Abstract
Background
Sphingosine-1-phosphate (S1P), a pleiotropic bioactive lipid mediator, has been implicated as a key regulatory molecule in cancer through its ability to promote cell proliferation, migration, angiogenesis and lymphangiogenesis. Previous studies suggested that S1P produced by sphingosine kinase 1 (SphK1) in breast cancer plays important roles in progression of disease and metastasis. However, the associations between S1P and clinical parameters in human breast cancer have not been well investigated to date.
Materials and Methods
We determined levels of S1P and other sphingolipids in breast cancer tissue by electrospray ionization-tandem mass spectrometry. Associations between S1P levels and clinicopathological features of the tumors were analyzed. Expression of phospho-SphK1 (pSphK1) in breast cancer tissues was determined by immunohistochemical scoring.
Results
Levels of S1P in breast cancer tissues were significantly higher in patients with high white blood cell count in the blood than patients without. S1P levels were lower in patients with HER2 overexpression/amplification than patients without. Further, cancer tissues with high pSphK1 expression showed significantly higher levels of S1P than cancer tissues without. Finally, patients with lymph node metastasis showed significantly higher levels of S1P in tumor tissues than patients with negative nodes.
Conclusions
To our knowledge, this is the first study to demonstrate that high expression of pSphK1 is associated with higher levels of S1P, which in turn is associated with lymphatic metastasis in breast cancer.
Keywords: Sphingosine-1-phosphate, Phospho-sphingosine kinase 1, Breast cancer, Lymphatic metastasis, HER2, Inflammation
1. Introduction
Breast cancer is the most common cancer diagnosis, and the second most common cause of cancer death among women in the United States [1]. Lymph node metastasis is a hallmark of breast cancer and is one of the major determinants of clinical staging and prognosis [2]. There has been growing evidence that tumor lymphangiogenesis, formation of new lymphatic vessels from the preexisting ones, plays an important role during lymphatic metastasis [3-5]. A number of signaling proteins, such as vascular endothelial growth factor (VEGF)-C/D and angiopoietins, are reported to mediate lymphangiogenesis. In addition to these signaling proteins, it has been recently discovered that bioactive lipid mediators also play critical roles in lymphangiogenesis and lymph node metastasis, thus providing new insights into the mechanisms of cancer metastasis [6].
Sphingosine-1-phosphate (S1P), a bioactive lipid mediator, has been implicated as a key regulatory molecule in cancer through its ability to promote cell proliferation, migration, invasion, angiogenesis and lymphangiogenesis [7-12]. S1P is generated intracellularly by two sphingosine kinases, sphingosine kinase 1 and 2 (SphK1 and SphK2), and is exported out of the cells where it regulates many functions by binding to and signaling through a family of five G protein-coupled receptors (S1PR1–5) [8, 13]. This process, known as "inside-out" signaling, explains the autocrine and paracrine actions of S1P [13]. In breast cancer, we have previously demonstrated that SphK1, but not SphK2, is involved in S1P export from breast cancer cells through the action of ATP-binding cassette transporters, ABCC1 and ABCG2 [14-16]. It has been demonstrated that SphK1 mRNA expression is upregulated in breast cancer, correlates with poor prognosis, and is associated with resistance to chemotherapy [17-19]. These studies suggest that S1P plays important roles in cancer progression and metastasis. However, the actual quantification of S1P in human breast cancer tissues has not been well investigated to date, limiting its ability to be used clinically as a biomarker. Furthermore, quantification of S1P mass in breast cancer could allow more accurate comparisons and evaluation of function between studies.
The aim of this study is to determine the mass quantities of bioactive sphingolipids, including sphingosine, S1P, dihydro-sphingosine, and dihydro-S1P, in human patient breast cancer tissue samples using high sensitivity liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) and to investigate associations between the levels of sphingolipids and clinicopathological features of the tumor. Furthermore, we hypothesized that activation of SphK1 is associated with higher levels of S1P in tumor. To test this hypothesis, we performed immunohistochemical staining of active form of phospho-SphK1 (pSphK1) and compared it to the S1P levels in tumor.
2. Material and Methods
2.1. Human breast cancer tissue samples
From January 2013 to April 2015, 289 Japanese patients with breast cancer underwent surgical resection in Niigata University Medical and Dental Hospital. During this period, breast cancer tissue samples were collected from 47 patients who had invasive tumors larger than 1.5 cm after obtaining informed consent. We analyzed 35 of the 47 primary breast cancer tissue samples, excluding a total of 12 patients. The excluded tissue samples included five patients who received neoadjuvant chemotherapy, two patients with body mass index greater than 35, three patients with bilateral breast cancer, and four patients with cancer in other organs. These patients were excluded because the above factors in these patients possibly affect levels of S1P in the body [20]. The blood counts were measured as a part of preoperative workup, which is within 30 days prior to the operation. All tissue samples were snap-frozen and stored at −80°C. This study protocol was approved by the Institutional Review Board of Niigata University Medical and Dental Hospital.
2.2. Quantification of Sphingolipids by LC-ESI-MS/MS
Lipids were extracted from tissue samples and sphingolipids were quantified by liquid chromatography, electrospray ionization-tandem mass spectrometry (LC-ESIMS/MS, 4000 QTRAP, ABI) at the Virginia Commonwealth University Lipidomics Core as described previously [6, 15, 21]. Internal standards were purchased from Avanti Polar Lipids (Alabaster, AL) and added to samples in 20 μl ethanol:methanol:water (7:2:1) in a cocktail of 500 pmol each. The HPLC grade solvents were obtained from VWR (West Chester, PA).
2.3. Pathologic Examination
All of the human breast cancer tissue specimens were submitted to the Department of Surgical Pathology in our hospital and examined by two experienced pathologists who had no access to the clinical data. Paraffin-embedded blocks from each resected specimen were used for immunohistochemistry. Serial 4μm sections were re-cut and used for staining with hematoxylin and eosin, estrogen receptor (ER), progesterone receptor (PgR), Human epidermal growth factor receptor 2 (HER2), Ki-67 proliferation index marker, pSphK1, and negative control. The ER and PgR protein expression was scored using the weighted Allred score technique. Ki-67 index was scored by counting positive and negative nuclei in the tumor specimen and a proliferation index was obtained by calculating the percentage of positive cells. Tumors were classified as having low or high proliferation index with 14% positive cells as the cut-off. HER2 expression was also determined by immunohistochemistry and by fluorescence in situ hybridization (FISH) analysis. HER2 positive tumors were defined as 3+ on immunohistochemistry or as positive by FISH.
Antigen retrieval for pSphK1 was performed by microwaving the slides under pressure in a citrate buffer for 10 min (pH 9.0). Endogenous peroxidase was blocked using 0.3% hydrogen peroxide for 20 min. After blocking non-specific background, the sections were incubated overnight with the primary antibody (SphK1 polyclonal antibody; 1:100 dilution; ECM Biosciences LLC, Versailles, KY, USA) at 4°C. Then, the sections were incubated with biotinylated rabbit anti-mouse streptavidin-peroxidase complex for 10 min. Diaminobenzidine was used as the chromogen, and the sections were counterstained with hematoxylin. Normal mouse immunoglobulin was substituted as the primary antibodies in the negative control. The vascular and lymphatic endothelial cells of all vessels reacted with the antibody against pSphK1. As a result, the pSphK1 staining intensity of endothelial cells is considered strong staining, and semi-quantitative evaluation of the pSphK1 staining intensity of breast cancer cells was registered as follows: 0 (negative), 1 (weak), 2 (moderate), or 3 (strong), based on comparison with endothelial cell staining. The staining score 0 and 1 were considered as pSphK1 negative and 2 and 3 considered as pSphK1 positive.
2.4. Comparison of the sphingolipids levels in the breast cancer tissue with clinicopathological status of breast tumors
Histologic findings were described according to the AJCC Staging Manual [22]. We excluded micro metastasis (< 2 mm) from consideration as lymph node metastasis. Levels of S1P and other sphingolipids in the breast cancer tissue samples were compared with clinicopathological status of breast cancer patients.
2.5. Statistical Analysis
All statistical evaluations were performed using the SPSS 22.0J software package (SPSS Japan, Tokyo, Japan). Categorical variables were compared by the Fisher exact test or the Pearson χ2 test and continuous variables between two groups were compered by the Mann-Whitney U test. All tests were two-sided and P values < 0.05 were considered statistically significant.
3. Results
3.1. Determination of sphingosine-1-phosphate (S1P) and other sphingolipid levels from 35 human patients with breast cancer
The levels of sphingolipids including sphingosine, dihydro-sphingosine (DHSph), S1P, dihydro-S1P (DHS1P) were successfully determined in breast cancer tissue samples from 35 patients. Clinical characteristics and histopathological results of the 35 patients are shown in Tables 1 and 2. Sixty percent (n = 21) of patients were under 60 years old.
Table 1.
Clinical Background
| Characteristics | Number of patients (%) |
|---|---|
| Age (years) | |
| <60 | 21 (60) |
| ≧60 | 14 (40) |
| Menopause status | |
| Premenopausal | 14 (40) |
| Postmenopausal | 21 (60) |
| Body Mass Index | |
| <25 | 27 (77) |
| ≧25, <35 | 8 (23) |
| Type of surgery | |
| Mastectomy + SLNB | 15 (43) |
| Mastectomy + Ax | 13 (37) |
| Lumpectomy + SLNB | 2 (6) |
| Lumpectomy + Ax | 5 (14) |
Ax: axillary lymph node dissection; SLNB: sentinel lymph node biopsy
Table 2.
Pathological Background
| Characteristics | Number of patients (%) |
|---|---|
| Primary tumor* | |
| T1 | 11 (31) |
| T2 | 19 (54) |
| T3 | 5 (14) |
| Regional lymph nodes* | |
| N0 | 18 (51) |
| N1 | 9 (26) |
| N2 | 6 (17) |
| N3 | 2 (6) |
| Distant metastasis* | |
| M0 | 35 (100) |
| M1 | 0 (0) |
| Stage* | |
| I | 6 (17) |
| II | 19 (54) |
| III | 10 (29) |
| Lymphatic invasion* | |
| Absent | 27 (77) |
| Present | 8 (23) |
| Vascular invasion* | |
| Absent | 31 (89) |
| Present | 4 (11) |
| Ki-67 index | |
| <14 | 12 (34) |
| ≧14 | 23 (66) |
| NSAS nuclear grade | |
| 1 | 25 (71) |
| 2 | 5 (14) |
| 3 | 5 (14) |
| Estrogen receptor expression | |
| Negative | 7 (20) |
| Positive | 28 (80) |
| Progesterone receptor expression | |
| Negative | 6 (17) |
| Positive | 29 (83) |
| HER2 overexpression/amplification | |
| Negative | 30 (86) |
| Positive | 5 (14) |
According to the AJCC cancer staging system [22].
3.2. S1P levels in human breast cancer are higher in patients with leukocytosis
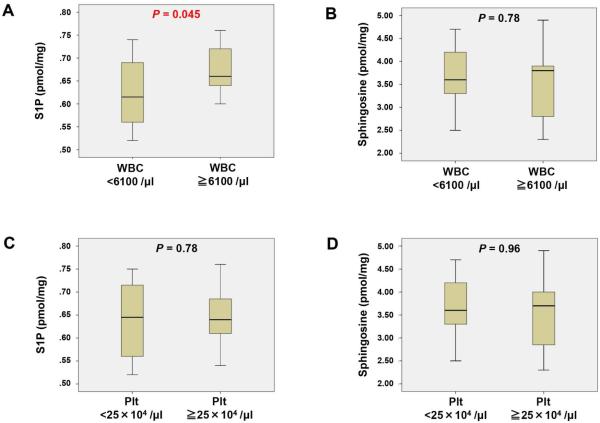
S1P is known to facilitate egress of lymphocytes from secondary lymphoid tissues such as the thymus, Peyer's patches in the small intestine, and lymph nodes into the bloodstream, thereby affecting the white blood cells (WBC) counts in the blood [3, 23-25]. Additionally, red blood cells (RBC) and platelets are known to store and release S1P into blood [26-29]. Therefore, it was of interest to investigate whether sphingolipids levels in breast tumor tissues correlate with blood cell counts (Fig.1 and Supplementary Fig. 1). We found that breast cancer tissue S1P levels were significantly higher in patients with high WBC count (WBC ≧ 6100/μl) as compared to patients without high WBC count (P = 0.045) while sphingosine levels did not show any difference (Fig. 1A, B). There were no significant associations between tissue S1P or sphingosine levels and platelet counts (Fig. 1C, D).
Fig. 1.
Levels of sphingosine-1-phosphate (S1P) and other sphingolipids in breast cancer tissue compared with the blood cell counts of the patients. The levels of S1P and sphingosine were determined by LC-ESI-MS/MS in patients with white blood cell (WBC) < 6100 /μl or ≧ 6100 /μl (A and B) and platelet (Plt) 25×104 /μl or ≧ 25×104/μl (C and D). Mean values are shown by horizontal lines. The statistical analysis was done by the Mann-Whitney U test.
3.3. S1P levels are affected by HER2 status, but not estrogen receptor (ER) or progesterone receptor (PgR) status
We have previously demonstrated that estradiol produces S1P in breast cancer cells via the estrogen receptor [15]. Thus, we investigated the association between levels of sphingolipids in breast cancer tissue and hormone receptors (ER, PgR) and HER2 expression status (Fig. 2 and Supplementary Fig. 2, 3A, B). Although the breast cancer tissue S1P levels were not associated with ER or PgR expression, it was higher in HER2 negative cancers as compared to cancers that overexpress HER2 (P = 0.001) (Fig. 2).
Fig. 2.
Levels of S1P and other sphingolipids in breast cancer tissue based on status of ER, PgR or HER2 overexpression/amplification. The levels of S1P were determined in the patients with ER negative or positive (A), and in the patients with PgR negative or positive (B). The levels of S1P and sphingosine were determined in the patients with HER2 overexpression/amplification negative or positive (C and D). Mean values are shown by horizontal lines. The statistical analysis was done by the Mann-Whitney U test.
3.4. High expression of phospho-SphK1 is associated with S1P levels
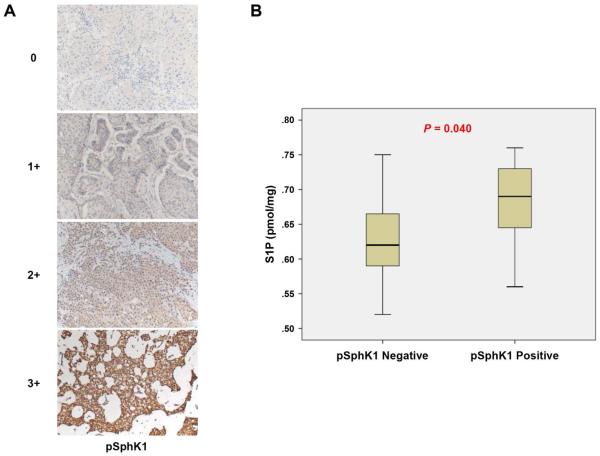
We have shown that SphK1, but not SphK2, is involved in S1P export from breast cancer cells [15]. Sphingosine is converted to S1P when SphK1 is activated by phosphorylation of Ser-225. We examined pSphK1 (Ser-225) expression in breast cancer tissues by immunohistochemistry. pSphK1 was expressed diffusely in the cytoplasm of cancer cells, which were scored as shown in Figure 3A. pSphK1 expression significantly associated with S1P levels in the breast cancer tissue (P = 0.040), which is in agreement with previously published reports using in vitro models (Fig. 3B). The levels of other sphingolipids in breast cancer tissue were not associated with tissue pSphK1 expression (Supplementary Fig. 3C, D and E)
Fig. 3.
S1P levels are associated with phospho-sphingosine kinase 1 (pSphK1) expression. (A) Staining intensity is determined as shown; the staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The staining score 0 and 1 was determined as pSphK1 negative and 2 and 3 determined as pSphK1 positive. (B) The levels of S1P were determined in the patients with pSphK1 negative or positive. Mean values are shown by horizontal lines. The statistical analysis was done by the Mann-Whitney U test.
3.5. All five tumors with HER2 overexpression/amplification displayed suppressed expression of pSphK1
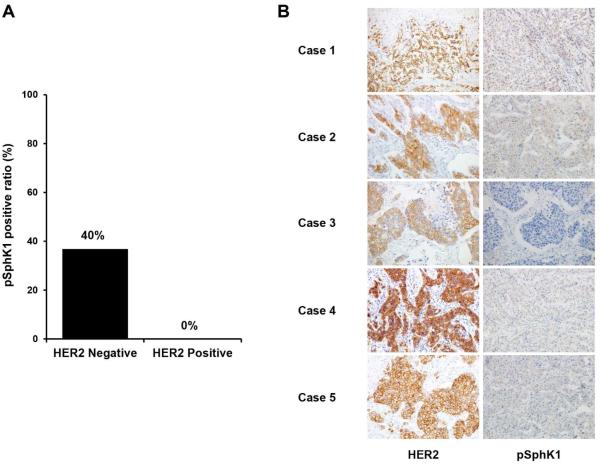
Next, we investigated the association between pSphK1 expression status and other sphingolipids in the breast cancer tissues and the clinicopathological factors (Table 3). pSphK1 expression status in breast cancer tissue was not associated with any clinicopathological factors, possibly due to the limited number of patients we examined. All of five patients with HER2 overexpression/amplification demonstrated negative expression of pSphK1 although there was no statistically significant association between HER2 status and SphK1 expression (Fig. 4).
Table 3.
Relationship between phospho-sphingosine kinase 1 (pSphK1) and clinicopathological factors
| pSphK1 (n=35) |
|||
|---|---|---|---|
| Negative | Positive | P-value | |
| Primary tumor* | |||
| T1 | 8 | 3 | 1.00 |
| T2,T3,T4 | 16 | 8 | |
| Regional lymph nodes* | |||
| Negative | 16 | 5 | 0.28 |
| Positive | 8 | 6 | |
| Estrogen receptor expression | |||
| Negative | 6 | 1 | 0.39 |
| Positive | 18 | 10 | |
| Progesterone receptor expression | |||
| Negative | 5 | 1 | 0.64 |
| Positive | 19 | 10 | |
| HER2 overexpression/amplification | |||
| Negative | 19 | 11 | 0.16 |
| Positive | 5 | 0 | |
| Ki-67 index | |||
| <14 | 6 | 6 | 0.13 |
| ≧14 | 18 | 5 | |
| NSAS nuclear grade | |||
| 1 | 16 | 9 | 0.45 |
| 2,3 | 8 | 2 | |
| Lymphatic invasion* | |||
| Absent | 19 | 8 | 0.69 |
| Present | 5 | 3 | |
| Vascular invasion* | |||
| Absent | 21 | 10 | 1.00 |
| Present | 3 | 1 | |
| White blood cell count (cells per uL) | |||
| <6100 | 13 | 9 | 0.15 |
| ≧6100 | 11 | 2 | |
| Platelet count (cells ×104 per μL) | |||
| <25 | 9 | 7 | 0.27 |
| ≧25 | 15 | 4 | |
According to the AJCC cancer staging system [22].
Fig. 4.
pSphK1 positive ratio in breast cancer tissue compared with HER2 overexpression. (A) pSphK1 positive ratio were determined in the patients with HER2 overexpression/amplification negative or positive. (B) Immunohistochemistry with HER2 and pSphK1 for patients with HER2 overexpression. All five cases of HER2 overexpression displayed pSphK1 negative status.
3.6. The expression of pSphK1 is low while the levels of S1P are high in the triple negative (TN) tumors
We compared the levels of S1P among the subtypes, classified as luminal, luminal-HER2, HER2, and TN types. We found that both luminal and TN shows high levels of S1P in the tumor while HER2 type shows lower levels of S1P compared to the others (Supplementary Fig. 7A). Interestingly, patients with TN breast cancer shows lower expression of pSphK1 on the tumor than those with luminal type breast cancer that contained similar levels of S1P as the TN tumors (Supplementary Fig. 7B).
3.7. S1P levels are higher in the patients with lymph node metastasis (pN)
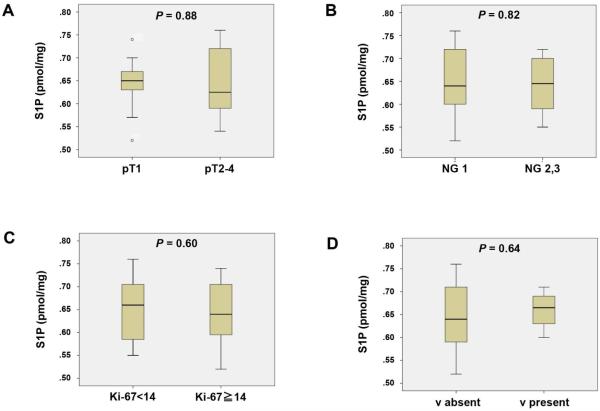
We further examined whether the levels of S1P in the breast cancer tissue are associated with tumor progression and lymph node metastasis in human patients (Fig. 5 and 6, and Supplementary Fig. 4-6). Somewhat surprisingly, S1P levels in the breast cancer tumor samples did not associate with tumor size, quantified as pT (Fig. 5A), cancer aggressiveness evaluated pathologically by nuclear grade (Fig. 5B), cancer cell proliferation quantified by Ki67 staining (Fig. 5C) or vascular invasion (Fig. 5D). On the other hand, S1P levels in breast cancer tissue samples among patients with lymph node metastasis were significantly higher than those without (P = 0.044) (Fig. 6C). Although there was no statistical difference, sphingosine levels in tumor tend to be lower in patients with lymph node metastasis, which is in agreement with the dogma that sphingosine is converted to S1P by pSphK1 (Fig. 6D). There was no association between S1P levels and lymphatic invasion (Fig. 6A).
Fig. 5.
Levels of S1P in breast cancer tissue compared with pathological primary tumor (pT), nuclear grade (NG), the Ki-67 index and vascular invasion (v). The levels of S1P were determined in patients with pT1 or pT2-4 (A), NG 1 or NG 2, 3 (B), Ki-67 index < 14 or Ki-67 index ≧ 14 (C), and v absent or v present (D). Mean values are shown by horizontal lines. The statistical analysis was done by the Mann-Whitney U test.
Fig. 6.
Levels of S1P and sphingosine in breast cancer tissues compared with the lymphatic invasion (ly) and pathological regional lymph nodes (pN) status. The levels of S1P and sphingosine were determined in the patients with ly absent or ly present (A and B), and in the patients with pN0 or pN1-3 (C and D). Mean values are shown by horizontal lines. The statistical analysis was done by the Mann-Whitney U test.
4. Discussion
A pleiotropic bioactive lipid mediator, S1P has emerged as a new player in the tumor microenvironment, where it regulates the processes affecting cancer progression, namely cell proliferation, invasion, angiogenesis, and lymphangiogenesis [30-33]. We have recently developed a sensitive and quantitative mass spectrometry (LC-ESI-MS/MS) method to quantify the S1P levels both in vitro and in vivo [6, 15, 21]. To our knowledge, this is the first study to quantify the levels of S1P in human breast cancer tissues utilizing state-of-art mass spectrometry technology. Further, we compared the levels of S1P in breast cancer tissue with the clinicopathological features of the tumors, and found significant associations between the levels of S1P and pSphK1 expression and lymph node metastasis.
Here, we demonstrate that levels of S1P in the breast cancer tissue in patients with lymph node metastasis were significantly higher than those in patients with negative nodes. Utilizing LC-ESI-MS/MS, we have previously revealed that S1P produced by SphK1 in cancer cells promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis, and subsequently lymph node metastasis in an in vivo model [6]. Therefore, higher levels of S1P in breast cancer is expected to promote lymphangiogenesis and lymph node metastasis. Taken together, our results suggest that higher levels of S1P in cancer influences lymphatic metastasis not only in animal models, but also in human patients with breast cancer.
We previously found that the serum S1P levels were significantly elevated in stage IIIA breast cancer patients who have lymph node metastases, compared with age/ethnicity-matched healthy volunteers [6]. This finding prompted us to hypothesize that S1P secreted from tumor stimulates blood endothelial cells to secrete S1P into the blood, which results in elevation of the levels of S1P in serum and promotion of metastasis. If this is the case, one would expect an association between the levels of S1P in tumors and in serum. In our samples, however, the association was not statistically significant, probably due to the small number of cases (data not shown). Further study with greater power is necessary to address this issue. In addition, there are other factors, such as IL-6 or TNF-α, which are secreted from tumor that potentially stimulates blood endothelial cells and immune cells in the tumor microenvironment to secrete S1P [34]. Similar to tumor tissue, chronic inflammation can also increase serum S1P. Previously, we have shown that the levels of S1P in the serum were elevated in chronic inflammation and found that the higher levels of S1P are associated with enhanced cancer progression using animal models [34]. Taken together, it is possible that tumor affects its microenvironment to increase the levels of S1P in the serum, and elevated levels of S1P in the serum can promote tumor progression.
In this study, we demonstrate the association between expression of pSphK1 and the levels of S1P as determined by LC-ESI-MS/MS in human breast cancer tissue. Previous studies showed that mRNA levels of SphK1 or expression levels of SphK1 measured by immunohistochemistry correlate with worse outcomes for breast cancer patients [17]. One may assume that high levels of SphK1 mRNA or high gene expression levels of SphK1 might result in high levels of S1P mediator in tissue. Importantly, in this study we revealed that pSphK1 positive-expression patients do indeed show significantly higher tissue levels of S1P molecule.
We found that breast cancer tissue S1P levels were lower in those with HER2 overexpression/amplification, and all five patients with HER2 overexpression/amplification demonstrated negative expression of pSphK1 although there was no statistically significant association between HER2 status and SphK1 expression. A few studies reported the relationship between SphK1 and HER2 expression [35-37]. Considering that both HER2 and SphK1 are strong activators of survival signaling pathways such as MAPK, and HER2 signal is a strong autonomous signal, it is tempting to speculate that negative feedback suppresses activation of SphK1 in HER2 positive breast cancer. It has been also reported that SphK1 gene expression is significantly higher in ER negative tumors than ER positive tumors [17, 38], however, we were unable to see that trend in our samples probably due to small number of patients.
We found that the expression of pSphK1 is low while the S1P levels are high in the TN tumors. This discrepancy between the low expression of pSphK1 and high S1P levels in the TN tumor suggests that the main provider of S1P in TN breast cancer may not be the tumor, but the tumor microenvironment. It has been reported that TN breast cancer is more immunogenic and has higher rate of tumor infiltrating lymphocytes [39]. Furthermore, given that immune cells express SphK1 and secrete S1P, we cannot help but speculate that the tumor microenvironment with more immune cells, but not the tumor itself, may provide S1P in the TN tumors.
We demonstrated an association between S1P and serum white blood cell count. S1P and its receptors have long been known to be crucial regulators of inflammatory and immune cell movement [40-42]. It is now recognized that in both homeostatic and disease settings, the SphK1-S1P-S1PRs axis controls the trafficking and migration of numerous types of immune cells, including T and B lymphocytes, natural killer T cells, dendritic cells, macrophages, neutrophils, hematopoietic progenitors, mast cells and osteoclasts [43-46]. This is in agreement with a previous report that the levels of S1P increase in patients with high WBC count [3, 23-25]. In the current study, we found that higher levels of tumor S1P are significantly associated with high WBC count in breast cancer patients, although the absolute difference in the levels is not large. We have previously shown that S1P levels of serum were affected by breast tumor [6]. Moreover, it has been reported that the blood S1P levels are maintained within a relatively small range, and minimal changes in S1P levels affect the immune system and human conditions. For instance, it was reported that slight decreases in S1P in high-density lipoprotein of serum was associated with type 1 diabetes mellitus [47]. It is also known that slight decreases in S1P in high density lipoproteins-containing serum fractions is associated with ischemic heart disease [48]. These findings suggest that even the small change of S1P levels can be related to the human disease.
The main limitations of this study are the retrospective nature of the analysis of a limited number of patients. To our knowledge, however, this is one of the largest series describing with the quantified mass levels of bioactive sphingolipids and the staining intensity of pSphK1 from human breast cancer tumor tissues and evaluating those levels with clinical data. Considering that breast cancer tissue S1P levels are associated with lymphatic metastasis, we believe that S1P in tumor may be useful as one of the biomarkers of lymphatic metastasis. Further, given the fact that lipid mediators including S1P have been overlooked as signaling molecules that play important roles in cancer progression until recently, it is possible that S1P and SphK1 may become one of the additional therapeutic targets in breast cancer.
5. Conclusion
This is the first study to our knowledge to demonstrate that breast cancer tissue S1P levels are associated with activated SphK1 expression in cancer and lymphatic metastasis. Our results suggest that higher levels of S1P in cancer tissue are correlated with lymph node metastasis in human patients with breast cancer.
Supplementary Material
Acknowledgement
We gratefully acknowledge the VCU Lipidomics Core, which is supported in part by funding from the NIH-NCI Cancer Center Support Grant P30CA016059. We thank Drs. Miki Hasegawa and Yu Koyama for sample collection.
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number 16K19888 for J.T., 15H05676 and 15K15471 for M.N. and 15H04927 for W.T. M.N. is supported by the Uehara Memorial Foundation, Nakayama Cancer Research Institute, Takeda Science Foundation, and Tsukada Medical Foundation. K.T. is supported by NIH/NCI grant R01CA160688 and Susan G. Komen Investigator Initiated Research Grant IIR12222224.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
J.T. and M.Nagahashi conceptualized and performed experiments. J.T. prepared the article. M.Nakajima carried out phospho-sphingosine kinase 1 staining. K.M. and K.T. performed data acquisition. R.R. revised the manuscript. M.Nagahashi, K.T. and T.W. provided supervision of experiments and revised the article.
Disclosure
The authors have no conflicts of interest related to this work.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- [2].Mumprecht V, Honer M, Vigl B, Proulx ST, Trachsel E, et al. In vivo imaging of inflammation- and tumor-induced lymph node lymphangiogenesis by immuno-positron emission tomography. Cancer Res. 2010;70:8842. doi: 10.1158/0008-5472.CAN-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yoon CM, Hong BS, Moon HG, Lim S, Suh PG, et al. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J Gastroenterol. 2010;16:4003. doi: 10.3748/wjg.v16.i32.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- [8].Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anelli V, Gault CR, Snider AJ, Obeid LM. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. Faseb j. 2010;24:2727. doi: 10.1096/fj.09-150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nagahashi M, Matsuda Y, Moro K, Tsuchida J, Soma D, et al. DNA damage response and sphingolipid signaling in liver diseases. Surg Today. 2015 doi: 10.1007/s00595-015-1270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55:1839. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nagahashi M, Takabe K, Terracina KP, Soma D, Hirose Y, et al. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed Res Int. 2014;2014:651727. doi: 10.1155/2014/651727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamada A, Ishikawa T, Ota I, Kimura M, Shimizu D, et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res Treat. 2013;137:773. doi: 10.1007/s10549-012-2398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- [18].Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8:e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th Springer; New York: 2010. [Google Scholar]
- [23].Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- [24].Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- [25].Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci U S A. 2010;107:20447. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ono Y, Kurano M, Ohkawa R, Yokota H, Igarashi K, et al. Sphingosine 1-phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1-phosphate concentration. Lipids Health Dis. 2013;12:20. doi: 10.1186/1476-511X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yatomi Y, Yamamura S, Hisano N, Nakahara K, Igarashi Y, et al. Sphingosine 1-phosphate breakdown in platelets. J Biochem. 2004;136:495. doi: 10.1093/jb/mvh143. [DOI] [PubMed] [Google Scholar]
- [28].Bode C, Sensken SC, Peest U, Beutel G, Thol F, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem. 2010;109:1232. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- [29].Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb j. 2007;21:1202. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- [30].Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- [31].Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- [32].Aoyagi T, Nagahashi M, Yamada A, Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat Res Biol. 2012;10:97. doi: 10.1089/lrb.2012.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takabe K, Yamada A, Rashid OM, Adams BJ, Huang WC, et al. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland Surg. 2012;1:80. doi: 10.3978/j.issn.2227-684X.2012.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Long JS, Edwards J, Watson C, Tovey S, Mair KM, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol Cell Biol. 2010;30:3827. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Long JS, Fujiwara Y, Edwards J, Tannahill CL, Tigyi G, et al. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J Biol Chem. 2010;285:35957. doi: 10.1074/jbc.M110.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ohotski J, Long JS, Orange C, Elsberger B, Mallon E, et al. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2012;106:1453. doi: 10.1038/bjc.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Datta A, Loo SY, Huang B, Wong L, Tan SS, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5:5920. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- [40].Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, et al. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- [41].Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer, and Lymphangiogenesis. Biomolecules. 2013;3:408. doi: 10.3390/biom3030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, et al. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv Biol Regul. 2014;54:112. doi: 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- [44].Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Denimal D, Pais de Barros JP, Petit JM, Bouillet B, Verges B, et al. Significant abnormalities of the HDL phosphosphingolipidome in type 1 diabetes despite normal HDL cholesterol concentration. Atherosclerosis. 2015;241:752. doi: 10.1016/j.atherosclerosis.2015.06.040. [DOI] [PubMed] [Google Scholar]
- [48].Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, et al. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011;10:70. doi: 10.1186/1476-511X-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.