Abstract
Objective
There are limited data on the role of activin A and its binding protein, follistatin, in nonalcoholic fatty liver disease (NAFLD). The main aim was the evaluation of serum activin A and follistatin levels in patients with biopsy-proven NAFLD vs. controls.
Methods
This was a case-control study. Fifteen patients with nonalcoholic simple steatosis (SS), 16 with steatohepatitis (NASH), and 52 (24 lean and 28 obese) controls were recruited. Activin A and follistatin were measured using ELISA.
Results
Activin A levels showed a trend towards progressive increase (p=0.010) from the controls (lean: 356±25, 95% CI 305–408; obese 360±20, 95% CI 320–401 pg/ml) to SS (407±28, 95% CI 347–466 pg/ml) and NASH patients (514±70 95% CI 364–664 pg/ml); this association became non-significant after adjusting for adiposity. Follistatin was not different between groups (lean controls: 1.11±0.08, 95% CI 0.95–1.28; obese controls: 1.00±0.07, 95% CI 0.86–1.14; SS: 0.86±0.07, 95% CI 0.70–1.02; NASH: 1.14±0.09, 95% CI 0.90–1.37 ng/ml; p=0.13). Within the NAFLD group of patients, follistatin was associated with NASH independently from activin A, gender and age, a relationship however likely reflecting the effect of adiposity.
Conclusions
Activin A is higher in patients with NASH than both lean and obese controls. Future clinical studies are needed to confirm and expand these findings, whereas mechanistic studies exploring underlying mechanisms are also warranted.
Keywords: activin A, follistatin, nonalcoholic fatty live disease, nonalcoholic steatohepatitis, steatosis
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a disease gaining increasing interest worldwide [1]. It ranges from simple nonalcoholic steatosis (SS) to nonalcoholic steatohepatitis (NASH), is characterized by steatosis, inflammation and fibrosis [2], and may lead to liver cirrhosis and hepatocellular carcinoma [3]. NAFLD shares common pathogenetic mechanisms with other components of the insulin resistance (IR) or metabolic syndrome [4], with adipokines playing a crucial role [5]. The prevalence of NAFLD increases in parallel with the epidemics of obesity and type 2 diabetes mellitus (T2DM) [6]. NASH diagnosis requires liver biopsy, an invasive method, thereby developing noninvasive markers for NAFLD represents a field of extensive research, targeting to replace liver biopsy or identify candidates for liver biopsy [7]. Furthermore, despite the high prevalence of the disease, NAFLD treatment remains an unmet medical need [8].
Activin A is a member of the tumor growth factor (TGF)-β superfamily and is regarded as a multifunctional cytokine expressed in a wide range of tissues and cells, where it regulates cellular differentiation, homeostasis of cell number and tissue architecture, inflammation, cell proliferation and apoptosis [9]. In hepatocytes, a complex role has been attributed to activin A; it is reported to be beneficial against lipid accumulation, but it may promote hepatic inflammation and fibrosis [10]. It enhances the expression of collagen and TGF-β1, induces mitochondrial β-oxidation, downregulates fatty acid synthase activity, promotes decreased weight percentage of saturated fatty acids, alters the composition of polyunsaturated fatty acids and promotes matrix metalloproteinase activity. Its expression is elevated in the fibrotic liver and it has been proposed to contribute to liver fibrosis through induction of matricellular proteins, including connective tissue growth factor, in hepatocytes and hepatic stellate cells. Moreover, it inhibits the proliferation and induces apoptosis of hepatocytes, contributing to the termination of liver regeneration; the proliferation of liver progenitor cells and their mediated liver regeneration are controlled by activin A [10,11].
Follistatin, the natural antagonist of activin A, is a widely expressed protein that binds and inactivates members of the TGF-β family, including activins [12]. It has been recently proposed that the liver is a major contributor to the circulating levels of follistatin in humans [13]. Deregulated expression of follistatin and activins has been implicated in hepatic diseases, including inflammation, fibrosis, liver failure and hepatocellular carcinoma [14].
In clinical terms, it has been proposed that activin antagonists, such as follistatin, may offer future therapeutic approaches in various conditions, including liver diseases [12]. The pathogenetic link between activin A, follistatin and NAFLD is of interest, since it may provide evidence for noninvasive assessment of the disease, but also the basis for future targeted treatment.
The primary aim of this study was the evaluation of serum activin A, follistatin levels and their ratio in patients with biopsy-proven NAFLD vs. lean and/or obese controls. Secondary aims were: a) the association of activin A and follistatin levels with IR, cardiometabolic risk factors, liver function tests, selected adipokines and irisin separately in patients and controls; b) the evaluation of serum activin A, follistatin levels and their ratio in specific histological lesions within NAFLD patients.
2. Patients and methods
2.1. Patients
NAFLD patients were consecutively recruited on an outpatient basis at a single center (Second Medical Clinic, Aristotle University of Thessaloniki, Greece). Determination of eligibility was based on medical history, physical examination, liver function tests (serum aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyl transferase [GGT], alkaline phosphatase) and liver ultrasound imaging performed during the screening visit.
Inclusion criteria for the NAFLD patients were: 1) age >18 years; 2) bright liver on ultrasound imaging and abnormal liver function tests for at least 6 months before liver biopsy; and 3) patient’s consent for liver biopsy. Individuals without NAFLD, living in the same region and being of similar gender and age to the patients, were recruited as controls from the same catchment area/study base at the Second Medical Clinic of Aristotle University of Thessaloniki and the Department of Endocrinology of 424 General Military Hospital, Thessaloniki, Greece. Inclusion criteria for the controls were: 1) age >18 years; 2) no history of abnormal liver ultrasound imaging or abnormal liver function tests; 3) currently normal liver ultrasound imaging; and 4) currently normal liver function tests. The controls were subsequently divided into two groups: the obese control group, which included those of similar body mass index (BMI) and waist circumference to NAFLD patients, and the lean control group, in which those of lower BMI and waist circumference compared to NAFLD patients were included. Controls did not undergo a liver biopsy, due to obvious ethical considerations. Exclusion criteria for both NAFLD patients and controls aimed to exclude conditions resulting in secondary fatty liver disease (e.g. alcoholic, viral, autoimmune, drug-induced etc.) and were described in detail in our previous publication [15]. Cirrhotic patients (fibrosis stage 4) were also excluded.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local ethics committees. All participants provided an informed consent.
2.2. Methods
Morning (8–9 am) fasting blood samples were collected 1–2h prior to liver biopsy. Serum AST, ALT, GGT, triglycerides, urea, creatinine, uric acid, total cholesterol and high-density lipoprotein (HDL-C) were measured within 1 h after blood drawing, with standard methods using an automated analyzer (Olympus AU2700; Olympus, Germany). Sera were also immediately frozen, initially at −30°C and later at −80°C, for the measurement in one batch at the end of the study of the remaining parameters, which included: glucose (Roche Cobas c311, Roche Diagnostics, IN), insulin (immunoassay, Immulite 1000, Siemens Healthcare Diagnostics, NJ), adiponectin (radioimmunoassay, Millipore, MA), leptin (enzyme-linked immunosorbent assay [ELISA], Millipore, MA), irisin (Phoenix Pharmaceuticals, CA), retinol-binding protein (RBP)-4 (Biovendor Research and Diagnostic Products, NC) and chemerin (Biovendor Research and Diagnostic Products, NC), as previously described [15]. Activin A and follistatin were measured with ELISA: activin A (ELISA, R&D Systems, MN; sensitivity 3.67 pg/ml, intra-assay coefficient of variation [CV] 4.2–4.4%, inter-assay CV 4.7–7.9%); follistatin (ELISA, R&D Systems, MN; sensitivity 0.03 ng/ml; intra-assay CV 4.9–7.5%; inter-assay CV 5.2–7.3%).
Liver biopsy was performed under computed tomography-guidance by an experienced radiologist and interpreted by two experienced pathologists. NAFLD patients were subdivided into those with SS or NASH according to the criteria of NAFLD Activity Score (NAS) [16]. Steatosis grade, fibrosis stage, lobular and portal inflammation, and ballooning were dichotomized [16].
BMI was calculated by the formula: body weight (kg)/height2 (m2). IR was quantified by the homeostasis model of assessment - insulin resistance (HOMA-IR) using the formula HOMA-IR = glucose (mmol/l) × insulin (µU/ml) / 22.5. Low-density lipoprotein cholesterol (LDL-C) was calculated by the formula of Friedewald. Activin A (ng/ml) to follistatin (ng/ml) and AST to ALT ratio were also calculated.
2.3. Statistical Analysis
Continuous data are presented as mean ± standard error of the mean (SEM). Categorical data are presented as absolute numbers or frequencies. Kolmogorov-Smirnov test was used to check the normality of distributions of continuous variables. Chi-square test was used for between group comparisons, in case of categorical variables. Spearman’s coefficient (rho; rs) was used for binary correlations. Partial correlation coefficient (rp) was used to adjust correlation for potential covariates. In case of continuous variables, between group comparisons were performed with independent samples T-test or Mann-Whitney test, in case of two groups, or one-way analysis of variance (ANOVA) or Kruskal-Wallis test, in case of more than two groups. In case of statistically significant difference in ANOVA or Kruskal-Wallis test, Bonferroni post-hoc correction was used for multiple pairwise comparisons. Analysis of covariance (ANCOVA) was used to adjust between group comparisons for covariates. Multiple binary logistic regression analysis (method “enter”) was used within NAFLD patients to identify whether activin A or follistatin were independently associated with NASH (dependent variable SS=0 and NASH=1) or specific histological lesions (i.e., for steatosis grade: ≤33%=0 and > 33%=1 etc.). For the need of logistic regression analysis, any of the included variables that did not follow normal distribution was logarithmically transformed. Statistical analysis was performed with SPSS 21 for Macintosh (IBM Corp., Armonk, NY). Significance was set at p<0.05 in most of the tests, apart from comparisons of activin A, follistatin levels and activin A to follistatin ratio (AFR) between specific histological lesions of NAFLD patients, in which it was set at p<0.01, whereas p-values between 0.01 and 0.05 were considered as suggestive (Bonferroni correction for 5 comparisons per variable).
3. Results
3.1. Comparisons between groups
Thirty-one patients with biopsy-proven NAFLD (15 with SS [aged 53.9±2.6; 10 women] and 16 with borderline or definite NASH [aged 53.9±2.9; 13 women]), 24 lean (aged 54.2±1.6; 20 women) and 28 obese controls (aged 52.6±1.6; 20 women) were included in this study. Comparative anthropometric and laboratory data of the study groups are presented in detail in our previous publication [15]. As selected, BMI and waist circumference were statistically lower in lean controls, but similar among patients with SS, NASH and obese controls. As expected, there were statistically significant differences between groups in ALT, AST, ALT to AST ratio, HDL-C, LDL-C, triglycerides, uric acid, insulin, HOMA-IR, leptin and adiponectin between groups, with NASH generally being the group with the higher metabolic burden [15].
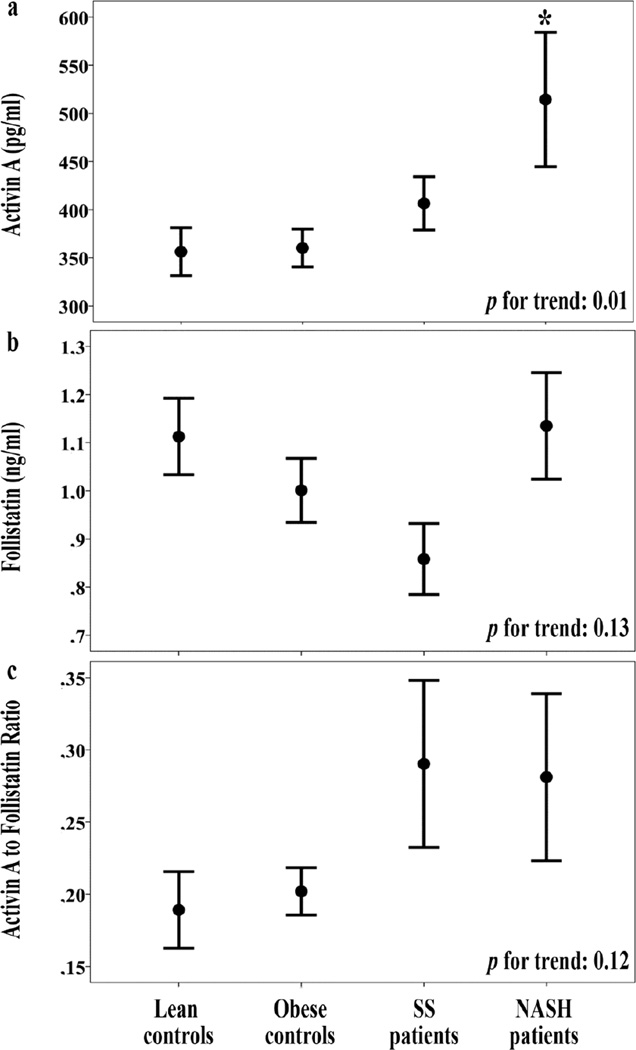
Serum activin A levels were escalating from the controls (being similar between lean and obese controls) to patients with SS and NASH (p=0.010 for trend; Table 1; Figure 1). In corrected pairwise comparisons, NASH patients had higher activin A than lean (p=0.015) and obese (p=0.014) controls. Serum follistatin levels were similar between groups, despite a trend towards lower levels in SS patients (p=0.13 for trend; Table 1; Figure 1). AFR did not differ between groups (lean controls: 0.38±0.05; obese controls: 0.40±0.03; SS; 0.58±0.12; NASH: 0.56±0.12; p=0.12 for trend; Figure 1).
Table 1.
Activin A and follistatin levels in study groups, unadjusted and after sequential adjustment for potential cofounders.
| Lean controls | Obese controls | SS | NASH | p-value for trend* |
|
|---|---|---|---|---|---|
|
Patients/Women (N) |
24 / 20 | 28 / 20 | 15 / 10 | 16 / 13 | 0.58 |
| Unadjusted | |||||
| Activin A (pg/ml) | 356 ± 25 | 360 ± 20 | 407 ± 28 | 514 ± 70 | 0.010 |
| Follistatin (ng/ml) | 1.11 ± 0.08 | 1.00 ± 0.07 | 0.86 ± 0.07 | 1.03 ± 0.04 | 0.13 |
| Model 1 (adjusted mutually) | |||||
| Activin A (pg/ml) | 359 ± 32 | 359 ± 29 | 401 ± 42 | 518 ± 40 | 0.009 |
| Follistatin (ng/ml) | 1.09 ± 0.08 | 0.99 ± 0.07 | 0.85 ± 0.10 | 1.16 ± 0.10 | 0.13 |
| Model 2 (adjusted mutually and for age) | |||||
| Activin A (pg/ml) | 357 ± 31 | 365 ± 28 | 402 ± 40 | 509 ± 39 | 0.014 |
| Follistatin (ng/ml) | 1.09 ± 0.08 | 1.00 ± 0.07 | 0.85 ± 0.10 | 1.16 ± 0.10 | 0.15 |
| Model 3 (adjusted mutually and for age and gender) | |||||
| Activin A (pg/ml) | 356 ± 31 | 365 ± 28 | 403 ± 41 | 509 ± 39 | 0.014 |
| Follistatin (ng/ml) | 1.09 ± 0.08 | 1.00 ± 0.07 | 0.85 ± 0.10 | 1.16 ± 0.10 | 0.16 |
| Model 4 (adjusted mutually and for age, gender and BMI) | |||||
| Activin A (pg/ml) | 393 ± 25 | 359 ± 19 | 397 ± 28 | 405 ± 29 | 0.43 |
| Follistatin (ng/ml) | 1.06 ± 0.10 | 1.02 ± 0.07 | 0.87 ± 0.11 | 1.19 ± 0.11 | 0.18 |
| Model 5 (adjusted mutually and for age, gender and waist circumference) | |||||
| Activin A (pg/ml) | 369 ± 28 | 363 ± 20 | 402 ± 30 | 410 ± 31 | 0.17 |
| Follistatin (ng/ml) | 1.11 ± 0.10 | 1.00 ± 0.07 | 0.84 ± 0.11 | 1.12 ± 0.12 | 0.20 |
| Model 6 (adjusted mutually and for age, gender and leptin) | |||||
| Activin A (pg/ml) | 408 ± 28 | 369 ± 24 | 386 ± 35 | 438 ± 35 | 0.39 |
| Follistatin (ng/ml) | 1.11 ± 0.09 | 1.00 ± 0.07 | 0.85 ± 0.10 | 1.14 ± 0.11 | 0.15 |
Data are estimated marginal mean ± standard error of the mean (SEM) or absolute numbers.
Between groups comparison (Analysis of covariance; ANCOVA).
Abbreviations: BMI, body mass index; NASH, nonalcoholic steatohepatitis; SS, simple steatosis.
Figure 1.
Serum activin A (a), follistatin (b) and activin A to follistatin ratio (c) (mean ± standard error of the mean) in lean and obese controls, SS and NASH patients.
P-values for trend refer to ANOVA results.
*: p=0.015 between NASH and lean controls, and p=0.014 between NASH and obese controls (multiple pairwise comparisons after Bonferroni correction).
When compared between total groups of patients (SS and NASH; n=31) and controls (lean and obese; n=52), activin A was higher in NAFLD patients (462±39 vs. 358±15 pg/ml, respectively; p=0.005), whereas follistatin was similar between patients and controls (1.00±0.07 vs. 1.05±0.05 ng/ml, respectively; p=0.55). Moreover, AFR was higher in NAFLD patients than controls (0.57±0.08 vs. 0.39±0.03, respectively; p=0.040).
After mutual adjustment (i.e., activin A for follistatin and follistatin for activin A) activin A, but not follistatin, remained significant, between groups (Table 1; model 1). Moreover, differences between groups did not change following sequential mutual adjustment plus adjustment for age (model 2), or for age and gender (model 3). However, when BMI (model 4), waist circumference (model 5) or leptin (model 6) were added to the model, differences between groups became non-significant for both activin A and follistatin (Table 1).
3.2. Association of activin A, follistatin and their ratio with other parameters
Correlation matrices, separately for total groups of controls (n=52) and patients (n=31) are presented in Table 2 and Table 3, respectively. Activin A was not correlated with follistatin in either NAFLD patients or controls.
Table 2.
Correlations matrix between activin A, follistatin and their ratio, and other study’s parameters for all controls (n=52).
| Activin A (pg/ml) | Follistatin (ng/ml) | Activin A to Follistatin ratio |
|
|---|---|---|---|
| Age (years) | 0.52 (<0.001)* | 0.20 (0.15) | 0.18 (0.22) |
| BMI (kg/m2) | 0.01 (0.92) | −0.16 (0.27) | 0.18 (0.20) |
| Waist circumference (cm) | −0.03 (0.82) | −0.04 (0.79) | 0.06 (0.69) |
| AST (U/l) | 0.05 (0.75) | −0.17 (0.22) | 0.16 (0.27) |
| ALT (U/l) | −0.19 (0.18) | −0.13 (0.35) | 0.00 (1.00) |
| AST/ALT ratio | 0.29 (0.15) | −0.05 (0.83) | 0.14 (0.50) |
| GGT (U/l) | −0.39 (0.005)* | −0.22 (0.11) | −0.09 (0.54) |
| Total cholesterol (mg/dl) | 0.12 (0.42) | 0.08 (0.60) | 0.05 (0.75) |
| HDL-C (mg/dl) | 0.17 (0.24) | −0.17 (0.25) | 0.21 (0.15) |
| LDL-C (mg/dl) | 0.15 (0.31) | 0.09 (0.51) | 0.07 (0.65) |
| Triglycerides (mg/dl) | −0.17 (0.42) | 0.01 (0.96) | −0.10 (0.48) |
| Urea (mg/dl) | 0.33 (0.10) | 0.09 (0.67) | 0.19 (0.35) |
| Creatinine (mg/dl) | −0.17 (0.41) | −0.40 (0.042)* | 0.13 (0.53) |
| Uric acid (mg/dl) | 0.08 (0.60) | −0.13 (0.40) | 0.09 (0.54) |
| Glucose (mg/dl) | −0.06 (0.70) | −0.10 (0.47) | 0.02 (0.91) |
| Insulin (µU/ml) | 0.08 (0.60) | −0.10 (0.50) | 0.15 (0.31) |
| HOMA-IR | 0.06 (0.68) | −0.09 (0.54) | 0.12 (0.39) |
| Leptin (ng/ml) | 0.18 (0.21) | 0.10 (0.49) | 0.10 (0.49) |
| Adiponectin (µg/ml) | 0.53 (<0.001)* | 0.06 (0.67) | 0.31 (0.027)* |
| RBP-4 (µg/ml) | −0.29 (0.036)* | −0.08 (0.57) | −0.13 (0.36) |
| Chemerin (ng/ml) | −0.03 (0.82) | 0.16 (0.26) | −0.12 (0.42) |
| Irisin (ng/ml) | −0.18 (0.21) | 0.15 (0.78) | −0.33 (0.016)* |
| Activin A (pg/ml) | - | 0.02 (0.88) | 0.63 (<0.001)* |
| Follistatin (ng/ml) | - | - | −0.73 (<0.001)* |
Data are presented as Spearman’s rho coefficient (p-value).
: Correlations with p-value<0.05.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; GGT, gamma-glutamyl transferase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model of assessment insulin resistance; LDL-C, low density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; RBP, retinol-binding protein.
Table 3.
Correlations matrix between activin A, follistatin and their ratio, and other study’s parameters for all NAFLD patients (n=31; sum of SS and NASH patients).
| Activin A (pg/ml) | Follistatin (ng/ml) | Activin A to Follistatin ratio |
|
|---|---|---|---|
| Age (years) | 0.32 (0.10) | −0.05 (0.79) | 0.22 (0.26) |
| BMI (kg/m2) | 0.51 (0.005)* | −0.04 (0.83) | 0.42 (0.026)* |
| Waist circumference (cm) | 0.12 (0.57) | 0.01 (0.95) | 0.14 (0.48) |
| AST (U/l) | 0.35 (0.06) | 0.17 (0.36) | 0.08 (0.67) |
| ALT (U/l) | −0.03 (0.87) | 0.26 (0.15) | −0.22 (0.26) |
| AST/ALT ratio | 0.51 (0.005)* | −0.14 (0.45) | 0.40 (0.031)* |
| GGT (U/l) | 0.21 (0.27) | 0.18 (0.34) | 0.04 (0.84) |
| Total cholesterol (mg/dl) | −0.35 (0.06) | 0.33 (0.07) | −0.43 (0.020)* |
| HDL-C (mg/dl) | 0.01 (0.97) | 0.14 (0.47) | −0.03 (0.89) |
| LDL-C (mg/dl) | −0.26 (0.18) | 0.27 (0.15) | −0.32 (0.09) |
| Triglycerides (mg/dl) | 0.06 (0.75) | 0.21 (0.25) | −0.17 (0.39) |
| Urea (mg/dl) | −0.31 (0.10) | −0.12 (0.51) | −0.14 (0.48) |
| Creatinine (mg/dl) | −0.31 (0.10) | −0.08 (0.66) | −0.17 (0.38) |
| Uric acid (mg/dl) | −0.27 (0.15) | −0.04 (0.85) | −0.17 (0.38) |
| Glucose (mg/dl) | 0.48 (0.009)* | 0.10 (0.58) | 0.19 (0.33) |
| Insulin (µU/ml) | 0.50 (0.006)* | 0.11 (0.55) | 0.24 (0.20) |
| HOMA-IR | 0.54 (0.003)* | 0.12 (0.51) | 0.27 (0.16) |
| Leptin (ng/ml) | 0.59 (0.001)* | 0.13 (0.48) | 0.31 (0.10) |
| Adiponectin (µg/ml) | 0.22 (0.25) | −0.50 (0.005)* | 0.48 (0.008)* |
| RBP-4 (µg/ml) | −0.31 (0.11) | 0.21 (0.27) | −0.36 (0.06) |
| Chemerin (ng/ml) | 0.32 (0.09) | 0.12 (0.54) | 0.02 (0.92) |
| Irisin (ng/ml) | −0.29 (0.13) | 0.28 (0.12) | −0.33 (0.09) |
| Activin A (pg/ml) | - | −0.10 (0.59) | 0.67 (<0.001)* |
| Follistatin (ng/ml) | - | - | −0.76 (<0.001)* |
Data are presented as Spearman’s rho coefficient (p-value).
: Correlations with p-value<0.05.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; GGT, gamma-glutamyl transferase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model of assessment insulin resistance; LDL-C, low density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RBP, retinol-binding protein; SS, simple steatosis.
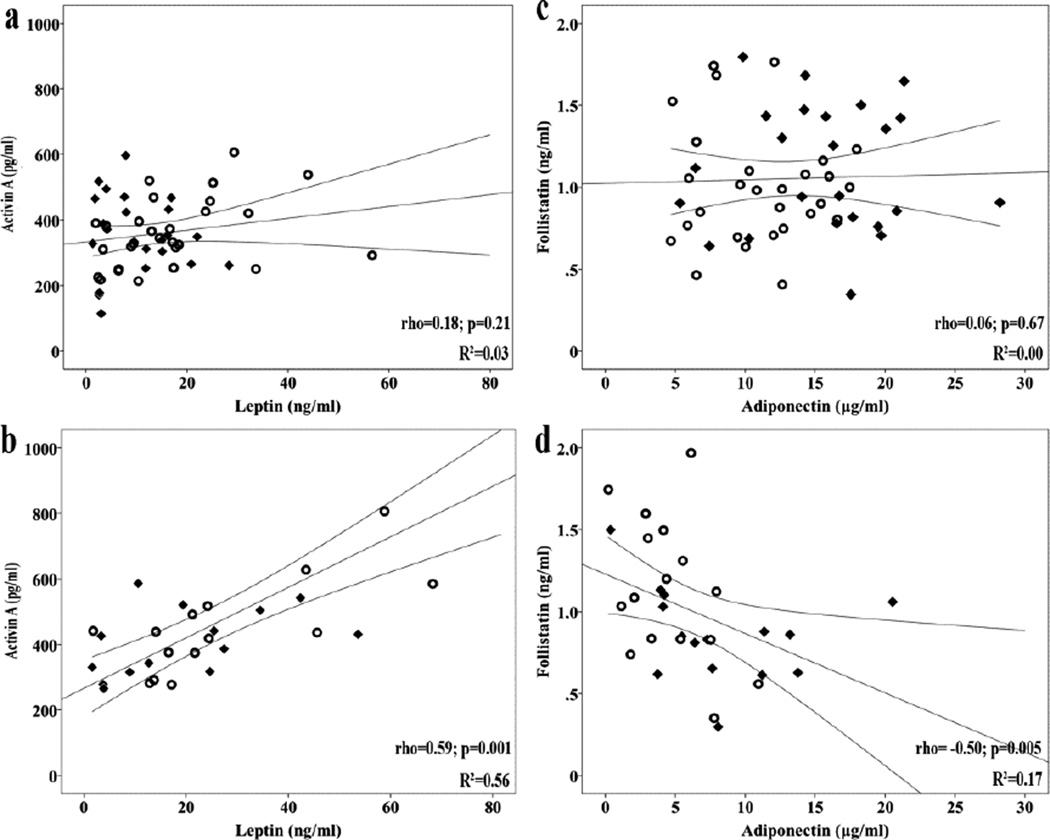
Within group of NAFLD patients (n=31), activin A was positively correlated with BMI, AST to ALT ratio, glucose, insulin, HOMA-IR, and leptin (Figure 2). Follistatin was negatively correlated with adiponectin (Figure 2). AFR was positively correlated with BMI, AST to ALT ratio and adiponectin, whereas negatively with total cholesterol.
Figure 2.
Correlations between activin A and leptin in controls (a) and in NAFLD patients (b), and between follistatin and adiponectin in controls (c) and in NAFLD patients (d). Rhombuses represent lean controls and open circles represent obese controls within controls (a, c). Rhombuses represent SS patients and open circles represent NASH patients within NAFLD patients (b, d). Lines represent linear equation line and its 95% Confidence Intervals.
rho: Spearman’s coefficient.
Within the group of controls (n=52), activin A was positively correlated with age and adiponectin, whereas negatively with GGT and RBP-4. Follistatin was negatively correlated with creatinine. AFR was positively correlated with adiponectin, whereas negatively with irisin (Table 2). After adjustment for the control subgroup (lean vs. obese), activin A remained significantly positively correlated with age (rp=0.53; p<0.001) and adiponectin (rp=0.57; p<0.001), whereas negatively with GGT (rp= −0.33; p=0.021), but not with RBP-4 (rp= −0.26; p=0.07). Follistatin did not remain significantly correlated with creatinine (rp = −0.34; p=0.10). AFR remained positively correlated with adiponectin (rp=0.33; p=0.019), but not with irisin (rp= −0.13; p=0.36).
3.3. Activin A, follistatin and their ratio in relation to specific hepatic histological lesions
The results of activin A, follistatin levels and AFR in relation to the histological lesion of NAFLD patients (n=31) are presented in Supplementary Table 1. Serum follistatin, but not activin A or their ratio, showed a trend towards higher levels (approximately 39%) in NAFLD patients with higher than lower grade of steatosis (p=0.017), though it did not reach the predefined level of statistical significance due to multiple comparisons (p<0.01). No other statistically significant difference was observed between groups.
3.4. Predictive value of activin A, follistatin or their ratio for NASH or hepatic histological lesions
In the sequential models of binary logistic regression analysis within NAFLD patients (n=31), the presence of NASH was selected as dependent variable. Apart from the necessary logarithmic transformations, activin A levels were transformed to ng/ml for regression analyses. Follistatin was significantly associated with the presence of NASH, independently from activin A, gender, age and BMI, though the significance was lost, when waist circumference or leptin levels replaced BMI (models 5b and 5c; Table 4). The association between follistatin and NASH was further attenuated when log(HOMA-IR) was entered into the model [model 6a: B=2.57; exp(B)=13.0; 95% CI: 0.93–183; p=0.06; model 6b: B=2.50; exp(B)=12.1; 95% CI: 0.83–177; p=0.07; model 6c: B=2.95; exp(B)=19.1; 95% CI: 0.87–419; p=0.06]. No other variable could independently predict NASH (Table 4), indicating that central adiposity and/or IR underlie the observed associations. Furthermore, AFR was not independently associated with NASH.
Table 4.
Sequential models of binary logistic regression analysis evaluating independent predictors of NASH within NAFLD patients (n=31; sum of SS and NASH patients).
| Variable | B | Exp(B) | p-value | 95% CI for Exp(B) |
|---|---|---|---|---|
| Model 1α | ||||
| Activin A (ng/ml) | 3.62 | 37.3 | 0.21 | 0.13 – 10962 |
| Model 1b | ||||
| Follistatin (ng/ml) | 2.12 | 8.3 | 0.063 | 0.89 – 78.1 |
| Model 2 | ||||
| Activin A (ng/ml) | 4.39 | 80.3 | 0.21 | 0.08 – 77055 |
| Follistatin (ng/ml) | 2.36 | 10.6 | 0.053 | 0.97 – 114 |
| Model 3 | ||||
| Activin A (ng/ml) | 3.85 | 46.8 | 0.16 | 0.22 – 9883 |
| Follistatin (ng/ml) | 2.77 | 16.0 | 0.046 | 1.06 – 242 |
| Gender (0: men; 1: women) | 1.25 | 3.48 | 0.235 | 0.44 – 27.3 |
| Model 4 | ||||
| Activin A (ng/ml) | 3.73 | 41.6 | 0.16 | 0.22 – 7911 |
| Follistatin (ng/ml) | 2.75 | 15.6 | 0.044 | 1.07 – 227 |
| Gender (0: men; 1: women) | 1.09 | 2.98 | 0.32 | 0.35 – 25.3 |
| Age (years) | 0.03 | 1.0 | 0.65 | 0.92 – 1.14 |
| Model 5a | ||||
| Activin A (ng/ml) | −0.17 | 0.8 | 0.97 | 0 – 16165 |
| Follistatin (ng/ml) | 2.83 | 17.0 | 0.038 | 1.17 – 247 |
| Gender (0: men; 1: women) | 1.38 | 4.0 | 0.27 | 0.34 – 46.2 |
| Age (years) | 0.04 | 1.0 | 0.53 | 0.93 – 1.2 |
| BMI (kg/m2) | 0.05 | 1.0 | 0.65 | 0.86 – 1.3 |
| Model 5b | ||||
| Activin A (ng/ml) | −0.16 | 0.9 | 0.97 | 0 – 7088 |
| Follistatin (ng/ml) | 2.73 | 15.3 | 0.050 | 1.00 – 233 |
| Gender (0: men; 1: women) | 1.39 | 4.0 | 0.26 | 0.36 – 44.6 |
| Age (years) | 0.04 | 1.0 | 0.50 | 0.93 – 1.16 |
| Waist circumference (cm) | 0.02 | 1.0 | 0.56 | 0.95 – 1.11 |
| Model 5c | ||||
| Activin A (ng/ml) | 5.32 | 204.6 | 0.19 | 0.07 – 612686 |
| Follistatin (ng/ml) | 3.10 | 22.2 | 0.054 | 0.95 – 516 |
| Gender (0: men; 1: women) | 1.50 | 4.49 | 0.28 | 0.30 – 66.6 |
| Age (years) | 0.03 | 1.0 | 0.64 | 0.92 – 1.14 |
| Leptin (ng/ml) | −0.02 | 1.0 | 0.60 | 0.91 – 1.06 |
SS was rated as 0 and NASH as 1 within dependent variable.
Abbreviations:: BMI, body mass index; HOMA-IR, homeostasis model of assessment insulin resistance, NASH, nonalcoholic steatohepatitis; SS, simple steatosis.
When we applied the same models of multiple linear regression analysis in specific histological lesions, follistatin (B=3.48; exp(B)=32.3; 95% CI: 1.11–939; p=0.043), but not activin A, provided a trend towards association with steatosis grade independently from gender, age and BMI, though it did not reach the predefined level of statistical significance due to multiple comparisons (p<0.01). No other variable could independently predict steatosis grade. It is underlined that higher steatosis grade was observed in NASH (69% of the group had steatosis grade ≥33%) than SS (all in the group had steatosis grade <33%) patients (p<0.001). Neither follistatin nor activin A were independently associated with fibrosis stage, ballooning, lobular and portal inflammation. Likewise, AFR was not independently associated with any histological lesion.
4. Discussion
We observed a trend towards progressive increase of activin A levels from the lean to obese controls, to patients with SS and NASH. This significance was lost when BMI or waist circumference or leptin were added as potential cofounders (Table 1, Figure 1). Although follistatin was not different between groups (Table 1), follistatin was significantly associated with NASH within the group of NAFLD patients independently from activin A, gender and age. However, the significance was lost after adjustment for fat mass or its correlates such as HOMA-IR (Table 4), indicating that adiposity may underlie the association between follistatin levels and NASH. The AFR was not different between subgroups, possibly due to lack of power, but was higher in the sum of NAFLD patients (n=31) as compared with the sum of controls (n=52). Future clinical studies are needed to confirm and expand our findings, whereas mechanistic studies exploring underlying mechanisms are also warranted.
There are very limited data on activin A and follistatin levels in NAFLD patients. Similar to our findings, Yndestad et al. observed a gradual increase in activin A levels from controls to SS patients and then to NASH patients [10]. On the contrary, follistatin was higher in their NAFLD (both SS and NASH) patients than controls and could not differentiate NASH, which however was independently associated with activin A [10]. Furthermore, activin A was positively associated with fibrosis stage, a finding, which was also not replicated in our study. Population and methodological differences may be responsible for the partial discrepancy between the results of this study [10] and ours. For example, Yndestad et al. recruited one control group of lower BMI and age than patients, whereas we had two (lean and obese) control groups of similar age.
Based on their findings, Yndestad et al. proposed that activin A may have a dual role in the pathogenesis of NAFLD: it may protect from steatosis, but, when the disease progresses, may worsen inflammation and fibrosis [9,10]. This hypothesis resembles the role of leptin in NAFLD, as we have proposed elsewhere: leptin seems to have anti-steatotic effect in early NAFLD, but may contribute to inflammation and fibrosis, when the disease progresses [17,18]. It is highlighted that activin A and leptin were positively correlated in our study (Table 3), and follistatin did not remain independently associated with NASH when leptin was entered in the model (Table 4). Previous clinical studies have shown that energy deprivation in healthy individuals alters activin A and leptin towards the same directions, although the reported changes in activin A were leptin-independent [19,20].
Early experimental studies showed that activin A contributes to the restoration of tissue architecture during liver regeneration [21]. On the other hand, activin A induces hepatocyte apoptosis and inhibits hepatocyte growth [22]; apart from its pro-apoptotic effect, in adult hepatocytes activin A has been shown to induce growth arrest and block DNA synthesis. Notably, a higher apoptosis rate is a hallmark of NASH pathogenesis [23]. Follistatin is a well-characterized binding protein of activin A. It prevents activin A, from interacting with its signaling receptors. It was shown that follistatin binds activin A almost irreversibly, thereby activin A is practically inactivated upon binding [9]. Importantly, follistatin is expressed in the same tissues as activin A and its synthesis is induced by activin A, suggesting that follistatin may constitute a negative loop to prevent activin A hyperactivity [24]. Based on aforementioned findings and our results, we could speculate that activin A production is increased with NAFLD progression, initially trying to limit steatosis, but then contributing to the pathogenesis of NASH. At the stage of SS, when activin A may have an anti-steatotic effect [9], follistatin levels decline, to allow activin A to exert its anti-steatotic effect. However, when the disease progresses, increased activin A induces follistatin production in an attempt to self-limit its inflammatory and fibrogenic effects on the liver. Notably, our findings indicate that follistatin loses its independent association with NASH, when central adiposity or IR are taken into account, which may imply either that follistatin is a marker reflecting central obesity and/or IR status or that follistatin is induced by central obesity and IR, but the potential counterbalancing follistatin action is not sufficient to limit disease progression. This is important in the light of the recent observation that the liver is a major contributor to circulating follistatin, where insulin inhibits and glucagon increases follistatin secretion in the hepatocyte [13]. However, mechanistic studies and long-term prospective studies are required to clarify the validity of our hypotheses.
Recently, follistatin levels were shown to increase less in patients with cirrhosis than healthy controls during glucagon/somatostatin infusion, although baseline follistatin levels were similar between groups [25]. Glucagon/somatostatin infusion experimentally increases the glucagon-insulin ratio, thereby mimicking the hormonal effect of exercise. Despite its small sample size (eight patients and eight controls) and the fact that cirrhosis was of various underlying liver diseases, this study was well performed and provided statistically significant results. The authors speculated that the inhibitory effect of follistatin on myostatin and other TGF-members might be diminished in liver cirrhosis, which may explain the loss of muscle mass observed in these patients, a hypothesis warranting further research.
Regarding the secondary aims of this study, leptin was associated with activin A in patients, but not in the controls, possibly indicating a parallel increase in the disease, as above mentioned. Furthermore, the associations between activin A and glucose, insulin and HOMA-IR were evident only in NAFLD patients, suggesting an important role only in the disease. The positive association between adiponectin, a hormone negatively associated with central adiposity and IR, and activin A in our controls, but not in NAFLD patients, should also be highlighted. Activin A may have a different role in health than disease, being beneficial (e.g. tissue regeneration [21]) in health, but harmful as the disease progresses (e.g. hepatocyte apoptosis [22]). In this regard, activin A may be associated with adiponectin (dependently or independently) in health, when both exert a beneficial effect, but not in disease. Adiponectin was previously reported to upregulate activin A in monocytes [26], whereas this action was impaired by lipid accumulation in human hepatocytes [27], findings in line with our results. However, this is an observational study, thereby only raising hypotheses; further mechanistic studies are required to elucidate the complex relationship between adiponectin and activin A in health and disease. A negative association was also observed between AFR and irisin, which however was abolished after adjustment for control type (lean vs. obese), indicating adiposity as a mediator of the relationship. A positive correlation between circulating follistatin and irisin in healthy males, and between muscle fibronectin type III domain containing (FNDC)5 (the precursor of circulating irisin [28]) mRNA and follistatin mRNA expression in morbidly obese individuals were previously reported [29]. Finally, activin A increased with age in the controls, possibly reflecting higher tissue regenerating needs with increasing age; however, this relationship is not evident in NAFLD patients, in whom other parameters (e.g., BMI and IR) may blunt the physiologic increase of activin A with age, a hypothesis also needing validation.
It should be highlighted that this study warrants further research on follistatin isoforms, other activins and myostatin in NAFLD, possibly in one multivariable model. This may further help to elucidate the mechanisms of the disease, but may also provide the soil for future treatment. In this regard, new medications are now being developed to manipulate the follistatin-myostatin-activin axis and, when mechanisms are elucidated, research on such medications may provide novel therapeutic possibilities.
Strengths of this study are the histological confirmation of NAFLD, the existence of two control groups (lean and obese, since NAFLD is closely related to obesity) and the fact that samples were run using state of the art techniques by personnel blinded to the study hypothesis. However, this study has certain limitations. The sample size is relatively small, though it was sufficient to provide statistically significant difference for activin A between groups, and show that, within NAFLD patients, follistatin was independently higher in NASH after adjustment for potential cofounders. Moreover, the case-control design of the study cannot prove causality, but can certainly raise credible hypotheses to be confirmed by future studies. Furthermore, the controls were not subjected to liver biopsy due to ethical considerations, since their liver function tests and liver ultrasonography were negative.
In conclusion, serum activin A showed a trend towards progressive increase from lean to obese controls, to SS and NASH patients, but the significance was lost after adjustment for measures of adiposity. Activin A was not independently associated with NASH or any specific hepatic lesion within NAFLD patients. Follistatin levels were not different between groups, but they were associated with NASH (within NAFLD patients) independently from activin A, gender and age. Although this association was blunted when adjusted for adiposity, these data suggest that follistatin may enhance progression from SS to NASH. Prospective studies are needed to confirm the hypothesis raised herein, before mechanistic studies attempt to elucidate mechanism and prove causality.
Supplementary Material
Acknowledgments
The authors feel more than grateful to Dr Efthimia Zafeiriadou (experienced radiologist), who performed the liver biopsies under computed tomography-guidance, and to Dr Kalliopi Patsiaoura and Dr Evangelia Katsiki (experienced pathologists), who interpreted the histological specimens.
Funding: This study was supported in part by a NIH grant (ID: DK081913).
Abbreviation list
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- FNDC
fibronectin type III domain containing
- GGT
gamma-glutamyl transferase
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model of assessment insulin resistance
- IR
insulin resistance
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- LDL-C
low density lipoprotein cholesterol
- RBP
retinol-binding protein
- SS
simple steatosis
- T2DM
type 2 diabetes mellitus
- TGF
tumor growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to disclose.
Author contributions
S.A. Polyzos: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; final approval
J. Kountouras: study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; final approval
A.D. Anastasilakis: acquisition of data; critical revision of the manuscript for important intellectual content; final approval
G. Triantafyllou: acquisition of data; critical revision of the manuscript for important intellectual content; final approval
C.S. Mantzoros: study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; final approval
References
- 1.Polyzos SA, Mantzoros CS. Nonalcoholic fatty future disease. Metabolism. 2015;65:1007–1016. doi: 10.1016/j.metabol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of nonalcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism. 2016;65:1151–1160. doi: 10.1016/j.metabol.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polyzos SA, Mantzoros CS. Necessity for timely noninvasive diagnosis of nonalcoholic fatty liver disease. Metabolism. 2014;63:161–167. doi: 10.1016/j.metabol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Polyzos SA, Kountouras J, Zavos C, Deretzi G. Nonalcoholic fatty liver disease: Multimodal treatment options for a pathogenetically multiple-hit disease. J Clin Gastroenterol. 2012;46:272–284. doi: 10.1097/MCG.0b013e31824587e0. [DOI] [PubMed] [Google Scholar]
- 9.Yndestad A, Haukeland JW, Dahl TB, Halvorsen B, Aukrust P. Activin A in nonalcoholic fatty liver disease. Vitam Horm. 2011;85:323–342. doi: 10.1016/B978-0-12-385961-7.00015-9. [DOI] [PubMed] [Google Scholar]
- 10.Yndestad A, Haukeland JW, Dahl TB, Bjoro K, Gladhaug IP, Berge C, et al. A complex role of activin A in non-alcoholic fatty liver disease. Am J Gastroenterol. 2009;104:2196–2205. doi: 10.1038/ajg.2009.318. [DOI] [PubMed] [Google Scholar]
- 11.Ding ZY, Jin GN, Wang W, Sun YM, Chen WX, Chen L, et al. Activin A-Smad Signaling Mediates Connective Tissue Growth Factor Synthesis in Liver Progenitor Cells. Int J Mol Sci. 2016;17:408. doi: 10.3390/ijms17030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedger MP, de Kretser DM. The activins and their binding protein, follistatin-Diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–295. doi: 10.1016/j.cytogfr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JS, Rutti S, Arous C, Clemmesen JO, Secher NH, Drescher A, et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J Clin Endocrinol Metab. 2016;101:550–560. doi: 10.1210/jc.2015-3668. [DOI] [PubMed] [Google Scholar]
- 14.Kreidl E, Ozturk D, Metzner T, Berger W, Grusch M. Activins and follistatins: Emerging roles in liver physiology and cancer. World J Hepatol. 2009;1:17–27. doi: 10.4254/wjh.v1.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. 2014;63:207–217. doi: 10.1016/j.metabol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Polyzos SA, Kountouras J, Zavos C, Deretzi G. The Potential Adverse Role of Leptin Resistance in Nonalcoholic Fatty Liver Disease: A Hypothesis Based on Critical Review of Literature. J Clin Gastroenterol. 2011;45:50–54. doi: 10.1097/MCG.0b013e3181ec5c66. [DOI] [PubMed] [Google Scholar]
- 18.Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism. 2015;64:60–78. doi: 10.1016/j.metabol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Moragianni VA, Aronis KN, Chamberland JP, Mantzoros CS. Short-term energy deprivation alters activin a and follistatin but not inhibin B levels of lean healthy women in a leptin-independent manner. J Clin Endocrinol Metab. 2011;96:3750–3758. doi: 10.1210/jc.2011-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab. 2011;96:3416–3423. doi: 10.1210/jc.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Bleser PJ, Niki T, Xu G, Rogiers V, Geerts A. Localization and cellular sources of activins in normal and fibrotic rat liver. Hepatology. 1997;26:905–912. doi: 10.1002/hep.510260416. [DOI] [PubMed] [Google Scholar]
- 22.Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- 23.Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;72:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 24.Rodgarkia-Dara C, Vejda S, Erlach N, Losert A, Bursch W, Berger W, et al. The activin axis in liver biology and disease. Mutat Res. 2006;613:123–137. doi: 10.1016/j.mrrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Rinnov AR, Plomgaard P, Pedersen BK, Gluud LL. Impaired follistatin secretion in cirrhosis. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-1923. [DOI] [PubMed] [Google Scholar]
- 26.Weigert J, Neumeier M, Wanninger J, Schober F, Sporrer D, Weber M, et al. Adiponectin upregulates monocytic activin A but systemic levels are not altered in obesity or type 2 diabetes. Cytokine. 2009;45:86–91. doi: 10.1016/j.cyto.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Wanninger J, Neumeier M, Hellerbrand C, Schacherer D, Bauer S, Weiss TS, et al. Lipid accumulation impairs adiponectin-mediated induction of activin A by increasing TGFbeta in primary human hepatocytes. Biochim Biophys Acta. 2011;1811:626–633. doi: 10.1016/j.bbalip.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Polyzos SA, Kountouras J, Shields K, Mantzoros CS. Irisin: a renaissance in metabolism? Metabolism. 2013;62:1037–1044. doi: 10.1016/j.metabol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Vamvini MT, Aronis KN, Panagiotou G, Huh JY, Chamberland JP, Brinkoetter MT, et al. Irisin mRNA and circulating levels in relation to other myokines in healthy and morbidly obese humans. Eur J Endocrinol. 2013;169:829–834. doi: 10.1530/EJE-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.