Abstract
Objectives: To evaluate the efficacy and safety of adapalene 0.1% benzoyl peroxide 2.5% gel in women aged 25 years or older via subgroup analysis of existing Phase 2 and 3 study data. Methods: Meta-analysis of pooled data from three multicenter, randomized, double-blind, vehicle-controlled, parallel-group, clinical trials compared results of treatment with either adapalene 0.1% benzoyl peroxide 2.5% gel or vehicle gel in adult females and teen-aged females. Efficacy assessments included investigator’s global assessment and median percent change in acne lesions. Safety assessments included skin tolerability and adverse events. Results: Two hundred fifty-four adult females and 488 teen-aged females were included in the analyses, and baseline characteristics were comparable between subjects receiving adapalene 0.1% benzoyl peroxide 2.5% or vehicle. Both adult females and teen-aged females in the adapalene 0.1% benzoyl peroxide 2.5% arm were significantly more often rated clear/almost clear compared with those in the vehicle arm at Weeks 8 (P=0.016) and 12 (P<0.001); at endpoint, success was achieved in 39.2 percent with adapalene 0.1% benzoyl peroxide 2.5% and 18.5 percent with vehicle. Comparison of the amount of difference between active and vehicle reductions in investigator’s global assessment showed that efficacy was similar for adult females versus teen-aged females (20.7% vs. 19.9%, respectively). Adapalene 0.1% benzoyl peroxide 2.5% had a rapid onset of action, with statistically significant reductions in all acne lesion types versus vehicle observed by Week 1. Adapalene 0.1% benzoyl peroxide 2.5% was safe and well-tolerated by adult females with a tolerability profile consistent with that seen in teen-aged females. Conclusions: The once-daily fixed-dose combination product adapalene 0.1% benzoyl peroxide 2.5% is an efficacious, safe, and well-tolerated treatment for adult female acne, with a profile similar to that in teen-aged females.
Although acne severity and prevalence is believed to peak in girls between 14 and 17 years, data suggest that the prevalence of acne among adult females (≥25 years) is increasing; in 2008, a study found acne in more than 50 percent of women in their third decade of life.1,2 Clinically, adult female (AF) acne can have variable presentations.2-4 In the literature, AF acne is often described as manifesting primarily as inflammatory papules and nodules that are localized to the lower third of the face, jawline, and neck; diffuse comedonal acne that includes numerous closed comedones with a small number of inflammatory lesions is another presentation of AF acne.3,4 In adult acne, the shoulders and back may be involved and postinflammatory erythema or pigmentation and scarring may be present.2-4 Hyperseborrhea is a typical feature with comedonal acne in adult women.2 Acne in adults may persist from adolescence to adulthood, may relapse after being absent for a long period of time, or may have a late onset.2,4 Recent data from a large-scale international study show that the mandibular form of acne traditionally associated with AF is less prevalent than previously thought and that most acne in AF more closely resembles teen-aged (young) acne (YF; in approximately 90% of cases).5
Acne in adult women has a negative impact on quality of life (QoL). AF acne patients report greater overall effects on QoL compared with adolescent acne patients.4,6 Further, adults treated with isotretinoin do not demonstrate the same improvements in social appraisal and assertiveness as younger patients.7 Adult acne can be associated with discomfort and cosmetic concerns, including inflammation, pigmentary changes, and scarring.4 It can also affect work productivity and social functioning.8 Finally, adult women may express concerns about photoaging, which is typically treated with topical retinoid therapy.9-11
Historically, clinicians have viewed AF acne as poorly responsive to topical or systemic therapy. However, several studies and recent meta-analyses of clinical trial data indicate that topical acne agents have good efficacy in the AF population.12-15 Benzoyl peroxide (BPO) in combination with a retinoid has been recommended as first-line therapy for AF acne.3,4,12 Systemic therapy is also often required in AF acne,3,4 but it has become increasingly clear that there is a substantial proportion of adult women with acne who respond to topical therapy, particularly those who have a clinical presentation similar to YF acne. A fixed-dose combination gel of adapalene/ BPO, 0.1%/2.5% (A-BPO) has been shown to be efficacious and safe in subjects as young as nine years old. A meta-analysis of three pivotal A-BPo studies (1 Phase 2 and 2 Phase 3) was conducted to assess the efficacy and safety of this treatment in AF.16-18 The efficacy of A-BPo gel therapy versus vehicle gel was evaluated in AF, as was efficacy in A-BPo gel-treated AF versus YF.
METHODS
Meta-analysis design. Data of two populations (AF ≥25 and YF <18 years) were pooled from three multicenter, randomized, double-blind, parallel group, vehicle-controlled studies conducted at 157 centers in the United States, Canada, Puerto Rico, and Europe. These studies had similar design and methodology. Subjects with acne rated mild, moderate, or severe on Investigator global Assessment (IGA) were randomized to receive adapalene 0.1% benzoyl peroxide 2.5% (A-BPo) gel (Epiduo® gel, galderma Laboratories), adapalene gel, BPo gel, or vehicle gel, once daily in the evening for 12 weeks.16.18 Efficacy assessments included IGA (clear to severe) and median lesions counts (inflammatory lesions [IL], noninflammatory lesions [nIL], and total). Safety assessments included tolerability signs and symptoms (dryness, erythema, scaling, and stinging/burning) assessed on a scale of 0 (none) to 3 (severe) and adverse events (AEs).
The meta-analysis evaluated the efficacy and safety of ABPo gel relative to those of the vehicle gel in each population (AF and YF). Efficacy was evaluated in the intent-to-treat (ITT) population, which included randomized subjects who were dispensed study medication. Safety was evaluated in the safety population, which included randomized subjects who applied at least one dose of study medication.
IGA in terms of success rate (clear and almost clear) and lesion counts in terms of mean percentage change were analyzed using the Cochran-Mantel-Haenszel (CMH) test stratified by center, using general association for success rates and row mean differences relative to identified distribution transformed scores for percentage change in lesion counts. All tests were 2-sided, with significance declared at 0.05 level.
RESULTS
Subject population. A total of 254 AF and 488 YF with acne were included in the meta-analysis (Table 1). The majority of subjects had moderate acne. Demographic variables were generally similar between the adults and adolescents treated with A-BPO and vehicle gel.
TABLE 1.
Demographic and baseline acne characteristics
| AF | YF | |||
|---|---|---|---|---|
| Characteristics | A-BPO (n = 130) | Vehicle (n = 124) | A-BPO (n = 243) | Vehicle (n = 245) |
|
Age (years) Mean ± SD (range) |
32 ± 6.8 (25-58) | 31.9 ± 6.9 (25-51) | 14.5 ± 1.6 (12,17) | 14.4 ± 1.6 (12, 17) |
Skin phototype, n (%)
|
|
|
|
|
Race, n (%)
|
|
|
|
|
|
Inflammatory lesion counts Mean ± SD |
26.5 ± 6.3 | 27 ± 7.0 | 28 ± 7.3 | 28.8 ± .0 |
|
Noninflammatory lesion counts Mean ± SD |
51.2 ± 21 | 45.2 ± 14.3 | 52.4 ± 18.8 | 52.6 ± 19.5 |
|
Total lesion counts Mean ± SD |
77.7 ± 23.5 | 72.2 ± 17.2 | 80.4±21.7 | 81.4±23.1 |
IGA at Baseline, n (%)
|
|
|
|
|
Efficacy. IGA success rate was significantly better in the A-BPO gel group compared to the vehicle gel groups (Figure 1). At study endpoint, 39.2 percent of the AF population in the A-BPO gel group were rated clear or almost clear compared to 18.5 percent of those in the vehicle gel group (Δ=20.7%). Week 12 success rates in the YF population were 34.2% for A-BPO gel and 14.3% in the vehicle gel group (Δ=19.9%).
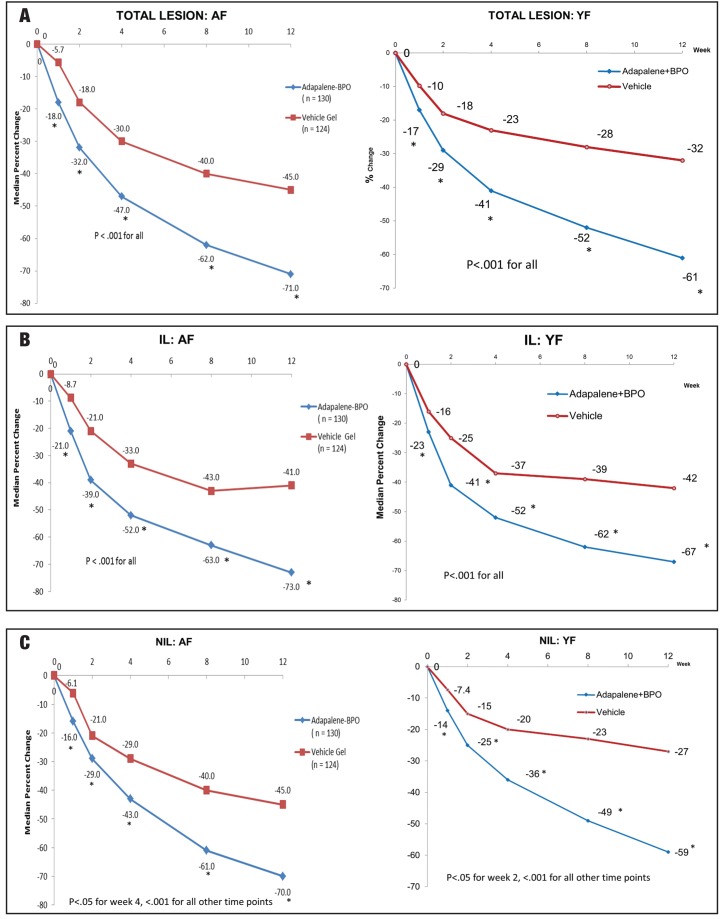
Figure 1.
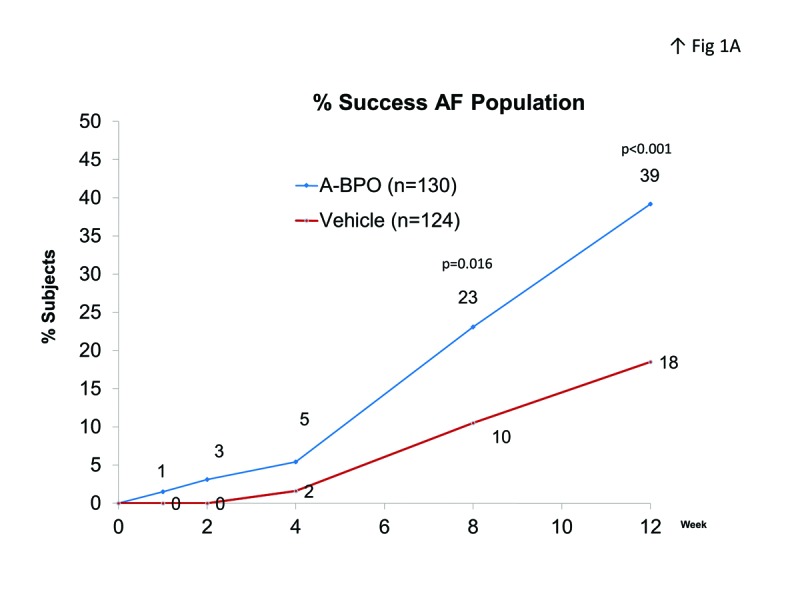
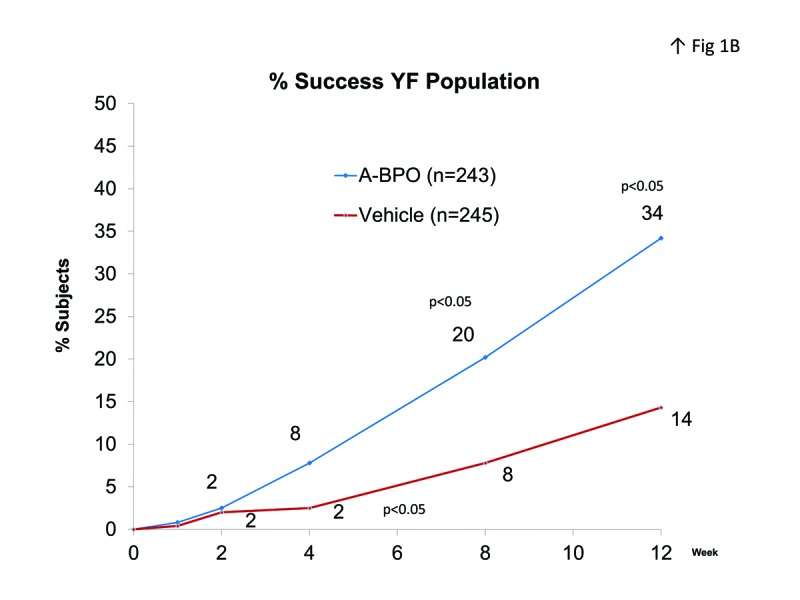
IGA success rate (clear or almost clear) in A) AF population and B) YF population.
A-BPO gel had an early onset of action with a statistically significant reduction in median percent change in total lesions, inflammatory lesions, and noninflammatory lesions observed after one week of treatment (Figure 2). In the AF population, A-BPO gel was significantly better than vehicle gel (P<0.001 for all) in Week 12 median percent change in total lesions (-71.0% vs. -45.0%; Δ=26%), IL (-73.0% vs. -41.0%; Δ=32%), and NIL (-70.0% vs. -45.0%; Δ=25%). A-BPO gel treatment also achieved significantly better reductions in Week 12 lesions than vehicle in the YF population: median percent change in total lesions (-61.0% vs. -32.0%; Δ=29%), IL (67.0% vs. -42.0%; Δ=25%), and NIL (-59.0% vs. -27.0%; Δ=32%).
Figure 2.
Median % change in lesion counts. A) Total lesions; B) IL; C) NIL.
Safety and tolerability: A-BPO gel versus vehicle gel in AF population. As expected from the overall clinical trials, treatment with A-BPO gel was safe in both the AF and YF populations. No serious related adverse events were reported, and there were fewer adverse events leading to discontinuation in the A-BPO gel group compared with the vehicle gel group. Table 2 summarizes the adverse events in adults treated with A-BPO gel and vehicle gel. A similar safety profile was observed in the AF and YF populations.
TABLE 2.
Summary of adverse events
| AF | YF | |||||||
|---|---|---|---|---|---|---|---|---|
| A-BPO (n = 130) | Vehicle (n = 124) | A-BPO (n = 243) | Vehicle (n = 245) | |||||
| Events | Subjects** | Events | Subjects** | Events | Subjects** | Events | Subjects** | |
| All AEs | 100 | 53 (40.8%) | 60 | 40 (32.3%) | 198 | 198 106 (43.6%) | 125 | 72 (29.4%) |
| Related* AEs | 42 | 36 (27.7%) | 14 | 11 (8.9%) | 85 | 60 (24.7%) | 19 | 17 (6.9%) |
| Related dermatologic AEs | 34 | 34 32 (24.6%) | 11 | 10 (8.1%) | 66 | 66 53 (21.8%) | 17 | 17 (6.9%) |
Related = possibly, probably, or definitely related
Subjects with at least one event
Numbers in columns cannot be added because a given subject may have reported more than one AE
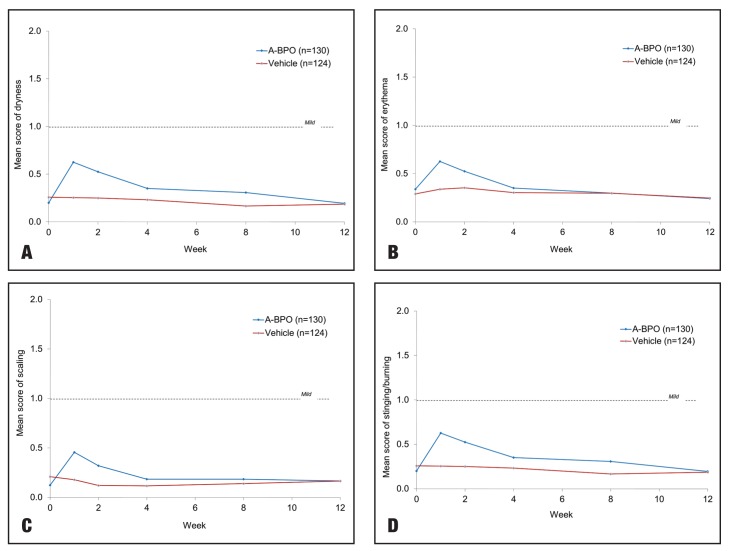
A-BPO gel was well-tolerated with mean scores below 1 (mild) for dryness, erythema, scaling, and stinging/ burning (Figure 3). These cutaneous signs/symptoms usually appeared in the first two weeks of treatment and diminished through Weeks 4 to 12 to a level near baseline. The most common related adverse events occurring in at least three percent of subjects in the A-BPO gel group were dry skin, contact dermatitis, and application-site irritation. Tolerability profile was similar also in the AF versus YF populations.
Figure 3.
Mean tolerability scores
DISCUSSION
Although historically it has been thought that AF with acne are not amenable to topical therapy, this meta-analysis joins the growing body of evidence showing that topical agents are efficacious for this population. The authors found A-BPO gel once daily to be an efficacious and well-tolerated treatment for acne in women aged 25 years and older, with a profile similar to that in younger females. AF in the A-BPO gel group were significantly more often rated clear/almost clear compared with those in the vehicle arm at Weeks 8 (P=0.016) and 12 (P<0.001); at endpoint, success was achieved in 39.2 percent AF with A-BPO gel and 18.5 percent AF with vehicle gel. The net treatment effect on IGA success rates versus vehicle (Δ) was 20.7 percent in AF and 19.9 percent in YF. Treatment effects on individual lesion types were also comparable between AF and YF with median percent change of total lesions Δ=26 percent and 29 percent; IL Δ=32 percent and 25 percent; NIL Δ=25 percent and 32 percent, respectively.
Systemic therapy in the AF population offers benefits and also risks. Low-dose isotretinoin has been studied in adult patients with acne, and efficacy has been found to be comparable in adults versus teens.19 However, isotretinoin is associated with risk for side effects and does not offer the additional photoaging benefits that are provided by a topical retinoid.20 The oral contraceptives with the best safety profile (e.g., second generation, levonorgestrel) have pro-androgenic progesterone and may cause acne.
Recent studies have also evaluated topical therapies in AF subjects, although the definition of “adult” has been variable. The current standard of care for the majority of individuals with acne is a topical retinoid plus antimicrobial;21,22 as discussed below, data now support this as first-line therapy for most AF as well. Stein Gold et al23 reported a post-hoc analysis of responses of AF versus YF treated with adapalene 0.1% gel in a large-scale, open-label, community-based study that included 481 YF aged 12 to 19 years, and 611 AF aged 20 or older. In this analysis, the proportion of subjects achieving a global assessment of clear/almost clear/marked improvement was 64.8 percent in the AF and 50.3 percent in the YF group (P<0.001). In addition, the AF group had a higher percentage of individuals who reported being “very satisfied” with treatment (45.5% vs. 35.8% for adolescents). Patient-reported treatment adherence and tolerability were similar between AF and YF.23 Subgroup analysis of pooled data from adapalene gel 0.3% also showed good efficacy in AF.24 The overall Phase 3 population from adapalene 0.3% studies included 258 AF acne subjects (18 years or older); in this group, the median reduction in total lesions was -55.7 percent (for comparison, reduction in the vehicle group was -37.7%). IL were reduced by -61.2 percent and NIL by -50.7 percent (versus -45.8% and -34.8%, respectively, in the vehicle group). In the AF population, the signs and symptoms of skin irritation were mostly mild or moderate in severity and were transient in duration, peaking in the first weeks of treatment and decreasing over time.24
Berger et al25 conducted a double-blind, randomized, parallel-group, vehicle-controlled study of tretinoin 0.04% in adults (both females and males aged 19-45 years) with mild-to-moderate acne. In this smaller study (tretinoin n=88, vehicle n=90), tretinoin was significantly greater in reducing both IL and NIL compared with vehicle (P<0.05).25 Dreno et al conducted an open-label, multicenter study of 397 AF with mild acne (mean 33 total acne lesions) aged 30 to 40 years who were treated with retinaldehyde 0.1%/glycolic acid 6% cream (RAL/GA) applied once daily for 90 days.14 Subjects also were allowed to continue any existing acne treatments without change. Efficacy was assessed by lesion counts and IGA, and safety assessments included AEs and cutaneous tolerability. RAL/GA was associated with significant reductions in IL and NIL. The treatment was also well-tolerated.14
Del Rosso et al12 performed a subgroup analysis of topical dapsone gel 5% in AF (older than 18 years) versus YF. This study analyzed data from two 12-week, multicenter, double-blind Phase 3 studies of twice-daily dapsone versus vehicle and compared YF (12-17 years, n=347) with AF 18 or older (n=434). At baseline, the YF group had significantly higher NIL (50.1 vs. 42.3) and total lesion counts (79.0 vs. 69.8). Topical dapsone was associated with a significant reduction in mean global assessment score in both AF and YF, but the effect in clinical success rates (clear/almost clear) was greater in AF (53.5%) compared with YF (45.3%). The reductions in inflammatory lesion counts were similar in both groups and vehicle (AF: -60 dapsone, -56.8 vehicle; YF: -56.2 dapsone, -48.0 vehicle; P=0.3). Greater reductions were reported for AF versus YF populations in both noninflammatory (AF: -47.4 dapsone, -42.3 vehicle; YF: -35.5 dapsone, -23.5 vehicle; P<0.0001 AF vs YF) and total lesions (AF: -52.8 dapsone, -48.3 vehicle; YF: -44.0 dapsone, -33.7 vehicle; P=0.0008 AF vs YF).12 Lynde et al26 reported an uncontrolled cohort study of dapsone 5% gel for treatment of mild-to-moderate inflammatory acne in 93 AF aged 18 or older (mean age 31.1 years) with sensitive skin. At Week 12, 69.4 percent of the AF subjects were considered treatment successes (clear or almost clear on global assessment), and dapsone was well-tolerated.26 It would be interesting to see results in the subgroup of women aged 25 and older for comparison with our population. In addition, we would not recommend using dapsone as monotherapy since topical dapsone gives subpar noninflammatory lesion reduction, which necessitates the addition of a topical retinoid.
Del Rosso et al13 also conducted a subgroup analysis of clindamycin/BPO in AF versus YF, again using data from two 12-week, Phase 3 studies with four treatment arms (clindamycin/BPO, BPO, clindamycin, or vehicle).13 AF were defined, as in this study, as aged 25 years or older. The pooled data included 1,080 YF less than 25 years of age and 395 AF. Clindamycin/BPO was shown to have comparable efficacy and tolerability in the AF and YF populations; of interest, there were also robust lesion reductions in the vehicle arm for both AF and YF subjects. The authors note that clindamycin/BPO gel should be used with caution below the jawline, since BPO can bleach clothing (note this should be considered also with A-BPO); they also recommend avoiding a combination regimen of clindamycin/BPO plus topical dapsone to minimize risks for temporary skin discolorations.13 Del Rosso’s work is supported by data from a subset of a large, open-label community-based study of clindamycin/BPO in subjects (n=1,389) with mild or moderate acne reported by Fernandez-Obregon et al.27 Efficacy in female and male subjects 18 years or younger (n=965) was compared to that in the group of both genders aged 21 to 30 years (n=185) and results showed better improvements in global assessment scores in the older group versus the younger group.27 Clindamycin/BPO is not recommended as monotherapy; addition of a topical retinoid is recommended to target more pathophysiologic factors and improve results.21,22
Thielitz et al15 compared the efficacy and safety of azelaic acid 15% gel and adapalene 0.1% gel in a long-term study of AF (18-45 years old, n=55) with acne. Both treatments were administered for three months, and subjects continued in the study for an additional six months either continuing adapalene treatment (n=19) or, if they were in the original azelaic acid arm by continuing azelaic acid (n=17) or receiving no treatment and being observed for six months (n=19). In the initial three-month period, both adapalene and azelaic acid significantly reduced acne lesion counts with comparable effects; similarly, there were no significant differences at nine months of active treatment with either adapalene or azelaic acid.15 Similar to dapsone and clindamycin/BPO, azelaic acid is not recommended as monotherapy.21,22 In addition to conventional retinoid/antimicrobial therapy, some AF may need the addition of an oral antibiotic or hormonal therapeutic approach.21
In the authors’ experience, AF with acne also often are concerned with photoaging. In addition to serving as the cornerstone of acne therapy, many women are aware of the benefits of retinoids for treating signs of photoaging and are pleased to utilize retinoids as part of an acne regimen. As clinicians, the authors agree with the recent article published by Dreno et al5 recommending a general treatment approach that targets the clinical presentation and incorporating general lifestyle factors, such as desire for treatment of photoaging, presence of pigmentary problems, and presence of scars.
CONCLUSION
A large proportion of AF with acne have a clinical presentation similar to teen-aged (youth) acne. The general treatment approach in AF with acne should include agents that target the lesions present and for most patients likely should include a topical retinoid plus antimicrobial as is standard for younger acne sufferers. The acne therapeutic strategy for AF also has to take into account the risk of adverse events linked to hormonal treatment or even low doses of isotretinoin. Available studies demonstrate that at least a subset of AF responds as well, if not better, than YF treated with topical therapies. The fixed-dose combination product A-BPO gel is a suitable treatment for acne in AF 25 years and older as it targets multiple pathophysiologic factors of acne and has a rapid onset of action. It has an overall profile in AF that is similar to that observed in YF. A-BPO gel is efficacious, well-tolerated, and has a good safety profile in a wide range of acne subjects, including AF.
Footnotes
DISCLOSURE:Financial support for data analysis and writing services provided by Galderma Laboratories, Fort Worth, Texas.
REFERENCES
- 1.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–580. [PubMed] [Google Scholar]
- 2.Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277–282. doi: 10.1111/j.1468-3083.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 3.Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063–1070. doi: 10.1111/jdv.12061. [DOI] [PubMed] [Google Scholar]
- 4.Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281–290. doi: 10.2165/00128071-200607050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Dreno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106. doi: 10.1111/jdv.12757. Epub 2014 Oct 8. [DOI] [PubMed] [Google Scholar]
- 6.Kokandi A. Evaluation of acne quality of life and clinical severity in acne female adults. Dermatol Res Pract. 2010 doi: 10.1155/2010/410809. July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myhill JE, LSR, Burnett JW. Self-esteem and social assertiveness in patients receiving isotretinoin treatment for cystic acne. Cutis. 1988;41:171–173. [Google Scholar]
- 8.Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Ascenso A, Ribeiro H, Marques HC, et al. Is tretinoin still a key agent for photoaging management? Mini Rev Med Chem. 2014;14:629–641. doi: 10.2174/1389557514666140820102735. [DOI] [PubMed] [Google Scholar]
- 10.Katsambas AD, Katoulis AC. Topical retinoids in the treatment of aging of the skin. Adv Exp Med Biol. 1999;455:477–482. doi: 10.1007/978-1-4615-4857-7_70. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177–182. [PubMed] [Google Scholar]
- 14.Dreno B, Castell A, Tsankov N, et al. Interest of the association retinaldehyde/glycolic acid in adult acne. J Eur Acad Dermatol Venereol. 2009;23:529–532. doi: 10.1111/j.1468-3083.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 15.Thielitz A, Lux A, Wiede A, et al. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. J Eur Acad Dermatol Venereol. 2015;29(4):789–796. doi: 10.1111/jdv.12823. Epub 2014 Nov 14. [DOI] [PubMed] [Google Scholar]
- 16.Gold LS, Tan J, Cruz-Santana A, et al. A North American study of adapalene-benzoyl peroxide combination gel in the treatment of acne. Cutis. 2009;84:110–116. [PubMed] [Google Scholar]
- 17.Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180–1189. doi: 10.1111/j.1365-2133.2009.09209.x. [DOI] [PubMed] [Google Scholar]
- 18.Thiboutot DM, Weiss J, Bucko A, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57:791–799. doi: 10.1016/j.jaad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644–646. doi: 10.1016/j.jaad.2005.11.1061. [DOI] [PubMed] [Google Scholar]
- 20.Bagatin E, Guadanhim LR, Enokihara MM, et al. Low-dose oral isotretinoin versus topical retinoic acid for photoaging: a randomized, comparative study. Int J Dermatol. 2014;53:114–122. doi: 10.1111/ijd.12191. [DOI] [PubMed] [Google Scholar]
- 21.Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49:S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 22.Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60:S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Colon LE, Johnson LA, et al. Do adults respond better to acne treatment than adolescents? Presented at the 2008 Annual Meeting of the American Academy of Dermatology; February 1-5, 2008; San Antonio, Texas.
- 24.Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Berger R, Barba A, Fleischer A, et al. A double-blinded, randomized, vehicle-controlled, multicenter, parallel-group study to assess the safety and efficacy of tretinoin gel microsphere 0.04% in the treatment of acne vulgaris in adults. Cutis. 2007;80:152–157. [PubMed] [Google Scholar]
- 26.Lynde CW, Andriessen A. Cohort study on the treatment with dapsone 5% gel of mild to moderate inflammatory acne of the face in women. Skinmed. 2014;12:15–21. [PubMed] [Google Scholar]
- 27.Fernandez-Obregon A, Davis MW. The BEST study: evaluating efficacy by selected demographic subsets. Cutis. 2003;71:18–26. [PubMed] [Google Scholar]