA. Total input chromatin DNA for immunoprecipitation was included as positive control for PCR amplification. hMPV infection dramatically increased NF-κB/p65 binding to the IL-8 promoter and IRF-3 binding to the RANTES promoter, which was significantly reduced by both resveratrol and quercetin treatment (Fig.3B), leading to reduced expression of cytokines and chemokines. NF-κB must undergo a variety of post-translational modification, including phosphorylation and acetylation, to achieve its full biological activity (Chen & Greene, 2004), (Chen et al., 2005). Acetylation of specific lysine residues has been shown to modulate distinct NF-κB functions (Chen & Greene, 2004;Schmitz et al., 2004). A possible mechanism by which resveratrol could affect hMPV-induced p65 activation is by inducing Sirtuin1 (SIRT1) (Park et al., 2012), which has histone deacetylase activity. SITR1 has been shown to antagonize NF-κB activation (Kauppinen et al., 2013), and reduced SIRT1 activity by HIV TAT protein was associated with increased binding of NF-κB to the promoter of proinflammatory genes (Blazek & Peterlin, 2008;Kwon et al., 2008;Yeung et al., 2004). Reduced SIRT1 activity has also been shown to be associated with increased activity of IRF-3 in adipose inflammation (Gillum et al., 2011), suggesting an antagonistic crosstalk between SIRT1 and IRF-3 also.
Figure 3. Effect of resveratrol and quercetin on transcription factor activation.
A549 cells were infected with hMPV at MOI of 1 for 24h in the presence or absence of 50 µM resveratrol or 10 µM quercetin. (A) Nuclear proteins were analyzed for NF-κB/p65 and IRF-3 levels by Western blot. For loading controls, membranes were stripped and reprobed with anti lamin B antibody. (B) ChIP-QgPCR analysis of p65 occupancy of the IL-8 gene promoter NF-κB site (left panel) and IRF-3 occupancy of the RANTES gene promoter ISRE site (right panel). Data shown is representative of three independent experiments. *P < 0.05 relative to 24h hMPV infected cells.
In conclusion, we found that resveratrol and quercetin treatment significantly reduced hMPV-induced oxidative damage, inflammatory responses and viral replication, suggesting their potential use as prophylactic and possibly therapeutic intervention for modulating hMPV-associated inflammatory lung disease, which deserve further testing in vivo, in an animal model of infection for example.
Acknowledgments
This project was supported by R01 AI062885, P01 AI07924602 and P30 ES006676. The authors would like to thank Tianshuang Liu for technical assistance and Cynthia Tribble for manuscript submission.
Reference List
- Angeloni C, Spencer JP, Leoncini E, Biagi PL, Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89:73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Bao X, Kolli D, Ren J, Liu T, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G disrupts mitochondrial signaling in airway epithelial cells. PLoS ONE. 2013;8:e62568. doi: 10.1371/journal.pone.0062568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008a;4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. Airway epithelial cell response to human metapneumovirus infection. Virology. 2007;368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Sinha M, Liu T, Hong C, Luxon BA, Garofalo RP, Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology. 2008b;374:114–127. doi: 10.1016/j.virol.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Berardi V, Ricci F, Castelli M, Galati G, Risuleo G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: potential clinical use. J Exp Clin Cancer Res. 2009;28:96. doi: 10.1186/1756-9966-28-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek D, Peterlin BM. Tat-SIRT1 tango. Mol Cell. 2008;29:539–540. doi: 10.1016/j.molcel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Boivin G, De SG, Hamelin ME, Cote S, Argouin M, Tremblay G, Maranda-Aubut R, Sauvageau C, Ouakki M, Boulianne N, Couture C. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44:1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- Boyer J, Brown D, Liu RH. Uptake of quercetin and quercetin 3-glucoside from whole onion and apple peel extracts by Caco-2 cell monolayers. J Agric Food Chem. 2004;52:7172–7179. doi: 10.1021/jf030733d. [DOI] [PubMed] [Google Scholar]
- Campagna M, Rivas C. Antiviral activity of resveratrol. Biochem Soc Trans. 2010;38:50–53. doi: 10.1042/BST0380050. [DOI] [PubMed] [Google Scholar]
- Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with Respiratory Syncytial Virus: role in viral-induced Interferon Regulatory Factor activation. J Biol Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant Treatment Ameliorates Respiratory Syncytial Virus-induced Disease and Lung Inflammation. Am J Respir Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9:263–285. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De LA, Arena G, Stecca C, Raciti M, Mattia E. Resveratrol inhibits proliferation and survival of Epstein Barr virus-infected Burkitt's lymphoma cells depending on viral latency program. Mol Cancer Res. 2011;9:1346–1355. doi: 10.1158/1541-7786.MCR-11-0145. [DOI] [PubMed] [Google Scholar]
- Djordjevic VB. Free radicals in cell biology. Int Rev Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999;43:145–155. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 2006;72:171–177. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DL, Wang X, Huong SM, Huang DY, Huang ES. 3,4',5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 2004;63:85–95. doi: 10.1016/j.antiviral.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Faith SA, Sweet TJ, Bailey E, Booth T, Docherty JJ. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Faris AN, Comstock AT, Wang Q, Nanua S, Hershenson MB, Sajjan US. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S, Shulman GI. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. 2011:1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jartti T, van den Hoogen BG, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul TN, Middleton E, Jr, Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Kim Y, Narayanan S, Chang KO. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Kolli D, Bao X, Casola A. Human metapneumovirus antagonism of innate immune responses. Viruses. 2012;4:3551–3571. doi: 10.3390/v4123551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravelli N, Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. 2014 doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Brent MM, Getachew R, Jayakumar P, Chen LF, Schnolzer M, McBurney MW, Marmorstein R, Greene WC, Ott M. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3:158–167. doi: 10.1016/j.chom.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Bao X, Liu T, Lai S, Li K, Garofalo RP, Casola A. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J Gen Virol. 2008;89:1978–1986. doi: 10.1099/vir.0.2008/000778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zang N, Zhou N, Li W, Xie X, Deng Y, Ren L, Long X, Li S, Zhou L, Zhao X, Tu W, Wang L, Tan B, Liu E. Resveratrol inhibits the TRIF- dependent pathway by upregulating sterile alpha and armadillo motif protein, contributing to anti-inflammatory effects after respiratory syncytial virus infection. J Virol. 2014;88:4229–4236. doi: 10.1128/JVI.03637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Saito H, Ikeda M, Hokari R, Kato N, Hibi T, Miura S. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J Gastroenterol. 2010;16:184–192. doi: 10.3748/wjg.v16.i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamara AT, Nencioni L, Aquilano K, De CG, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res. 1997;56:107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- Pollack RM, Crandall JP. Resveratrol: Therapeutic Potential for Improving Cardiometabolic Health. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt165. [DOI] [PubMed] [Google Scholar]
- Sato F, Martinez NE, Shahid M, Rose JW, Carlson NG, Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am J Pathol. 2013;183:1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen V, van den Hoogen B, Fouchier R, Tripp RA, Alvarez R, Manoha C, Williams J, Schildgen O. Human Metapneumovirus: Lessons Learned over the First Decade. Clinical Microbiology Reviews. 2011;24:734–754. doi: 10.1128/CMR.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz ML, Mattioli I, Buss H, Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. Chembiochem. 2004;5:1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Yang J, Brasier AR. Two-step cross-linking for analysis of protein-chromatin interactions. Methods Mol Biol. 2012;809:105–120. doi: 10.1007/978-1-61779-376-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchide N, Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules. 2011;16:2032–2052. doi: 10.3390/molecules23100000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueba O. Respiratory syncytial virus: I. concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–272. [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XH, Zang N, Li SM, Wang LJ, Deng Y, He Y, Yang XQ, Liu EM. Resveratrol Inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation. 2012;35:1392–1401. doi: 10.1007/s10753-012-9452-7. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang N, Xie X, Deng Y, Wu S, Wang L, Peng C, Li S, Ni K, Luo Y, Liu E. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J Virol. 2011;85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]