Abstract
Objective
To review the evidence on and estimate the risk of myocardial infarction and stroke in bipolar disorder.
Method
A systematic search using MEDLINE, EMBASE, PsycINFO, Web of Science, Scopus, Cochrane Database of Systematic Reviews, and bibliographies (1946 – May, 2013) was conducted. Case-control and cohort studies of bipolar disorder patients age 15 or older with myocardial infarction or stroke as outcomes were included. Two independent reviewers extracted data and assessed quality. Estimates of effect were summarized using random-effects meta-analysis.
Results
Five cohort studies including 13 115 911 participants (27 092 bipolar) were included. Due to the use of registers, different statistical methods, and inconsistent adjustment for confounders, there was significant methodological heterogeneity among studies. The exploratory meta-analysis yielded no evidence for a significant increase in the risk of myocardial infarction: [relative risk (RR): 1.09, 95% CI 0.96–1.24, P = 0.20; I2 = 6%]. While there was evidence of significant study heterogeneity, the risk of stroke in bipolar disorder was significantly increased (RR 1.74, 95% CI 1.29–2.35; P = 0.0003; I2 = 83%).
Conclusion
There may be a differential risk of myocardial infarction and stroke in patients with bipolar disorder. Confidence in these pooled estimates was limited by the small number of studies, significant heterogeneity and dissimilar methodological features.
Keywords: bipolar disorder, cardiovascular diseases, myocardial infarction, stroke, meta-analysis
Introduction
It is increasingly recognized that the morbidity and mortality associated with bipolar disorder (BD) is not simply the result of psychiatric symptoms such as psychosis or suicide, but also the consequence of a wide range of medical comorbidity (1, 2). The standardized mortality ratio (SMR) for BD in general [2.5 for men, 2.7 for women (3); 3.3 overall (4)] and cardiovascular [CVD; SMR 1.9 for men, 2.6 for women (3); 2.1 overall (4)] or neurovascular disease [SMR 1.6 for men, 2.3 for women (3); 3.5 overall (4)], in particular, underscore the public health priority to determine the morbidity burden, risk factors and pathophysiological mechanisms of cardiovascular and neurovascular disease in BD.
The mechanisms that would potentially explain the link between BD and MI and stroke have not been established, but may actually be shared between MI and stroke because both diseases are associated with atherosclerosis (5, 6). Not unique to BD, classic risk factors for CVD (7, 8) include high body mass index driven by unhealthy diet and low levels of physical activity (9), high rates of smoking (9, 10), hypertension (11), and diabetes (12). There may be additional risk for patients with BD from the standpoint of druginduced weight gain (13) and metabolic disturbances (14–16) and relatively poor access to health care (17).
The association between coronary heart disease and major depressive disorder has been well established in longitudinal studies (18). In BD, there is evidence from cross-sectional studies to suggest an association with CVD [odds ratios (OR) between 1.4 and 5.0] (11, 19, 20). Cerebrovascular disease association with BD has been less systematically studied with only one cross-sectional study that showed a higher risk of vascular diseases (including stroke) (20). Longitudinal studies, a better estimation of the risk, however, have had variable study designs and have shown conflicting results. Almost all studies were conducted using registers developed with administrative codes, which carries a risk of misclassification of the exposure and the outcome (21). Furthermore, covariates used in the multivariate models were dissimilar and some important potential confounders were not always considered.
Aims of the study
i) Obtain and review all the available evidence on this topic, ii) conduct an exploratory meta-analysis to estimate whether individuals with bipolar disorder are at greater risk, compared with individuals without bipolar disorder, for developing myocardial infarction or stroke, and iii) suggest future directions for translational research, focusing on epidemiology and biomarkers.
Material and methods
Data sources and searches
A comprehensive search strategy was used to identify relevant studies that were published between 1946 through May 2013. This search strategy, executed by an expert medical librarian with expertise in systematic reviews, accessed the following databases: MEDLINE, EMBASE, PsycINFO, Web of Science, Scopus, and Cochrane Database of Systematic Reviews. The following search terms were used: bipolar disorder, manic, depression, cerebrovascular disorders, cardiovascular diseases, incidence, prevalence, comorbid, case-control or longitudinal. The exact search terms utilized and the specific method by which they were combined are available upon request. A manual review of bibliographies from selected reports was conducted to identify additional studies.
Study selection
Two reviewers (MLP and ABCB) conducted an independent review of titles and abstracts from the references obtained by the search strategy and bibliography review. Eligibility criteria used to select reports for systematic review included: studies in English, French, or Spanish, case-control or cohort study design, people age 15 or older, BD diagnosis (as out-patient or in-patient) defined by the Diagnostic Statistical Manual for Mental Disorders (DSM-III through IV-TR) or by the International Classification of Diseases (ICD 8 through 10) as the exposure, and a comparison group of individuals without a BD diagnosis. Our outcome measures were i) MI or ii) stroke defined by ICD-8, 9, or 10 criteria, clinical diagnosis, or self-report diagnosis. If the event of interest was contained in a composite outcome, such as ‘cardiovascular diseases’, the author was contacted to obtain the specific outcomes of interest.
As cross-sectional studies may overestimate the risk of stroke due to post-stroke mania, only longitudinal case-control and cohort studies were included (22). The lower age limit of 15 years was chosen to accommodate the first BD incidence peak (23). Broad methods of outcome ascertainment (including self-report) were chosen because MI and stroke are relatively acute and well-defined outcomes that reliably come to medical attention and are therefore less subject to recall or measurement bias.
Full texts of all abstracts selected by at least one of the reviewers were screened for eligibility. Both reviewers assessed the full text reports that fulfilled the eligibility criteria. Inter-reviewer agreement was evaluated at this stage using kappa statistics. A third investigator was not needed for adjudication of any study as consensus regarding inclusion or exclusion of each report was achieved.
Data extraction and quality assessment
The two reviewers independently reviewed each report, extracting data on study characteristics according to the STROBE statement for reports of observational studies (24). Relative risks (RR) were preferred over odds ratios (OR) and adjusted measures of effect were preferred over unadjusted measures. If a report did not include the data needed for the meta-analysis, the corresponding author was contacted to obtain the missing information. Although initially planned, assessment for possible publication bias could not be performed because of the small number of studies and significant heterogeneity.
Data synthesis and analysis
Separate analyses were performed for MI and stroke. Adjusted OR was assumed to be equivalent to adjusted RR when event rates were low (<10%) (25). Incidence Rate Ratios (IRR), Admission Rate Ratios (ARR) and Hazards Ratios (HR) were treated as RR. Subgroup analysis comparing estimates of effect that account for follow-up time (IRR, ARR and HR) vs. the ones that do not account for time (OR) were conducted to confirm whether this assumption impacted the results. If it was not possible to obtain adjusted estimates for the specific outcome by contacting the study author, unadjusted RRs and 95% confidence intervals (CIs) were calculated using the number of events reported. If not available, the study was subsequently excluded.
Meta-analyses were performed calculating RR using random-effects models, if all included studies were cohort studies, as RR can be calculated for cohort studies and it is easier to interpret than OR (26). Inconsistency and statistical heterogeneity were measured by the Q statistic and the I2 test (27). Causes of heterogeneity were explored by doing sensitivity/subgroup analyses. REVMAN software version 5.2 (28) and JMP version 9 (SAS Institute Inc., Cary, NC, USA) were utilized for all statistical analyses. This manuscript followed MOOSE statement for reporting of systematic reviews and meta-analyses of observational studies (29).
Results
Systematic review
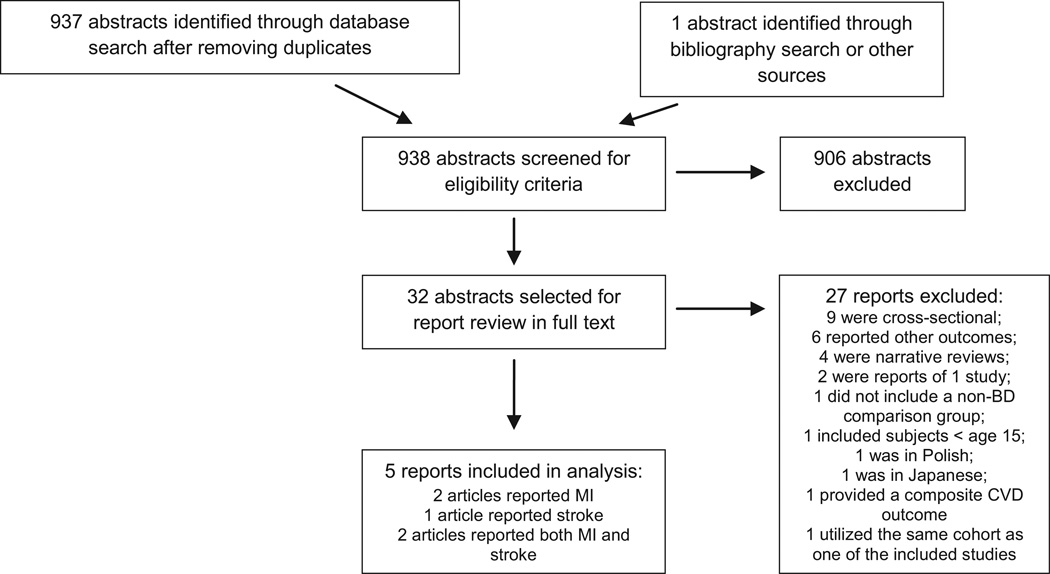
As summarized in Fig. 1, of 938 abstracts obtained, 32 were selected at least by one of the reviewers for full text review (19, 20, 30–58). Seven reports that included (30, 31, 44, 46, 50, 51, 58) [two reports included MI and stroke (44, 58); both counted as two reports] met criteria for further review. All these studies were cohort studies; we did not find case-control studies. Agreement for included studies was high, with a kappa of 0.85. One study (30) reported a composite CVD outcome. The corresponding author was unable to provide a specific estimate for MI, and thus, the study was excluded. One study (58) included 58 individuals under age 15 (0.34% of participants with BD and 0.0004% of total sample); given this miniscule contribution from underage individuals, the study was retained for this meta-analysis. Two studies (44, 50) were conducted using the same register within a similar timeframe, thus the samples overlapped. Because the study by Nilsson and collaborators (50) encompassed a shorter timeframe and thus a smaller sample size as compared to the study by Laursen and collaborators, and was more prone to bias due to their osteoarthritis comparison cohort, it was removed from the analyses.
Fig. 1.
Flow diagram.
These five cohort studies totaled 13 115 911 individuals (27 092 with BD) from four countries, with a maximum follow-up of 20 years (Table 1). Two studies (44, 58) were conducted in Scandinavian registers (Denmark and Sweden), which combined provided more than 13 000 000 participants and more than 23 000 patients with BD, while three smaller studies (31, 46, 51) were carried out in other countries (Taiwan and USA, respectively).
Table 1.
Studies included in the systematic review
| Study | Country | Setting | Time frame | Total Sample (BD sample) |
Exposed group | Unexposed group | Outcome | Follow-up (year) | Reported estimate† | 95% C.I† | Co-variates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laursen TM et al. 2011 (44) | Denmark | Registry | 1955–1995 | 2,450,812 (6,215) |
Patients w/BD age ≥15‡ |
Individuals age ≥15 without psychiatric admissions |
Ml | 12 | Ml: aIRR: 1.32 | 0.88–1.99 | Sex, age and calendar time |
| Lin HC et al. 2008 (31) | Taiwan | Registry | 1997–2002 | 6,422(1,429) | Hospitalized patients w/BD age ≥45 |
Appendectomy patients ≥18 year w/o psych diag. |
Ml | 6 | uOR:1.31§ | 0.87–1.97 | Age and comorbid HTN, hyperlipidemia and renal disease |
| Ramsey CM et al. 2010 (51) | USA | Population-based 2-wave survey |
1981–1996 | 2,768 (58) | Patients w/BD age ≥18 |
Individuals without hx of mania/hypomania |
CVD/MI | 11.5 | CVD: aOR: 2.97 MI:uRR:1.68¶ |
1.40–6.34 0.63–4.47 |
None for Ml |
| Westman J et al. 2013 (58) | Sweden | Registry | 1987–2006 | 10,631,208 (17,101) |
Hospitalized patients w/BD, no SCZ |
Individuals without BD or SCZ admissions |
Ml | 20 | MI:aARR:1.03 | 0.92–1.16 | Sex, calendar year and age |
| Laursen TM et al. 2011 (44) | Denmark | Registry | 1955–1995 | 2,450,812 (6,215) |
Patients w/BD age ≥15‡ |
Individuals age ≥15 without psychiatric admissions |
Stroke | 12 | S.:alRR:2.0’ | 1.61–2.52 | Sex, age and calendar time |
| Lin HC et al. 2007 (46) | Taiwan | Registry | 1998–2003 | 18,702(2,289) | Hospitalized patients w/BD age ≥18 |
Appendectomy patients ≥18 year w/o psych diag. |
Stroke | 6 | aOR:2.05 | 1.73–3.54 | Age, HTN, hyperlipidemia, renal dis., alcoho and substance dependence |
| Westman J et al., 2013 (58) | Sweden | Registry | 1987–2006 | 10,631,208 (17,101) |
Hospitalized patients w/BD, no SCZ |
Individuals without BD or SCZ admissions |
Stroke | 20 | S: aARR: 1.39 | 1.26–1.52 | Sex, calendar year and age |
BD, bipolar disorder; SCZ, schizophrenia; MDD, major depressive disorder; w/o, without; psych diag, psychiatric diagnosis; hx, history; CVD, cardiovascular disease; Ml, myocardial infarction; S, stroke; aHR, adjusted hazard ratio; aIRR, adjusted incidence rate ratio; aOR, adjusted odds ratio; aARR, adjusted admission rate ratio; uOR, unadjusted odds ratio; uRR, unadjusted relative risk; HTN, hypertension.
Rounded to 2 decimal places.
Only part of the cohort included out-patients with bipolar disorder.
aOR not reported, but stated that did not change after adjustment.
Calculated using raw data.
Three register studies (31, 44, 58) utilized administrative diagnostic codes using ICD 9 or 10 codes for diagnosis of the exposure (BD) and the outcomes (MI and stroke). One study (51) interviewed patients directly using a structured diagnostic interview administered by lay interviewers. The cohort by Westman and collaborators (58) had the longest follow-up (20 years). Two studies (44, 51) included in- and out-patients as their exposed cohorts, although the study by Laursen et al. included out-patients only for a part of the cohort timeframe. Four studies (31, 44, 46, 58) utilized comparison cohorts obtained from the same registers (excluding patients with schizophrenia or with history of psychiatric admissions) or utilized participants with surgical conditions (i.e. appendicitis). One survey study (51) used a comparison cohort that included all patients without BD.
For the risk of MI, the estimates of effect were dissimilar, although non-significant. The largest study by Westman and collaborators reported a lack of an increased risk of MI among patients with BD, after adjusting for age, sex, and calendar time (adjusted ARR: 1.03, 95% CI 0.92–1.16). The other three studies also did not show a statistically significant increased risk of MI; however, the estimates of effect were farther from the null [adjusted IRR: 1.32, 95% CI 0.88–1.99 (44); unadjusted OR: 1.31, 95% CI 0.87–1.97 (31); unadjusted RR: 1.68, 95% CI 0.63–4.47 (51)].
For the risk of stroke, all studies reported statistically significant increased risk of stroke among patients with BD, reaching up to two times the risk of stroke compared with the unexposed group.
The statistical methodology and the employed estimates of effect varied greatly among these studies. While all the included studies were cohort studies, only two studies utilized time-to-event analyses (i.e. Kaplan–Meier or Cox Proportional Hazards Regression). The statistical adjustment was diverse. Four studies (31, 44, 46, 58) adjusted for age and only two studies (44, 58) further adjusted for sex. No studies adjusted for diabetes.
Assessment of bias
In general, the included studies were at risk of at least moderate bias. The study by Ramsey et al. (51) was the only work that utilized a more representative sample of the full spectrum of patients with BD, because it included out-patients and non-treatment-seeking individuals who responded to their survey. The other studies included hospital-based samples. One study (43) comprised outpatients seen at the hospitals’ clinics, but only for a part of their cohort timeframe. Among the included studies, only two studies (44, 58) reported prior validity studies of diagnostic codes. The non-exposed cohorts excluded patients with schizophrenia (58), psychiatric admissions (43), or any psychiatric diagnosis (31, 46). One study used a structured interview conducted by lay interviewers to assess history of mania or hypomania (51). The rest of the studies relied on administrative registers (31, 43, 46, 58). Two studies (31, 46) did not report whether the BD cohort was free of MI or stroke before the start of the follow-up. The degree of statistical adjustment varied greatly. All studies adjusted for age and sex. However, no studies controlled for diabetes and one study (31) adjusted for hypertension. The length of the follow-up ranged from 6 to 20 years, and then the follow-up may have been insufficient for the development of coronary artery disease or stroke. Only one study (51) reported the number of participants who were lost to follow-up.
Exploratory meta-analysis
Data synthesis
Of the five included reports (31, 44, 46, 51, 58), two reported MI outcomes, one reported stroke outcomes and two reported both MI and stroke outcomes. It was not possible to obtain from the authors specific adjusted measures of effect for MI in one study (51). In this case, the unadjusted RR of MI was calculated using the number of events obtained from the article.
Risk of myocardial infarction
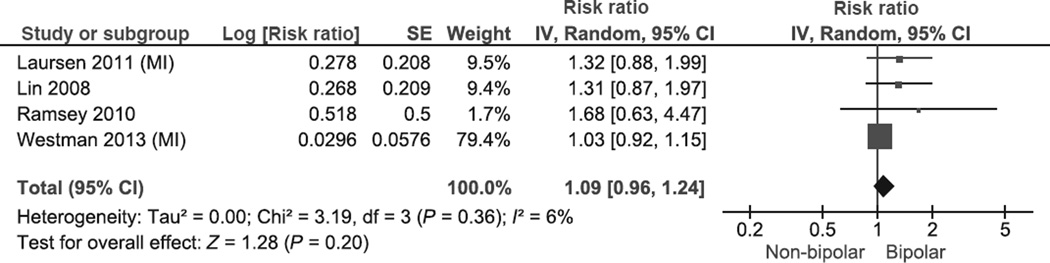
The pooled RR of MI in BD was 1.09 (95% CI 0.96–1.24, P = 0.20; heterogeneity: χ2 = 3.19, P = 0.36; I² = 6%; Fig. 2). A sensitivity analysis that excluded studies that reported only unadjusted estimates of effect yielded a pooled RR of 1.08 (95% CI 0.92–1.26, P = 0.35; heterogeneity: χ2 = 2.20, P = 0.33; I² = 9%). There was no evidence of significant differences in the RR of MI when comparing studies with general population non-exposed cohorts (RR 1.11, 95% CI 0.90–1.37, heterogeneity P = 0.28, I2 = 22%), vs. non-exposed cohorts with medical conditions (appendectomy) [RR 1.31, 95% CI 0.87–1.97, heterogeneity (not applicable, only one study), I2 = 0%; Relative Risk Ratio (RRR) 0.85, 95% CI 0.54–1.34; test for subgroup differences: P = 0.49]. This was also the case when comparing studies that reported IRR or HR (RR 1.08, 95% CI 0.89–1.29, heterogeneity P = 0.25, I2 = 24%) vs. the studies that reported RR or OR (RR 1.38, 95% CI 0.94–2.01, heterogeneity P = 0.52, I2 = 0%; RRR 0.78, 95% CI 0.51–1.20; test for subgroup differences: P = 0.25). In addition, there was no evidence of significant differences in the RR of MI between studies that only included BP in-patients at the index episode (RR 1.09, 95% CI 0.94–1.27) and those that included BP in-patients as well as out-patients (RR 1.85, 95% CI 0.69– 4.94; RRR 0.59, 95% CI 0.22–1.60; test for subgroup differences: P = 0.30). We did not perform subgroup analysis by adjustment for psychotropic medication, because none of the studies reported estimates for MI after controlling for medication use.
Fig. 2.
Meta-analysis of risk of myocardial infarction in bipolar disorder. SE, standard error; IV, inverse variance; CI, confidence interval.
Risk of stroke
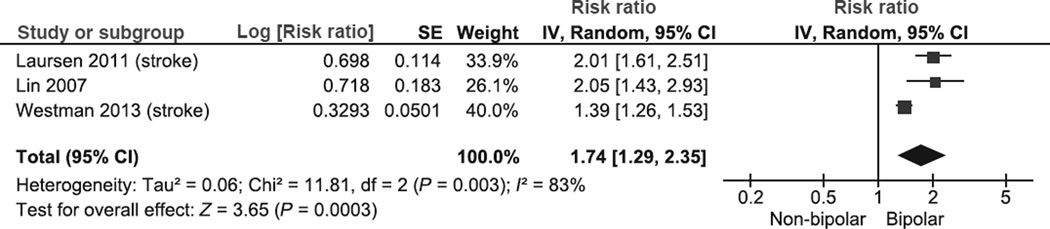
The pooled RR of stroke in BD was 1.74 (95% CI 1.29–2.35; P = 0.0003) (Fig. 3). In this analysis, there was significant heterogeneity (χ2 = 11.81, P = 0.003, I2 = 83%). Subgroup analysis revealed significant differences when comparing studies with referent cohorts that excluded patients with schizophrenia vs. cohorts that excluded all patients with psychiatric admissions (excluded patients with schizophrenia: RR 1.39, 95% C.I. 1.26–1.53; excluded all patients with any psychiatric admission: RR 2.02, 95% C.I. 1.67– 2.64; RRR: 0.69, 95% C.I. 0.56–0.85; test for subgroup differences: P = 0.0006). This analysis resolved heterogeneity. Conversely, there was no significant difference between studies that used general population non-exposed cohorts with studies that utilized non-exposed cohorts with medical conditions (general population: RR 1.65, 95% C.I. 1.15–2.36; appendectomy: RR 2.05, 95% C.I. 1.43–2.93; RRR: 0.81, 95% C.I. 0.48–1.34; test for subgroup differences: P = 0.40). We did not perform subgroup analyses comparing in-patients and out-patients and by psychotropic medication adjustment because all stroke studies were conducted on BP in-patients and none of these studies controlled for medication.
Fig. 3.
Meta-analysis of risk of stroke in bipolar disorder. SE, standard error; IV, inverse variance; CI, confidence interval.
Discussion
This first systematic review and exploratory meta-analysis on the risk of MI and stroke in BD suggests that there may be a differential risk. This is not a definitive conclusion but a call for further study as the number of studies was small with significant methodological heterogeneity. This first exploratory meta-analysis as a first estimation of the risk of MI and stroke in BD across published studies has the strength of sample size (over 13 million individuals and 29 000 with BD), but a number of limitations.
As all studies except one were conducted using administrative registers, they are all affected by the same problems registers have for epidemiological studies. First, it is assumed that diagnostic codes in administrative registers are valid, while indeed the validity has not been adequately assessed for all registers and for all diagnoses, because the quality of these validity studies has been highly heterogeneous (59, 60). In addition, the codes are contrasted to the clinical notes, that in general do agree, but there are very few validity studies comparing the clinical note against a structured clinical interview (61). Second, registers such as the Danish Psychiatric Central Register (62) are based on secondary or tertiary hospitals contacts, and then the patients captured by these databases are only those who seek treatment and who require a more complex care (63). This issue is particularly important in BD, and even more important in bipolar II disorder patients, who may never require a hospital admission or treatment in a hospital setting. Thus, the representativeness of the full spectrum of illness severity is somewhat limited in these registers. Third, the lack of detailed information in these registers, on important variables that are associated with CVD and BD (e.g. body mass index, smoking) impede the accurate exploration of potential confounders or mediators of this association. Fourth, diagnostic practices, diagnostic criteria and clinician awareness of the disease have changed over time. In a study conducted in New South Wales, covering the timeframe from 1967 to 1977, there was an increase in the diagnosis of mania along with a decrease in the diagnosis of paranoid schizophrenia, after lithium became available (64). Conversely, concordance between ICD-8 and ICD-10, and between ICD-8 and DSM-III for affective disorders have been reported to be high (65). Furthermore, cultural and ethnic differences have been reported for the clinical presentation of BD (66–68). All these issues may have impacted the diagnostic methods across the included studies and over time.
The comparison cohorts were also dissimilar across the included studies. Only one study (51) did not exclude any participants from the comparison cohort. The other studies excluded patients with schizophrenia or participants with prior psychiatric admissions or other psychiatric diagnoses. Of note, several psychiatric disorders have been associated with CVD in longitudinal studies, specifically major depressive disorder (18) and schizophrenia (69). Then, the exclusion of these patients from the comparison cohort may have introduced bias by selecting a referent group with a potentially lower risk of CVD than the general population (‘ultra-healthy controls’). This observation may explain why the study by Westman et al. (58), which only excluded patients with schizophrenia from the comparison cohort, found an IRR for MI of 1.03, while the study by Laursen et al. (70), in which participants with any psychiatric admission were excluded from the comparison cohort (assuming a ‘healthier’ referent cohort), found an IRR of 1.32. In the same vein, the heterogeneity of the stroke meta-analysis may be explained by this same reason [i.e. the estimate of effect for stroke in Westman et al. study (58) was significantly closer to the null than the estimate for the Laursen et al. study (43)].
Several different statistical methods were utilized in these studies, even though all employed cohort designs. Two studies from Taiwan conducted on the same register used logistic regression models for estimating the effect of BD on the risk of MI and stroke. A logistic regression does not take into account follow-up time or censoring. Therefore, it is a limited statistical approach for longitudinal data when the follow-up time for each participant is different (71), as it was the case in these studies. In addition, the covariates utilized in the multivariate models across studies varied dramatically. No studies adjusted for an important risk factor for CVD: diabetes (7). This medical disease is an important potential confounder of the association between BD and MI and stroke, because diabetes have been associated with MI (7), stroke (72) and also with BD (73, 74). Diabetes is even more important in BD due to the increased risk of diabetes with atypical antipsychotics (14, 16, 75). Further, the lack of assessment of other potential confounders (e.g. physical activity) may have introduced residual confounding that was not adjusted for in the multivariate models.
In the case of the risk of MI, one large study explained 79% of the pooled estimate of effect and may undermine the estimates of the other smaller studies, which were farther from the null, albeit non-significant. No studies reported estimates after adjusting for critical risk factors for MI such as diabetes. While the pooled estimate for stroke provided somewhat stronger evidence of an increase in the risk among patients with BD, this estimate was obtained from studies with significant heterogeneity. However, subgroup analyses comparing studies with comparison cohorts that excluded patients with schizophrenia vs. those that excluded patients with any psychiatric admission resolved heterogeneity, providing some evidence that the methods for selecting the comparison cohort may have had a large impact on the estimate of the risk of stroke.
The majority of the studies lacked sufficient reporting on patients lost to follow-up, which is important given i) the likelihood of higher dropout rates among patients with BD (76) and ii) the potential bias that could have been introduced if the dropout rates were different between exposed and non-exposed groups (77). Nevertheless, studies in which nationwide databases were utilized and patients were followed-up until death (44, 58), the likelihood of large proportions of lost to follow-up may be lower.
The number of studies that satisfied even our broad inclusion criteria was relatively small. Therefore, the statistical power to obtain an accurate and precise pooled estimate and to detect subgroup differences was limited. In addition, due to the small number of studies, different types of estimates of effect (ARR, IRR, HR, OR) were pooled. We compared the pooled estimate of effect obtained from ARR, IRR and HR and the estimate obtained from OR, without finding significant differences.
The pooled relative risk for MI was not elevated with no significant statistical heterogeneity, while the pooled relative risk for stroke in BD was significantly increased with a precise estimate, but significant study heterogeneity. While this meta-analysis did not confirm the higher rates of MI reported in cross-sectional studies (11, 19, 20), it did support the findings from prior cross-sectional studies of stroke in patients with BD (20). Cross-sectional designs are susceptible to bias and confounding, therefore longitudinal studies (particularly cohort studies) are more robust with regards to providing a valid estimate of the risk of an outcome given an exposure.
There is a discrepancy between our study which showed no evidence of increased risk of myocardial infarction in BD and the relatively higher mortality rate of myocardial infarction in BD, in comparison to the general population (3, 44, 78). We postulate three possible explanations: i) patients with BD and the general population have similar rates of MI, but the lethality is greater in BD; this is supported by data reporting higher mortality rates of patients with severe mental illness (including BD) after admission due to acute heart disease (43); ii) patients with BD receive less medical care for MI than general population, either because they have poor access to health care (17) or lower rates of cardiovascular health care utilization and invasive cardiac diagnostic/therapeutic procedures (43); iii) patients with BD may be at higher risk of post-MI arrhythmias and sudden cardiac death (58) out of the hospital, which may underestimate the true risk of myocardial infarction. This last explanation could be driven by the use of antipsychotics, which have been associated with QTc prolongation, torsade de pointes and sudden cardiac death (79), alterations of the autonomic nervous system (80) and abnormalities in the hypothalamus-pituitary-adrenal axis (81), both seen in BD and also associated with poor outcomes after MI (82). The limitations of out-of-hospital cardiac death ascertainment make it difficult to reliably test this hypothesis.
Although several classic risk factors for CVD and stroke (i.e. smoking (83), diabetes (74), hypertension (11), obesity (84), and low physical activity (9)) are associated with BD, we did not find sufficient evidence to support an increased risk of MI. Moreover, exposure to mood-stabilizer medications (mainly atypical antipsychotics) causes weight gain (84), diabetes (14), and dyslipidaemia (15), all of which are risk factors for MI and stroke. No studies in this meta-analysis provided estimates for MI after adjusting for other risk factors for MI besides age and sex, except for one study (31) that also adjusted for hypertension. It is unlikely that statistical adjustment would have had a large impact on the larger studies that reported estimates close to 1, because the presence of negative confounding (i.e. a variable positively associated with BD and negatively associated with MI or vice versa) is improbable, given that all CVD risk factors are also associated with BD. Finally, this meta-analysis focused on specific neurocardiovascular diseases; the increased risk of cardiovascular disease in broader terms (after adjusting for important confounders) in patients with BD reported in two studies (30, 51) may have been driven by other cardiovascular diseases besides MI (i.e. hypertension, stable coronary artery disease, heart failure, arrhythmias).
In the case of stroke, as patients with BD present with higher rates of CVD risk factors [i.e. smoking (83), diabetes (74), hypertension (11), obesity (84), and low physical activity (9)], a higher risk of stroke would be expected and was actually supported by all the included studies with moderate risk of bias (43, 46, 58). One stroke study (46), even after adjusting for hypertension and dyslipidemia, reported increased risk, which may support the hypothesis that other risk factors (i.e. inflammation) may contribute to the risk of stroke besides the classical cardiovascular risk factors.
Multi-systemic inflammation has also been proposed as one underlying explanation for this potential association (85). MI, stroke and bipolar diseases each have been associated with abnormal levels of pro- and anti-inflammatory cytokines and soluble receptors (e.g., sIL-2R, IL-6, IL-4, IL-1β, IL-10, tumor necrosis factor-α, C-reactive protein) (85, 86). Inflammatory response in BD may manifest in young adult life and contribute to or accelerate early vascular damage and subsequent atherosclerosis, a key pathophysiological mechanism underlying MI and ischemic stroke (87).
Because BD often develops in adolescence and young adulthood, an inflammatory dysregulation initiation could contribute to the development of early-onset cerebral atherosclerosis in brain regions implicated in BD. Longitudinal epidemiological databases with detailed phenotypic classification should be able to test this hypothesis.
This first systematic review and exploratory meta-analysis suggests a possible differential risk of myocardial infarction and stroke in BD, in the context of highly heterogeneous studies. The evidence, however, is sufficiently limited to quantify definitive levels of risk. Methodologically rigorous studies should be conducted in population-based samples, include bipolar out-patients, use referent cohorts from the general population, and incorporate longer follow-up timeframes and accurate ascertainment of the exposure and outcomes (using other methods besides administrative coding), including information regarding cardiac or post-stroke death and loss to follow-up. It is imperative to better understand the association of BD with MI, stroke, and other neurocardiovascular diseases because patients with BD are at a higher risk of mortality due to these diseases (3) and die 10 years earlier compared to the general population (58). Furthermore, the pathophysiological mechanisms underlying these associations, including inflammatory biomarkers and non-invasive assessment of atherosclerosis (88), should be explored to better understand the mechanisms that are responsible for the high mortality due to neurocardiovascular disease in patients with BD.
Summations.
In patients with bipolar disorder, the risk of subsequent myocardial infarction may not be increased, while the risk of stroke may be significantly elevated.
High mortality rates due to myocardial infarction in patients with bipolar disorder may not be explained by an increased risk of myocardial infarction in the same population.
More methodologically rigorous studies should be conducted to accurately estimate the risk of cardiovascular disease in bipolar disorder and identify biomarkers that may aid in the understanding of the underlying pathophysiology.
Considerations.
The available studies had methodological limitations that lowered the confidence on the reported estimates of effect.
There was high methodological heterogeneity among the included studies, and statistically significant heterogeneity among stroke studies, which may limit the interpretation of the results and pooled estimates of effect.
The predominance of Scandinavian registers, the use of hospital-based databases and regional specific diagnostic practices impede a global generalization of these findings to other countries or to the full spectrum of patients with bipolar disorder.
Acknowledgments
We thank the authors of the selected studies for providing the information needed for our meta-analysis. We also thank M. Hassan Murad, M.D., M.P.H,. and Victor M. Montori, M.D., M.Sc., from the Knowledge and Evaluation Research Unit (KER Unit) at Mayo Clinic for their contribution regarding systematic review and meta-analysis’ methodology. We thank Robert L. Frye, M.D. for critically reviewing the manuscript. Finally, we thank Patricia J. Erwin for her role in the design and execution of the search strategy.
Footnotes
Declaration of interest
This project was partially supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. It was also partially supported by Mayo Foundation for Medical Education and Research. Dr. Prieto has received honoraria for speaker activities and development of educational presentations from GlaxoSmithKline, has received travel support from GlaxoSmithKline, Lilly, Lundbeck, Pharmavita, and has received scholarship support from the Government of Chile. Dr. Bellivier has received honoraria for speaker activities from AstraZeneca, Bristol-Myers Squibb, Euthérapie, Lundbeck, Otsuka, European Space Agency, and for consulting from Bristol-Myers Squibb, Lundbeck, Otsuka, European Space Agency. Dr. Frye has been a consultant (unpaid) for Allergan, Merck, Myriad, Sanofi-Aventis, Sunovion, Takeda Global Research, Teva Pharmaceuticals, United Biosource Corporation, has received grant support from Myriad, Pfizer, National Alliance for Schizophrenia and Depression (NARSAD), National Institute of Mental Health (NIMH), National Institute of Alcohol Abuse and Alcoholism (NIAAA), Mayo Foundation, and has received travel support from Chilean Society of Neurology, Psychiatry and Neurosurgery (Sociedad de Neurologia, Psiquiatria y Neurocirugia), Advanced Health Media, GlaxoSmithKline, Colombian Society of Neuropsychopharmacology, AstraZeneca, Bristol-Myers-Squib, Otsuka, Sanofi-Aventis. For the remaining authors, no further conflict of interests were declared.
References
- 1.Frye MA. Clinical practice. Bipolar disorder–a focus on depression. N Engl J Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- 2.Magalhaes PV, Kapczinski F, Nierenberg AA, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand. 2012;125:303–308. doi: 10.1111/j.1600-0447.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 3.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 4.Castagnini A, Foldager L, Bertelsen A. Excess mortality of acute and transient psychotic disorders: comparison with bipolar affective disorder and schizophrenia. Acta Psychiatr Scand. 2013;128:370–375. doi: 10.1111/acps.12077. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 6.Madden JA. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology. 2012;79:S58–S62. doi: 10.1212/WNL.0b013e3182695836. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007;9:443–452. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 10.Heffner JL, Strawn JR, Delbello MP, Strakowski SM, An-thenelli RM. The co-occurrence of cigarette smoking and bipolar disorder: phenomenology and treatment considerations. Bipolar Disord. 2011;13:439–453. doi: 10.1111/j.1399-5618.2011.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre RS, Konarski JZ, Misener VL, Kennedy SH. Bipolar disorder and diabetes mellitus: epidemiology, etiology, and treatment implications. Ann Clin Psychiatry. 2005;17:83–93. doi: 10.1080/10401230590932380. [DOI] [PubMed] [Google Scholar]
- 13.Torrent C, Amann B, Sanchez-Moreno J, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. doi: 10.1111/j.1600-0447.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo JJ, Keck PE, Jr, Corey-Lisle PK, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among Medicaid patients with bipolar disorder: a nested case-control study. Pharmacotherapy. 2007;27:27–35. doi: 10.1592/phco.27.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’italien GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–1825. doi: 10.1176/ajp.2006.163.10.1821. [DOI] [PubMed] [Google Scholar]
- 16.Bobo WV, Cooper WO, Stein C, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- 17.Bradford DW, Kim MM, Braxton LE, Marx CE, Butter-field M, Elbogen EB. Access to medical care among persons with psychotic and major affective disorders. Psychiatr Serv. 2008;59:847–852. doi: 10.1176/ps.2008.59.8.847. [DOI] [PubMed] [Google Scholar]
- 18.van der Kooy K, van Hout H, Marwijk H, Marten H, Ste-houwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 19.Kilbourne AM, Brar JS, Drayer RA, Xu X, Post EP. Cardiovascular disease and metabolic risk factors in male patients with schizophrenia, schizoaffective disorder, and bipolar disorder. Psychosomatics. 2007;48:412–417. doi: 10.1176/appi.psy.48.5.412. [DOI] [PubMed] [Google Scholar]
- 20.Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res. 2011;70:145–154. doi: 10.1016/j.jpsychores.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allebeck P. The use of population based registers in psychiatric research. Acta Psychiatr Scand. 2009;120:386–391. doi: 10.1111/j.1600-0447.2009.01474.x. [DOI] [PubMed] [Google Scholar]
- 22.Santos CO, Caeiro L, Ferro JM, Figueira ML. Mania and stroke: a systematic review. Cerebrovasc Dis. 2011;32:11–21. doi: 10.1159/000327032. [DOI] [PubMed] [Google Scholar]
- 23.Bellivier F, Golmard JL, Rietschel M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. J Clin Epidemiol. 1994;47:881–889. doi: 10.1016/0895-4356(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.2. Copenhagen: 2012. [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Callaghan RC, Khizar A. The incidence of cardiovascular morbidity among patients with bipolar disorder: a population-based longitudinal study in Ontario, Canada. J Affect Disord. 2010;122:118–123. doi: 10.1016/j.jad.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Lin HC, Tsai SY, Lee HC. No higher risk of myocardial infarction among bipolar patients in a 6-year follow-up of acute mood episodes. Psychosom Med. 2008;70:73–76. doi: 10.1097/PSY.0b013e31815c1e93. [DOI] [PubMed] [Google Scholar]
- 32.Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Psychiatric comorbidity and mortality after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:213–220. doi: 10.1161/CIRCOUTCOMES.108.829143. [DOI] [PubMed] [Google Scholar]
- 33.Baune BT, Adrian I, Arolt V, Berger K. Associations between major depression, bipolar disorders, dysthymia and cardiovascular diseases in the general adult population. Psychother Psychosom. 2006;75:319–326. doi: 10.1159/000093955. [DOI] [PubMed] [Google Scholar]
- 34.Bellivier F, Medina E, Coulomb S, Lukasiewicz M. Observational, multinational study of bipolar I and II disorders (WAVE-bd): comparison of clinical data between France and other countries. Eur Neuropsychopharmacol. 2012;22:S281–S282. [Google Scholar]
- 35.Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med. 2006;68:684–691. doi: 10.1097/01.psy.0000237316.09601.88. [DOI] [PubMed] [Google Scholar]
- 36.Correll CU. Elevated cardiovascular risk in patients with bipolar disorder: When does it start and where does it lead? J Clin Psychiatry. 2008;69:1948–1952. doi: 10.4088/jcp.v69n1214. [DOI] [PubMed] [Google Scholar]
- 37.Corruble E. Bipolar disorders and somatic comorbidities. Encephale. 2008;34:S143–S145. doi: 10.1016/S0013-7006(08)80625-2. [DOI] [PubMed] [Google Scholar]
- 38.Ghadirian AM, Engelsmann F. Somatic illness in manicdepressive and schizophrenic patients. J Psychosom Res. 1985;29:281–286. doi: 10.1016/0022-3999(85)90055-8. [DOI] [PubMed] [Google Scholar]
- 39.Guo JJ, Patel NC, Li H, Keck JRPE. Prevalence of treated bipolar disorders and associated comorbidities in managed care and medicaid populations. Am Health Drug Benefits. 2010;3:171–178. [Google Scholar]
- 40.Hirose T. Comorbidity in depressive disorders. Seishin Shinkeigaku Zasshi. 2001;103:1046–1054. [PubMed] [Google Scholar]
- 41.Jerrell JM, McIntyre RS, Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J Clin Psychiatry. 2010;71:1518–1525. doi: 10.4088/JCP.09m05585ora. [DOI] [PubMed] [Google Scholar]
- 42.Kupfer DJ. The increasing medical burden in bipolar disorder. J Am Med Assoc. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 43.Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Morten-sen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66:713–720. doi: 10.1001/archgenpsychiatry.2009.61. [DOI] [PubMed] [Google Scholar]
- 44.Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One. 2011;6:e24597. doi: 10.1371/journal.pone.0024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laursen TM, Nordentoft M. Heart disease treatment and mortality in schizophrenia and bipolar disorder - changes in the Danish population between 1994 and 2006. J Psy-chiatr Res. 2011;45:29–35. doi: 10.1016/j.jpsychires.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 46.Lin HC, Tsai SY, Lee HC. Increased risk of developing stroke among patients with bipolar disorder after an acute mood episode: a six-year follow-up study. J Affect Disord. 2007;100:49–54. doi: 10.1016/j.jad.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Michalik E, Puzynski S. Waniek [Incidence of psychosomatic disorders in patients with bipolar psychoses (pilot studies)] J Psychiatr Pol. 1980;14:19–27. [PubMed] [Google Scholar]
- 48.Miller BJ. Hospital admission for schizophrenia and bipolar disorder. BMJ (Online) 2011;343:d5652. doi: 10.1136/bmj.d5652. [DOI] [PubMed] [Google Scholar]
- 49.Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67:e16. [PubMed] [Google Scholar]
- 50.Nilsson FM, Kessing LV. Increased risk of developing stroke for patients with major affective disorder-a registry study. Eur Arch Psychiatry Clin Neurosci. 2004;254:387–391. doi: 10.1007/s00406-004-0519-9. [DOI] [PubMed] [Google Scholar]
- 51.Ramsey CM, Leoutsakos JM, Mayer LS, Eaton WW, Lee HB. History of manic and hypomanic episodes and risk of incident cardiovascular disease: 11.5 year follow-up from the Baltimore Epidemiologic Catchment Area Study. J Affect Disord. 2010;125:35–41. doi: 10.1016/j.jad.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry. 2011;68:1135–1142. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slomka JM, Piette JD, Post EP, et al. Mood disorder symptoms and elevated cardiovascular disease risk in patients with bipolar disorder. J Affect Disord. 2012;138:405–408. doi: 10.1016/j.jad.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swartz HA, Fagiolini A. Cardiovascular disease and bipolar disorder: risk and clinical implications. J Clin Psychiatry. 2012;73:1563–1565. doi: 10.4088/JCP.12ac08227. [DOI] [PubMed] [Google Scholar]
- 55.Taylor V, McKinnon MC, Macdonald K, Jaswal G, Mac-queen GM. Adults with mood disorders have an increased risk profile for cardiovascular disease within the first 2 years of treatment. Can J Psychiatry. 2010;55:362–368. doi: 10.1177/070674371005500605. [DOI] [PubMed] [Google Scholar]
- 56.Weber NS, Fisher JA, Cowan DN, Niebuhr DW. Psychiatric and general medical conditions comorbid with bipolar disorder in the National Hospital Discharge Survey. Psy-chiatr Serv. 2011;62:1152–1158. doi: 10.1176/ps.62.10.pss6210_1152. [DOI] [PubMed] [Google Scholar]
- 57.Wu SI, Chen SC, Juang JJM, et al. Diagnostic procedures, revascularization, and inpatient mortality after acute myocardial infarction in patients with schizophrenia and bipolar disorder. Psychosom Med. 2013;75:52–59. doi: 10.1097/PSY.0b013e31827612a6. [DOI] [PubMed] [Google Scholar]
- 58.Westman J, Hallgren J, Wahlbeck K, Erlinge D, Alfreds-son L, Osby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3:e002373. doi: 10.1136/bmjopen-2012-002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byrne N, Regan C, Howard L. Administrative registers in psychiatric research: a systematic review of validity studies. Acta Psychiatr Scand. 2005;112:409–414. doi: 10.1111/j.1600-0447.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 60.Parker G. Register now: validity later. Acta Psychiatr Scand. 2005;112:407–408. doi: 10.1111/j.1600-0447.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 61.Kessing L. Validity of diagnoses and other clinical register data in patients with affective disorder. Eur Psychiatry. 1998;13:392–398. doi: 10.1016/S0924-9338(99)80685-3. [DOI] [PubMed] [Google Scholar]
- 62.Munk-Jorgensen P, Mortensen PB. The Danish Psychiatric Central Register. Dan Med Bull. 1997;44:82–84. [PubMed] [Google Scholar]
- 63.Munk-Jørgensen P, Okkels N, Ruggeri M, Thornicroft G. Fifty years’ development and future perspectives of psychiatric register research. Acta Psychiatr Scand. 2014;130:87–98. doi: 10.1111/acps.12281. [DOI] [PubMed] [Google Scholar]
- 64.Parker G, O’donnell M, Walter S. Changes in the diagnoses of the functional psychoses associated with the introduction of lithium. Br J Psychiatry. 1985;146:377–382. doi: 10.1192/bjp.146.4.377. [DOI] [PubMed] [Google Scholar]
- 65.Kessing L. A comparison of ICD-8 and ICD-10 diagnoses of affective disorder -a case register study from Denmark. Eur Psychiatry. 1998;13:342–345. doi: 10.1016/S0924-9338(99)80700-7. [DOI] [PubMed] [Google Scholar]
- 66.Vieta E, Pappadopulos E, Mandel FS, Lombardo I. Impact of geographical and cultural factors on clinical trials in acute mania: lessons from a ziprasidone and haloperidol placebo-controlled study. Int J Neuropsychopharmacol. 2011;14:1017–1027. doi: 10.1017/S146114571100040X. [DOI] [PubMed] [Google Scholar]
- 67.Kennedy N, Boydell J, van Os J, Murray RM. Ethnic differences in first clinical presentation of bipolar disorder: results from an epidemiological study. J Affect Disord. 2004;83:161–168. doi: 10.1016/j.jad.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Strakowski SM, Keck PE, Jr, Arnold LM, et al. Ethnicity and diagnosis in patients with affective disorders. J Clin Psychiatry. 2003;64:747–754. doi: 10.4088/jcp.v64n0702. [DOI] [PubMed] [Google Scholar]
- 69.Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47:1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Laursen TM, Wahlbeck K, H€allgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8:e67133. doi: 10.1371/journal.pone.0067133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bull K, Spiegelhalter DJ. Survival analysis in observational studies. Stat Med. 1997;16:1041–1074. doi: 10.1002/(sici)1097-0258(19970515)16:9<1041::aid-sim506>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 72.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–e923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 73.Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156:1417–1420. doi: 10.1176/ajp.156.9.1417. [DOI] [PubMed] [Google Scholar]
- 74.Regenold WT, Thapar RK, Marano C, Gavirneni S, Kond-apavuluru PV. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- 75.Kessing LV, Thomsen AF, Mogensen UB, Andersen PK. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197:266–271. doi: 10.1192/bjp.bp.109.076935. [DOI] [PubMed] [Google Scholar]
- 76.Moon E, Chang JS, Kim MY, et al. Dropout rate and associated factors in patients with bipolar disorders. J Affect Disord. 2012;141:47–54. doi: 10.1016/j.jad.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 77.Barry AE. How attrition impacts the internal and external validity of longitudinal research. J Sch Health. 2005;75:267–270. doi: 10.1111/j.1746-1561.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- 78.Angst J, Hengartner MP, Gamma A, von Zerssen D, Angst F. Mortality of 403 patients with mood disorders 48 to 52 years after their psychiatric hospitalisation. Eur Arch Psychiatry Clin Neurosci. 2012;263:425–434. doi: 10.1007/s00406-012-0380-1. [DOI] [PubMed] [Google Scholar]
- 79.Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 80.Cohen H, Kaplan Z, Kotler M, Mittelman I, Osher Y, Ber-sudsky Y. Impaired heart rate variability in euthymic bipolar patients. Bipolar Disord. 2003;5:138–143. doi: 10.1034/j.1399-5618.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 81.Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- 82.Manfrini O, Pizzi C, Trere D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. Eur Heart J. 2003;24:1560–1566. doi: 10.1016/s0195-668x(03)00345-2. [DOI] [PubMed] [Google Scholar]
- 83.Waxmonsky JA, Thomas MR, Miklowitz DJ, et al. Prevalence and correlates of tobacco use in bipolar disorder: data from the first 2000 participants in the Systematic Treatment Enhancement Program. Gen Hosp Psychiatry. 2005;27:321–328. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 84.McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–213. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- 85.Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141:1–10. doi: 10.1016/j.jad.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao D, Ning N, Guo Y, Ning W, Niu X, Yang J. Computed tomography for detecting coronary artery plaques: a meta-analysis. Atherosclerosis. 2011;219:603–609. doi: 10.1016/j.atherosclerosis.2011.08.022. [DOI] [PubMed] [Google Scholar]