Abstract
Cigarette smoke (CS) is a main source of oxidative stress and a key risk factor for emphysema, which consists of alveolar wall destruction. Alveolar type (AT) II cells are in the gas exchange regions of the lung. We isolated primary ATII cells from deidentified organ donors whose lungs were not suitable for transplantation. We analyzed the cell injury obtained from nonsmokers, moderate smokers, and heavy smokers. DJ-1 protects cells from oxidative stress and induces nuclear erythroid 2–related factor-2 (Nrf2) expression, which activates the antioxidant defense system. In ATII cells isolated from moderate smokers, we found DJ-1 expression by RT-PCR, and Nrf2 and heme oxygenase (HO)-1 translocation by Western blotting and immunocytofluorescence. In ATII cells isolated from heavy smokers, we detected Nrf2 and HO-1 cytoplasmic localization. Moreover, we found high oxidative stress, as detected by 4-hydroxynonenal (4-HNE) (immunoblotting), inflammation by IL-8 and IL-6 levels by ELISA, and apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in ATII cells obtained from heavy smokers. Furthermore, we detected early DJ-1 and late Nrf2 expression after ATII cell treatment with CS extract. We also overexpressed DJ-1 by adenovirus construct and found that this restored Nrf2 and HO-1 expression and induced nuclear translocation in heavy smokers. Moreover, DJ-1 overexpression also decreased ATII cell apoptosis caused by CS extract in vitro. Our results indicate that DJ-1 activates the Nrf2-mediated antioxidant defense system. Furthermore, DJ-1 overexpression can restore the impaired Nrf2 pathway, leading to ATII cell protection in heavy smokers. This suggests a potential therapeutic strategy for targeting DJ-1 in CS-related lung diseases.
Keywords: DJ-1, nuclear erythroid 2–related factor-2, alveolar type II cells, cigarette smoke
Clinical Relevance
Cigarette smoke is a major risk factor for emphysema development. Our results demonstrate that DJ-1 can restore the impaired antioxidant defense system regulated by nuclear erythroid 2–related factor-2 in human primary alveolar type II cells in heavy smokers. This may lead to potential therapeutic strategies against emphysema development.
Cigarette smoke (CS) contains mutagens, carcinogens, and toxic substances (1, 2). It has been linked to a variety of chronic lung disorders and is a major cause of morbidity and mortality. CS is also a main risk factor for emphysema development through generation of severe oxidative stress and chronic inflammation (3, 4). Oxidative stress is a state of imbalance between the production of free radicals and their clearance by the cellular antioxidant defense system (5, 6). Recent evidence also indicates that the apoptosis of alveolar epithelial cells is involved in the pathogenesis of pulmonary emphysema, characterized by the loss of the alveolar wall (7). Alveolar type (AT) II cells are in the gas exchange regions of the lung (8). They have a distinct morphology with apical microvilli and characteristic lamellar bodies. ATII cells make and secrete pulmonary surfactant. They also proliferate to restore the epithelium after lung injury.
DJ-1 is a small, conserved, multifunctional protein (∼20 kD) reported to be involved in diverse cellular processes, ranging from antioxidant defense to control of protein–RNA interaction to the oxidative stress response (9, 10). It has been detected in lung tissue (11) and reported to protect against reactive oxygen species (12, 13) and apoptosis in different cell types (14–17). It is expressed in the cytoplasm and nucleus. DJ-1 may possess chaperone-like activity; however, its physiological function is unclear (18).
The nuclear erythroid 2–related factor-2 (Nrf2) transcription factor is a key regulator of the cellular redox status and phase II detoxifying enzymes in the lung (6, 19). Nrf2 also regulates the expression of other antioxidant genes, such as heme oxygenase (HO)-1. We previously reported that Nrf2 protects human and murine ATII cells against injury by CS in vitro and in vivo (20–22). However, the role of DJ-1 in the activation of the Nrf2-mediated antioxidant defense system in the lung is not fully understood.
We hypothesize that ATII cells obtained from heavy smokers will have more injury than from moderate smokers or nonsmokers. Furthermore, we expect that DJ-1 will positively regulate the Nrf2-mediated antioxidant defense system against CS-induced oxidative stress in ATII cells. There is no report on this activation in human primary alveolar epithelial cells. We also anticipate that DJ-1 overexpression by adenovirus (Ad) DJ-1 construct will activate the Nrf2 pathway, which will provide protection against ATII cell injury by CS in vitro. To our knowledge, this is the first study of primary ATII cells isolated from nonsmokers, moderate smokers, and heavy smokers. This approach provides an excellent system to study the effects of ATII cell exposure to CS both ex vivo and in vitro.
Material and Methods
Human Primary ATII Cells
Human lungs from organ donors were obtained from the International Institute for the Advancement of Medicine. We selected donors without a history of chronic lung disease, with the ratio of partial pressure arterial oxygen and fraction of inspired oxygen of greater than 225, X-ray and clinical history not indicative of infection. Nonsmokers were individuals who never smoked, moderate smokers who smoked 5–10 cigarettes, and heavy smokers who smoked 25–40 cigarettes per day for at least 3 years (23). The Committee for the Protection of Human Subjects at National Jewish Health (Denver, CO) approved this research. ATII cells were isolated and cultured as we previously published (21, 22, 24, 25).
Infection with AdDJ-1
AdDJ-1 and AdGFP were prepared and used as we previously described (12, 25). We used virus diluted to a multiplicity of infection of 200 plaque forming unit (pfu)/cell in PBS for ATII cell infection. Cells were allowed to express transgenes for 24 hours before usage. All infected cell cultures were examined for adequate infection efficiency, as assessed by green fluorescence protein (GFP) fluorescence (91%) and by Western blotting for DJ-1.
ATII Cell Treatment with CS Extract In Vitro
The CS extract (CSE) was prepared as we previously described (21, 22). Briefly, the smoke of one 3R4F cigarette (Kentucky Tobacco Research and Development Center, Lexington, KY) was drawn into 12.5 ml Dulbecco’s modified Eagles medium with a peristaltic pump (Manostat 72-310-000; Barrington, IL). A 3% CSE concentration was selected for our study based on the viability assay.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
We used a combination of staining for antiprosurfactant protein C (proSP-C) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay as previously described (26–28). The percentages of TUNEL-positive apoptotic ATII cells were calculated per 10 high-power fields (magnification, 10 × 40 objective) (29). The same protocol (without proSP-C staining) was used to determine apoptosis by TUNEL assay in cultured ATII cells treated with CSE in vitro.
Western Blotting
Protein expression in ATII cells was measured by Western blotting, as we described previously (24). We used anti–HO-1, –lamin B1, -Nrf2, –DJ-1, I-kappa-B alpha (–IκB-α), –IL-8, and 4-hydroxynonenal (–4-HNE) antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Nuclear and Cytoplasmic Fraction Isolation
We used the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommendations to isolate nuclear and cytoplasmic fractions from ATII cells for subsequent Western blotting. We used lamin B1 and IκB-α as markers of nuclear and cytoplasmic fractions, respectively, as previously reported (30).
Immunocytofluorescence
We analyzed Nrf2 translocation in freshly isolated ATII cells. ATII cell cytospins were incubated with rabbit anti-Nrf2 antibody. The secondary antibody, Alexa Fluor 594 IgG, was applied for 1 hour. Cells were mounted with Vectashield medium (Burlingame, CA) containing 4′,6-diamidino-2-phenylindole.
ELISA
IL-8 and IL-6 levels were measured in freshly isolated ATII cells by ELISA (ELISA Tech., Aurora, CO) according to the manufacturer’s recommendations. We used a MicroQuant microplate spectrophotometer (BioTek, Winooski, VT) and analyzed data with KCjunior Data Analysis Software (BioTek).
Real-Time PCR
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD). Assay On-Demand DJ-1 probe (Hs00994896_g1) was purchased from Applied Biosystems (Thermo Fisher Scientific, Waltham, MA). Gene expression levels were calculated as a ratio to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1) expression.
Statistical Analysis
One-way ANOVA by GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA) was used. A Dunnett’s test was applied (P < 0.05). Data are shown here as the mean (±SEM) from at least three independent experiments.
Results
CS Induces ATII Cell Apoptosis in Heavy Smokers Ex Vivo
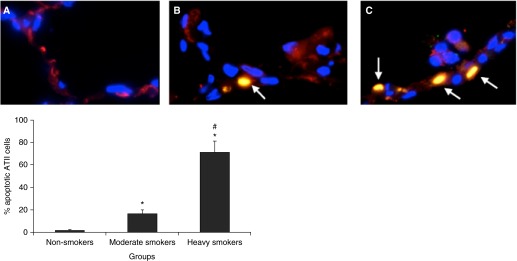
We have previously shown that CSE generates oxidative stress, DNA damage, and ATII cell injury in vitro and in vivo (20, 22). Here, we wanted to determine ATII cell injury induced by CS in moderate smokers and heavy smokers compared with nonsmokers ex vivo. We used a combination of TUNEL assay and staining for proSP-C to identify apoptotic ATII cells in lung tissue. We found the highest percentage of apoptotic ATII cells (71.2%) in heavy smokers in comparison with moderate smokers (16.2%) and nonsmokers (1.5%) (Figure 1). These results indicate that, first, CS induces apoptosis in ATII cells ex vivo, and, second, the percent of apoptotic ATII cells correlates with the smoking status of lung donors.
Figure 1.
Cigarette smoke (CS) induces apoptosis in alveolar type (AT) II cells isolated from heavy smokers ex vivo. (A) nonsmokers; (B) moderate smokers; (C) heavy smokers. ATII cells in the lung tissue sections were identified by staining with prosurfactant protein-C (red), and apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). Apoptotic ATII cells are shown by arrows. *Statistically significant increase compared with control (P < 0.05); #statistically significant increase compared with moderate smokers (n = 6). Data are shown as the mean (±SEM).
DJ-1 Expression in ATII Cells Obtained from Nonsmokers, Moderate Smokers, and Heavy Smokers Ex Vivo
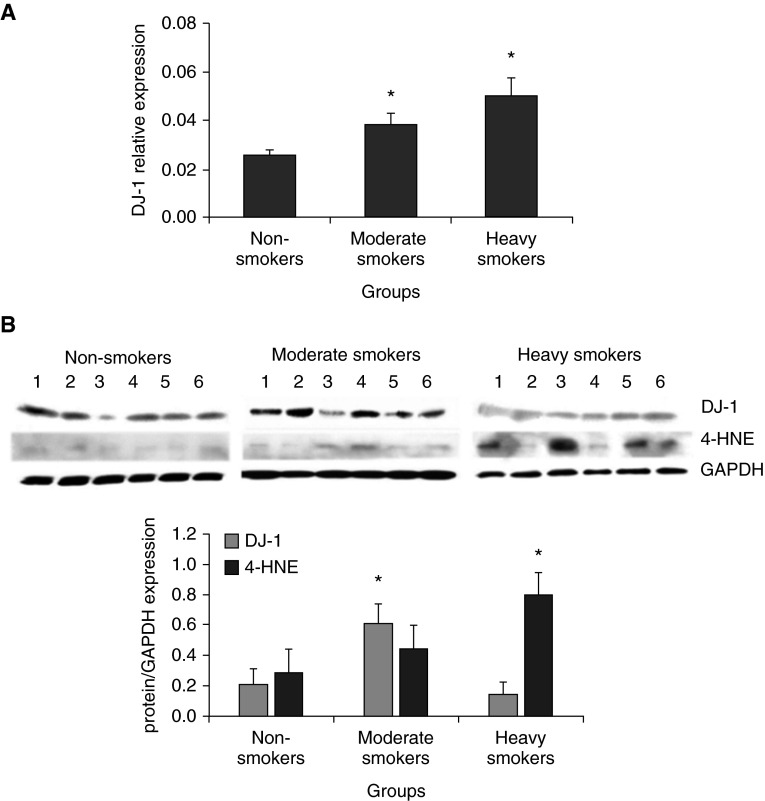
Because DJ-1 is involved in protection against oxidative stress, we wanted to determine its expression at the transcription and protein levels by RT-PCR and Western blotting, respectively. We found higher DJ-1 mRNA levels in ATII cells isolated from heavy smokers in comparison with moderate smokers or nonsmokers (Figure 2A). We also wanted to determine DJ-1 protein expression, and found lower DJ-1 levels in heavy smokers in comparison with moderate smokers and nonsmokers by Western blotting (Figure 2B). To determine the discrepancy between DJ-1 protein and gene levels in these lung donors, we measured the oxidative stress by using 4-HNE. We observed higher 4-HNE expression in heavy smokers in comparison with moderate smokers and nonsmokers (Figure 2B). These results suggest DJ-1 degradation during high oxidative stress in these lung donors. They are in agreement with the highest percentage of apoptotic ATII cells detected in heavy smokers. Our results show that DJ-1 is induced by CS. Furthermore, DJ-1 protects ATII cells against CS-induced oxidative injury in moderate smokers, but not in heavy smokers ex vivo.
Figure 2.
DJ-1 mRNA and protein expression in ATII cells ex vivo. DJ-1 mRNA expression by RT-PCR (A) and protein (B) levels (immunoblotting) were analyzed from freshly isolated ATII cells obtained from nonsmokers, moderate smokers, and heavy smokers. Relative expression is also shown. *Statistically significant increase in comparison with control (P < 0.05; n = 6). Data are shown as the mean (±SEM). 4-HNE, 4-hydroxynonenal.
Nrf2 Protects ATII Cells against Injury Induced by CS in Moderate Smokers Ex Vivo
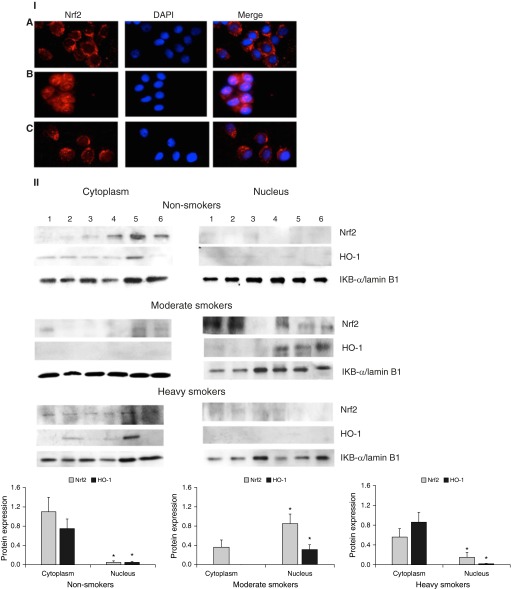
We found that DJ-1 is involved in ATII cell protection against injury induced by CS in moderate smokers. It has been reported that the DJ-1 pathway is widely associated with the antioxidant pathway induced by Nrf2 (11). Therefore, next, we wanted to determine whether DJ-1 expression correlates with Nrf2 levels, a key regulator of the antioxidant defense system. First, we analyzed cytospins of freshly isolated ATII cells from nonsmoker, moderate smoker, and heavy smoker lung donors. We found Nrf2 cytoplasmic localization in ATII cells isolated from nonsmokers and heavy smokers, and nuclear Nrf2 translocation in moderate smokers (Figure 3, panel I). This suggests Nrf2 activation in these latter lung donors. We also isolated nuclear and cytoplasmic fractions from ATII cells obtained from nonsmokers, moderate smokers and heavy smokers to determine expression of Nrf2 and its downstream target HO-1 by Western blotting. We found that both Nrf2 and HO-1 have mainly nuclear localization in moderate smokers and predominant cytoplasmic localization in heavy smokers and nonsmokers (Figure 3, panel II). Our results indicate that: first, the Nrf2 pathway is involved in the protection of ATII cells against injury by CS in moderate smokers ex vivo; second, nuclear localization of Nrf2 induces expression of phase 2 enzymes, such as HO-1 in ATII cells in moderate smokers; and third, Nrf2 and HO-1 cytoplasmic expression in heavy smokers indicates an impaired antioxidant defense system. These results are in agreement with the higher percentage of apoptotic ATII cells and oxidative stress observed in heavy smokers compared with moderate smokers and nonsmokers.
Figure 3.
Nuclear erythroid 2–related factor-2 (Nrf2) localization in ATII cells isolated from moderate smokers ex vivo. Panel I: representative pictures of Nrf2 localization in ATII cell cytospin on the day of isolation. (A) Nonsmoker; (B) moderate smoker; (C) heavy smoker (immunocytofluorescence). Panel II: Nrf2 and heme oxygenase (HO)-1 localization in nuclear and cytoplasmic fractions in freshly isolated ATII cells obtained from nonsmokers, moderate smokers, and heavy smokers (immunoblotting). IκB-α and lamin B1 were used for cytoplasmic and nuclear fractions, respectively. Relative expression is also shown. Data are shown as the mean (±SEM), n = 6, *P < 0.05.
CSE Induces Early DJ-1 and Late Nrf2 Expression in ATII Cells In Vitro
We identified that both DJ-1 and Nrf2 protect ATII cells against injury by CS in moderate smokers. Next, we sought to determine the time frame of their expression in vitro. We cultured ATII cells as described in the Materials and Methods section, followed by treatment with 3% CSE for 5 minutes, 10 minutes, 15 minutes, 30 minutes, 4 hours, and 24 hours to analyze DJ-1 and Nrf2 expression in a time-dependent manner. We found early DJ-1 expression with a maximum level at 15 minutes (Figure 4). Nrf2 levels increased later, with a maximum expression at 4 hours after CSE treatment. These data indicate that DJ-1 is involved in an early response to the oxidative stress response, whereas Nrf2 is activated later (Figure 4). In addition, these results suggest that DJ-1 may regulate the Nrf2 pathway.
Figure 4.
CS extract (CSE) induces early DJ-1 and late Nrf2 expression in ATII cells in vitro. ATII cells were isolated from nonsmokers, cultured, and treated with 3% CSE in vitro, as described in the Materials and Methods section for different times (immunoblotting; C, control). Relative expression of these proteins is also shown. *Statistically significant increase in comparison with control (P < 0.05; n = 3). Data are shown as the mean (±SEM).
High IL-8 and IL-6 Levels in ATII Cells Isolated from Heavy Smokers Ex Vivo
Next, we sought to determine whether low DJ-1 and Nrf2 expressions in heavy smokers contribute to the inflammatory response in these lung donors. We focused on CS-mediated regulation of IL-8 and IL-6, as these cytokines are known contributors to inflammation in emphysema (31). We found the highest IL-8 and IL-6 levels in ATII cells isolated from heavy smokers compared with moderate smokers or nonsmokers (Figure 5). These results suggest that impairment of the antioxidant defense system correlates with high proinflammatory response in heavy smokers ex vivo. Our data indicate that DJ-1 protects ATII cells against CS-induced injury, and may play an important role in modulating susceptibility to lung diseases.
Figure 5.
High IL-8 and IL-6 levels in ATII cells isolated from heavy smokers ex vivo. IL-8 and IL-6 levels were analyzed in freshly isolated ATII cells obtained from nonsmokers, moderate smokers, and heavy smokers by ELISA. *Statistically significant increase compared with nonsmokers; #statistically significant increase compared with moderate smokers (P < 0.05; n = 6). Data are shown as the mean (±SEM).
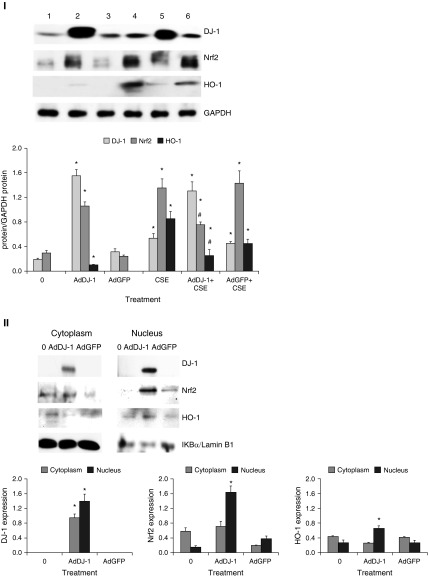
AdDJ-1 Can Restore Nrf2-Mediated Protection in Heavy Smokers
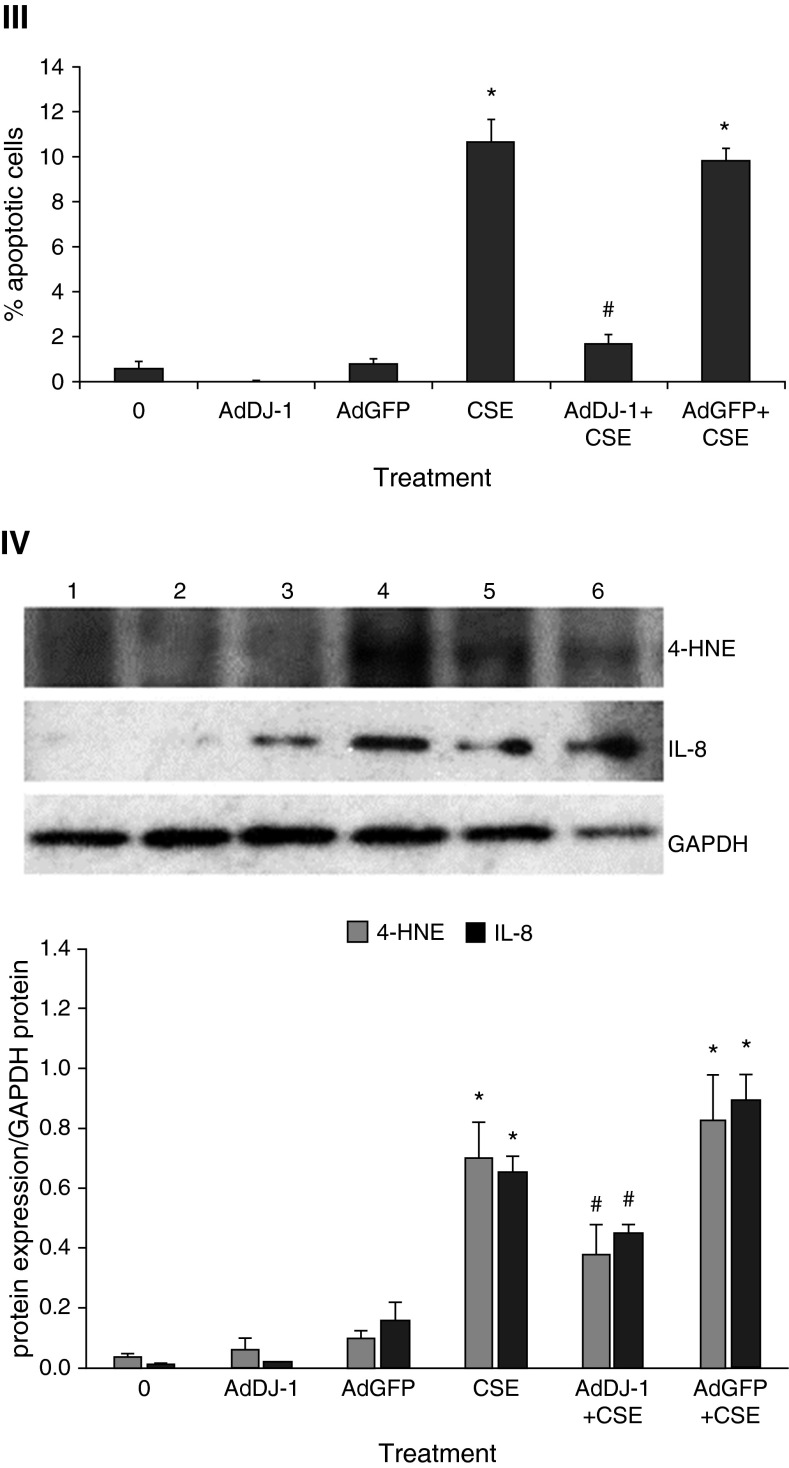
We found low DJ-1 and Nrf2 expression in ATII cells in heavy smokers ex vivo. Therefore, we wanted to examine whether DJ-1 overexpression using AdDJ-1 construct can activate Nrf2 levels in ATII cells in vitro. This is an important approach to determine whether we can restore the impaired Nrf2 pathway in ATII cells in heavy smokers. We observed Nrf2 induction by treatment with 3% CSE for 24 hours. We also found Nrf2 and HO-1 activation in ATII cells infected with AdDJ-1 after 24 hours (Figure 6, panel I). However, Nrf2 expression was decreased after infection with AdDJ-1 followed by treatment with CSE in comparison with CSE alone. This correlates with lower HO-1 levels. Moreover, we detected that AdDJ-1 induced Nrf2 and HO-1 nuclear translocation in ATII cells isolated from heavy smokers (Figure 6, panel II). We also performed TUNEL assay to determine the protective effect of DJ-1 overexpression against cell death. We found decreased apoptosis in ATII cells infected with AdDJ-1 followed by treatment with CSE in vitro (Figure 6, panel III). Our results indicate that: first, DJ-1 overexpression induces Nrf2 and HO-1 expression, which protects ATII cells against CSE-induced injury; second, DJ-1 overexpression induces Nrf2 and HO-1 nuclear translocation in ATII cells obtained from heavy smokers, which can restore antioxidant defense systems in these donors; and third, DJ-1 overexpression decreased ATII cell apoptosis induced by CSE.
Figure 6.
DJ-1 overexpression activates Nrf2 and decreases apoptosis of ATII cells induced by CSE in vitro. ATII cells were cultured, infected with adenovirus (Ad) DJ-1 or AdGFP, and treated with 3% CSE for 24 hours, as described in the Materials and Methods section. Panel I: AdDJ-1 increases Nrf2 and HO-1 expression. Lane 1, control; lane 2, AdDJ-1; lane 3, AdGFP; lane 4, CSE; lane 5, AdDJ-1 + CSE; lane 6, AdGFP + CSE. Relative expression is also shown. *Statistically significant increase in comparison with control. #Statistically significant decrease compared with CSE alone (P < 0.05; n = 3). Panel II: AdDJ-1 restores Nrf2 and HO-1 nuclear expression in ATII cells isolated from heavy smokers. Relative expression of these proteins is shown. *Statistically significant increase in comparison with control (P < 0.05; n = 6). Panel III: AdDJ-1 decreases apoptosis of ATII cells induced by 3% CSE in vitro. Cells were infected with AdDJ-1 followed by treatment with 3% CSE, as described in the Materials and Methods section. Apoptosis was analyzed by TUNEL assay. *Statistically significant increase in comparison with control. #Statistically significant decrease compared with CSE alone (P < 0.05; n = 3). Panel IV: AdDJ-1 decreases oxidative stress and inflammatory response induced by 3% CSE in ATII cells as measured by 4-HNE and IL-8 expression, respectively. Lane 1, control; lane 2, AdDJ-1; lane 3, AdGFP; lane 4, CSE; lane 5, AdDJ-1 + CSE; lane 6, AdGFP + CSE. Relative expression is also shown. *Statistically significant increase in comparison with control. #Statistically significant decrease compared with CSE alone (P < 0.05; n = 3). Data are shown as the mean (±SEM).
AdDJ-1 Inhibits 4-HNE and IL-8 Expression Induced by CSE in ATII Cells In Vitro
We found that AdDJ-1 decreases apoptosis of ATII cells induced by CSE (Figure 6, panel III). Next, we sought to determine the mechanism by which DJ-1 protects these cells. We infected ATII cells with AdDJ-1, followed by exposure to 3% CSE. We found that AdDJ-1 significantly lowers 4-HNE and IL-8 expression in cells exposed to CSE compared with CSE alone (Figure 6, panel IV). Our results suggest that DJ-1 overexpression decreases oxidative stress and proinflammatory response induced by CSE in ATII cells.
Discussion
CS is a very rich source of oxidants, and has been reported to induce epithelial cell death in emphysema (32). Nrf2, a key regulator of the antioxidant response system pathway, is critically important for a cellular defense system (33, 34). This work describes the important positive regulatory role of DJ-1 for the Nrf2 pathway, which protects against ATII cell injury by CS. To our knowledge, there are no studies on the comparison of ATII cell injury in moderate and heavy smokers. We confirmed our hypotheses that ATII cells isolated from heavy smokers have more injury than those obtained from moderate smokers or nonsmokers. We also found that DJ-1 induces Nrf2-mediated protection against ATII cell injury by CS. Our findings show that DJ-1 activation prevents cell injury induced by CS in moderate smokers. The obtained results improve our understanding of the DJ-1–Nrf2 pathway and oxidative stress in ATII cells. Using human primary ATII cells exposed to CS ex vivo supports the importance of our studies. Furthermore, this unique approach, including nonsmokers, moderate smokers, and heavy smokers, fills the gap in our knowledge on the mechanism of the impairment of the antioxidant defense system in heavy smokers, which may contribute to emphysema development.
It has been reported that higher apoptosis of ATII cells is associated with CS-induced lung diseases (35). Smoking is a dominating risk factor in the development of emphysema, which is characterized by alveolar wall destruction (36). We detected higher oxidative stress in ATII cells isolated from heavy smokers than in those from moderate smokers. Oxidative stress has been implicated in the initiation of lung inflammatory responses (37). ATII cells can produce inflammatory mediators, such as IL-6 and IL-8 (7, 38). Proinflammatory cytokines are increased in emphysema and appear to amplify inflammation in this disease (39, 40). We observed higher IL-8 and IL-6 levels in ATII cells obtained from heavy smokers than in those from moderate smokers or nonsmokers. Our results are in agreement with those of Garbin and colleagues (23), who observed higher IL-6 and NF-κB levels in peripheral blood mononuclear cells obtained from heavy smokers than in those from moderate smokers or nonsmokers. Our observations indicate a link between oxidative stress, apoptosis, and inflammation in ATII cells, depending on the smoking status.
Next, we wanted to determine the protective role of DJ-1 against ATII cell injury by CS. We found high DJ-1 mRNA expression in ATII cells obtained from both moderate and heavy smokers in comparison with nonsmokers. We also checked DJ-1 protein levels and found lower expression in ATII cells isolated from heavy smokers than in those from moderate smokers. This may suggest DJ-1 degradation. Lower DJ-1 levels were also observed in lung tissue obtained from patients with chronic obstructive pulmonary disease compared with that from control smokers (11). Moreover, it was previously reported that DJ-1 can protect cells from cell death in vitro (41). In our next approach, we wanted to determine the role of Nrf2 in ATII cell injury by CS. It was shown that Nrf2 activation induces expression of antioxidant genes, such as HO-1 (6). We found Nrf2 and HO-1 nuclear localization in ATII cells obtained from moderate smokers and their cytoplasmic localization in heavy smokers. This indicates Nrf2-mediated protection in the former group. Zhang and Forman (42) showed that HO-1 plays a critical role in resistance to oxidative stress induced by acrolein, a toxicant in CS, in bronchial epithelial cells. Garbin and colleagues (23) also observed higher Nrf2 levels in peripheral blood mononuclear cells obtained from moderate smokers than in those obtained from heavy smokers or nonsmokers. Impaired Nrf2 signaling was reported in lung tissue obtained from patients with chronic obstructive pulmonary disease (43). Our data indicate that low DJ-1 expression may at least partially contribute to Nrf2 decline, high apoptosis, and oxidative stress in ATII cells obtained from heavy smokers.
Our results also suggest a difference in the molecular signature of ATII cells obtained from nonsmokers, moderate smokers, and heavy smokers in vitro. Other studies indicate that nasal epithelial cells isolated from nonsmokers and smokers can maintain their different phenotype depending on smoking status during in vitro culture (44). We analyzed DJ-1 and Nrf2 expression in ATII cells exposed to CSE in vitro. It has been reported that CSE increased DJ-1 levels in A549 cells in vitro (45). We found early DJ-1 induction followed by late Nrf2 expression. Our results suggest that DJ-1 activation decreases oxidative stress and protects ATII cells through induction of the antioxidant response system mediated by Nrf2. This is in agreement with observations that DJ-1 is required to stabilize Nrf2 (17). We continued this approach using the AdDJ-1 construct. We found that DJ-1 overexpression increases Nrf2 and HO-1 expression in vitro. Moreover, our results indicate that AdDJ-1 inhibits oxidative stress and proinflammatory response induced by CSE, as measured by 4-HNE and IL-8 expression, respectively. We have previously reported that Nrf2 overexpression protected human ATII cells and ATI-like cells against oxidative stress–induced injury and proinflammatory response (25). It has also been observed that DJ-1 overexpression protects neuronal cells from oxidative stress (12, 46). Furthermore, in the critical experiment, we observed Nrf2 and HO-1 nuclear translocation after transfection of ATII cells obtained from heavy smokers with AdDJ-1. This suggests that DJ-1 overexpression can rescue the impaired Nrf2-mediated antioxidant defense system in these donors. It has been recently found that AdDJ-1 mitigated baseline loss of Nrf2 in bone marrow–derived macrophages obtained from DJ-1 knockout mice (47). However, to our knowledge, there is no report on the role of DJ-1 overexpression in human primary ATII cells.
In conclusion, our results improve our knowledge on the imbalance of the oxidative stress and antioxidant defense system in heavy smokers. The results also indicate the important role of DJ-1 in modulation of Nrf2-mediated protection of ATII cells against injury induced by CS. Our approach may provide a potential therapeutic strategy against CS-induced lung diseases.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Karen Edeen and Kelly Correll (National Jewish Health, Denver, CO) for their assistance with human alveolar type II cell isolations, and Liudmila Vlasenko (Temple University, Philadelphia, PA) for help with experiments.
Footnotes
This work was supported by National Institutes of Health (NIH) grant R01 HL118171 (B.K.), Flight Attendant Medical Research Institute grant CIA130046 (B.K.), American Lung Association (B.K.), and NIH grant R01 ES016285 (R.M.T.).
Author Contributions: K.B., E.M.M., R.M.T., and B.K. designed research; K.B., E.M.M., and W.Z. performed research; W.Z., C.R.F., H.W.C., S.G.K., and R.P.B. contributed new reagents/analytic tools; K.B., E.M.M., R.M.T., S.G.K., R.J.M., and B.K. analyzed data; K.B. and B.K. wrote the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0304OC on April 19, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18:575–586. doi: 10.1093/carcin/18.3.575. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev. 2009;12:45–64. doi: 10.1080/10937400802545094. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke–induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 5.Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, α-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 7.Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1:219–226. doi: 10.1186/1617-9625-1-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Hod Y. Differential control of apoptosis by DJ-1 in prostate benign and cancer cells. J Cell Biochem. 2004;92:1221–1233. doi: 10.1002/jcb.20159. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T α-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR. Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem. 2011;286:14941–14951. doi: 10.1074/jbc.M110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 17.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease–associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 19.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Messier EM, Day BJ, Bahmed K, Kleeberger SR, Tuder RM, Bowler RP, Chu HW, Mason RJ, Kosmider B. N-acetylcysteine protects murine alveolar type II cells from cigarette smoke injury in a nuclear erythroid 2–related factor-2–independent manner. Am J Respir Cell Mol Biol. 2013;48:559–567. doi: 10.1165/rcmb.2012-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messier EM, Bahmed K, Tuder RM, Chu HW, Bowler RP, Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013;4:e573. doi: 10.1038/cddis.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garbin U, Fratta Pasini A, Stranieri C, Cominacini M, Pasini A, Manfro S, Lugoboni F, Mozzini C, Guidi G, Faccini G, et al. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS One. 2009;4:e8225. doi: 10.1371/journal.pone.0008225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosmider B, Loader JE, Murphy RC, Mason RJ. Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells. Free Radic Biol Med. 2010;48:1513–1524. doi: 10.1016/j.freeradbiomed.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le A, Damico R, Damarla M, Boueiz A, Pae HH, Skirball J, Hasan E, Peng X, Chesley A, Crow MT, et al. Alveolar cell apoptosis is dependent on p38 MAP kinase–mediated activation of xanthine oxidoreductase in ventilator-induced lung injury. J Appl Physiol (1985) 2008;105:1282–1290. doi: 10.1152/japplphysiol.90689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosmider B, Osiecka R, Ciesielska E, Szmigiero L, Zyner E, Ochocki J. Induction of apoptosis and necrosis in lymphocytes by the cis-Pt(II) complex of 3-aminoflavone in comparison with cis-DDP. Mutat Res. 2004;558:169–179. doi: 10.1016/j.mrgentox.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Kosmider B, Zyner E, Osiecka R, Ochocki J. Induction of apoptosis and necrosis in A549 cells by the cis-Pt(II) complex of 3-aminoflavone in comparison with cis-DDP. Mutat Res. 2004;563:61–70. doi: 10.1016/j.mrgentox.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Ginzberg HH, Shannon PT, Suzuki T, Hong O, Vachon E, Moraes T, Abreu MT, Cherepanov V, Wang X, Chow CW, et al. Leukocyte elastase induces epithelial apoptosis: role of mitochondial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol. 2004;287:G286–G298. doi: 10.1152/ajpgi.00350.2003. [DOI] [PubMed] [Google Scholar]
- 30.Gill MB, Bockhorst K, Narayana P, Perez-Polo JR. Bax shuttling after neonatal hypoxia-ischemia: hyperoxia effects. J Neurosci Res. 2008;86:3584–3604. doi: 10.1002/jnr.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantin AM. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc Am Thorac Soc. 2010;7:368–375. doi: 10.1513/pats.201001-014AW. [DOI] [PubMed] [Google Scholar]
- 33.Boutten A, Goven D, Boczkowski J, Bonay M. Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway. Expert Opin Ther Targets. 2010;14:329–346. doi: 10.1517/14728221003629750. [DOI] [PubMed] [Google Scholar]
- 34.Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17:363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art: cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006;3:503–510. doi: 10.1513/pats.200603-054MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundbäck B, Lindberg A, Lindström M, Rönmark E, Jonsson AC, Jönsson E, Larsson LG, Andersson S, Sandström T, Larsson K Obstructive Lung Disease in Northern Sweden Studies. Not 15 but 50% of smokers develop COPD?—report from the obstructive lung disease in northern Sweden studies. Respir Med. 2003;97:115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 37.Min T, Bodas M, Mazur S, Vij N. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med (Berl) 2011;89:577–593. doi: 10.1007/s00109-011-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 39.Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, Jeyaseelan S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol. 2012;47:104–111. doi: 10.1165/rcmb.2011-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 41.Opsahl JA, Hjørnevik LV, Bull VH, Fismen L, Frøyset AK, Gromyko D, Solstad T, Fladmark KE. Increased interaction between DJ-1 and the Mi-2/nucleosome remodelling and deacetylase complex during cellular stress. Proteomics. 2010;10:1494–1504. doi: 10.1002/pmic.200900586. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-δ and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- 43.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. 2009;180:1196–1207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Carson JL, Lu TS, Brighton L, Hazucha M, Jaspers I, Zhou H. Phenotypic and physiologic variability in nasal epithelium cultured from smokers and non-smokers exposed to secondhand tobacco smoke. In Vitro Cell Dev Biol Anim. 2010;46:606–612. doi: 10.1007/s11626-010-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan X, Kelsen SG, Merali S. Proteomic analysis of oxidative stress–responsive proteins in human pneumocytes: insight into the regulation of DJ-1 expression. J Proteome Res. 2008;7:4955–4961. doi: 10.1021/pr800295j. [DOI] [PubMed] [Google Scholar]
- 46.Heo JY, Park JH, Kim SJ, Seo KS, Han JS, Lee SH, Kim JM, Park JI, Park SK, Lim K, et al. DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: involvement of mitochondrial complex I assembly. PLoS One. 2012;7:e32629. doi: 10.1371/journal.pone.0032629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shan Y, Akram A, Amatullah H, Zhou DY, Gali PL, Maron-Gutierrez T, González-López A, Zhou L, Rocco PR, Hwang D, et al. ATF3 protects pulmonary resident cells from acute and ventilator-induced lung injury by preventing Nrf2 degradation. Antioxid Redox Signal. 2015;22:651–668. doi: 10.1089/ars.2014.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.