Abstract
Protein S-glutathionylation (PSSG) is an oxidant-induced post-translational modification of protein cysteines that impacts structure and function. The oxidoreductase glutaredoxin-1 (Glrx1) under physiological conditions catalyzes deglutathionylation and restores the protein thiol group. The involvement of Glrx1/PSSG in allergic inflammation induced by asthma-relevant allergens remains unknown. In the present study, we examined the impact of genetic ablation of Glrx1 in the pathogenesis of house dust mite (HDM)-induced allergic airways disease in mice. Wild-type (WT) or Glrx1−/− mice were instilled intranasally with HDM on 5 consecutive days for 3 weeks. As expected, overall PSSG was increased in Glrx1−/− HDM mice as compared with WT animals. Total cells in bronchoalveolar lavage fluid were similarly increased in HDM-treated WT and Glrx1−/− mice. However, in response to HDM, mice lacking Glrx1 demonstrated significantly more neutrophils and macrophages but fewer eosinophils as compared with HDM-exposed WT mice. mRNA expression of the Th2-associated cytokines IL-13 and IL-6, as well as mucin-5AC (Muc5ac), was significantly attenuated in Glrx1−/− HDM-treated mice. Conversely, mRNA expression of IFN-γ and IL-17A was increased in Glrx1−/− HDM mice compared with WT littermates. Restimulation of single-cell suspensions isolated from lungs or spleens with HDM resulted in enhanced IL-17A and decreased IL-5 production in cells derived from inflamed Glrx1−/− mice compared with WT animals. Finally, HDM-induced tissue damping and elastance were significantly attenuated in Glrx1−/− mice compared with WT littermates. These results demonstrate that the Glrx1–PSSG axis plays a pivotal role in HDM-induced allergic airways disease in association with enhanced type 2 inflammation and restriction of IFN-γ and IL-17A.
Keywords: asthma, protein S-glutathionylation, IL-17A, IFN-γ, neutrophils
Clinical Relevance
This study demonstrates that the protein S-glutathionylation (PSSG)–glutaredoxin redox axis controls the nature of adaptive immune and inflammatory responses in an experimental model of allergic airways disease in mice. This advances our understanding the role that oxidative processes play in allergic disease and provides a platform for targeting PSSG chemistry to combat allergic airways disease.
Oxidative stress has been linked to the pathogenesis of a variety of diseases, and allergic inflammatory disorders, such as chronic asthma, are accompanied by changes in the oxidative environment (1). Inflammatory cells recruited to the asthmatic airways have an exceptional capacity for producing a battery of reactive oxygen and nitrogen species. These molecules have the potential to inflict oxidative damage to biomolecules, and also play a role in regulating redox-sensitive signal transduction pathways (2). Evidence for an oxidant–antioxidant imbalance in asthmatic airways has been demonstrated in a number of studies, revealing decreased total antioxidant capacity as well as lower levels of individual antioxidants in plasma and bronchoalveolar lavage (BAL) fluid of patients with asthma (3). Thiols in the form of sulfhydryl groups of proteins are among the most susceptible oxidant-sensitive targets, and can be reversibly oxidized to sulfenic acids (−SOH) or disulfides (S-S), or irreversibly oxidized to sulfinic (−SO2H) and sulfonic (−SO3H) acids (4).
Protein S-glutathionylation (PSSG) represents an oxidant-induced post-translational modification of reactive cysteines within proteins, and has emerged as a key regulatory mechanism whereby oxidants regulate (patho-)biological processes. PSSG occurs when the sulfhydryl group present in glutathione forms a mixed disulfide bond with a protein cysteine via mechanisms that remain incompletely understood. Increases in PSSG have been reported in different cell types under a variety of oxidative conditions (4), and numerous proteins have now been reported to be affected by PSSG. Glutaredoxin-1 (Glrx1; mammalian, cytosolic) is a well characterized, efficient, and specific catalyst of deglutathionylation responsible in part for the reversal of PSSG under physiological conditions (4). As a member of the thiol-disulfide oxidoreductase family, Glrx1 promotes reversible reduction of PSSG to free sulfhydryl groups through a monothiol mechanism. Our laboratories recently reported increases in GLRX1 expression in both the sputum from patients with asthma and the bronchiolar epithelium, in association with decreased PSSG (5). These data correspond with the increases in Glrx1 expression and activity observed predominantly in bronchial epithelium in the ovalbumin (OVA) model of allergic airways disease (6).
The impact of Glrx1–PSSG axis using asthma-relevant allergens and sensitization via the airways is unknown. House dust mite (HDM) is a complex allergen to which up to 85% of patients with asthma are allergic (7). The goal of the present study was to determine the impact of Glrx1 ablation on the development of HDM-induced allergic airways disease. Our results indicate that Glrx1 regulates HDM-induced adaptive immune responses and allergic airways disease by promoting type 2–driven inflammation and restricting IFN-γ and IL-17A. Some of the results of these studies have been previously reported in the form of an abstracts (8, 9).
Materials and Methods
Animal Studies
Glrx1−/− mice were backcrossed for over 10 generations into a BALB/cJ background (10) and were housed in the University of Vermont (Burlington, VT) animal facility. For all experiments, 8- to 12-week-old Glrx1−/− and littermate wild-type (WT) BALB/cJ control mice were used. All procedures were approved by the Institutional Animal Care and Use Committee.
Murine Model of HDM-Induced Allergic Asthma
WT and Glrx1−/− mice were subjected to intranasal instillations with 50 μg of HDM extract (cat. no. XPB70D3A2.5, lot no. 259585; Greer, Lenoir, NC) resuspended in PBS, or with PBS alone as a vehicle control, once a day for 5 consecutive days for 3 weeks. Mice were killed 72 hours after the final HDM challenge (Figure 1A).
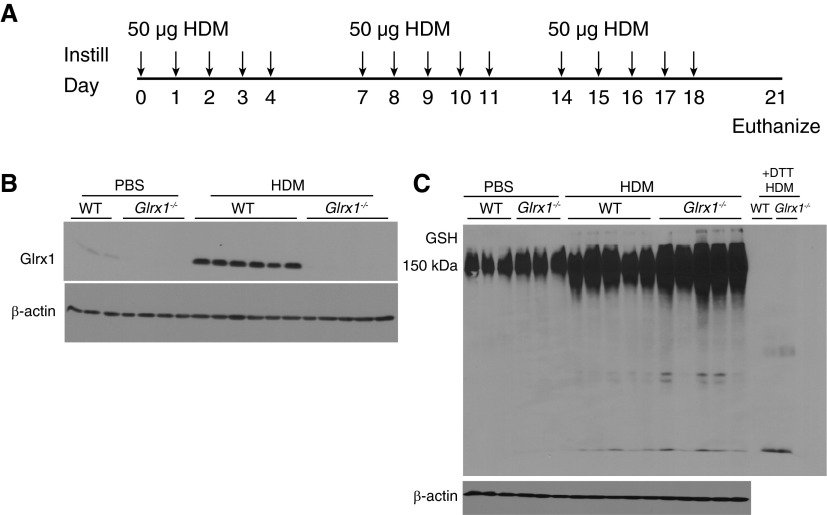
Figure 1.
Evaluation of protein S-glutathionylation (PSSG) and glutaredoxin-1 (Glrx1) expression in the whole lung after house dust mite (HDM) challenge. (A) Schematic depicting repeated intranasal instillation of 50 µg of HDM or PBS for the vehicle control and death on Day 21, 72 hours after the final challenge. (B) Immunoblot for Glrx1 in homogenized lung tissue; β-actin is shown as a loading control. Immunoblots are representative of three independent experiments; wild-type (WT) PBS (n = 3), Glrx1−/− PBS (n = 4), WT HDM (n = 6), and Glrx1−/− HDM (n = 5). (C) Immunoblot for total glutathione (GSH) in homogenized lung tissue. Lung tissue homogenates were electophoresed under nonreducing conditions, transferred into nitrocellulose, and probed with an anti-GSH antibody. +1,4-Dithiothreitol (DTT), representative samples from WT or Glrx1−/− mice exposed to HDM incubated with DTT before electrophoresis, to reduce PSSG as a reagent control; β-actin is shown as a loading control. Immunoblots are representative of three independent experiments; WT PBS (n = 3), Glrx1−/− PBS (n = 3), WT HDM (n = 5), and Glrx1−/− HDM (n = 5).
Assessment of Airway Hyperresponsiveness
All mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (90 mg/kg), tracheotomized, and mechanically ventilated at 200 breaths per minute. Respiratory mechanics were assessed at baseline (saline) and in response to increasing doses of methacholine (12.5, 25, 50 mg) via a forced oscillation technique using a computer-controlled small animal ventilator (FlexiVent; SCIREQ, Montreal, PQ, Canada), as previously described (11, 12).
Serum IgG1 and IgE
After mice were killed, blood was collected by heart puncture and immediately spun through a microtainer. Serum was collected and IgG1 and IgE content was determined using an ELISA-based method. Additional details are provided in the online supplement.
BAL
After mice were killed, BAL was collected using 1 ml PBS. Total cell counts were determined using the Advia 120 automated hematology analyzer (Siemens; Munich, Germany). For differential cell counts, cells collected by BAL were centrifuged onto glass slides at 600 rpm, and cytospins were stained using the Hema3 kit (Fisher Scientific, Kalamazoo, MI). A minimum of 300 cells were counted for every individual mouse, as previously described (12).
mRNA Analysis
Lobes of the right lung were flash frozen, pulverized, and total RNA was isolated and purified using the RNeasy kit (Qiagen, Valencia, CA). Detailed methods and primer sequences are provided in the online supplement.
Preparation of Lung Tissue for Single-Cell Suspension
Lung cells were prepared using a lung dissociation kit (Miltenyi Biotec, San Diego, CA) and GentleMACs dissociator (Miltenyi Biotec) per the manufacturer’s instructions. Detailed methods are provided in the online supplement.
Preparation of Splenocytes
Splenocytes were prepared from WT and Glrx1−/− mice as described in the online supplement.
ELISA
IL-5, IL-6, IL-13, IFN-γ, and IL-17A were detected by ELISA in lung homogenates (normalized for protein) or supernatants from single-cell suspensions, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Histopathology
Tissue histopathology and mucus metaplasia were assessed as described in the online supplement.
Statistical Analysis
All data were evaluated using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). Statistical significance was calculated with one-way, two-way, or three-way ANOVA followed by either a Bonferroni or Tukey post hoc test, and P values less than 0.05 were considered statistically significant. Scoring of histological staining was analyzed by Kruskal–Wallis test.
Results
Absence of Glrx1 Results in Increases in Protein PSSG after Repeated Challenge with HDM
Based on our previous observations using the OVA model of allergic airways disease (6), we first sought to determine whether Glrx1 and PSSG were affected in an HDM model of allergic airways disease, using the intranasal sensitization regimen shown in Figure 1A. In response to repeated intranasal administration of HDM, Glrx1 expression was robustly increased in the lung tissue homogenates of WT mice, compared with PBS vehicle controls (Figure 1B). Under physiological conditions, Glrx1 acts to reduce S-glutathionylated proteins to their sulfhydryl forms (6). Despite increases in Glrx1 (Figure 1B), PSSG content was also increased in lung tissues after HDM exposure compared with PBS control mice (Figure 1C). As expected, genetic ablation of Glrx1 resulted in greater increases in overall PSSG in response to HDM compared with WT littermates (Figure 1C), consistent with the deglutathionylating activity of Glrx1.
Modulation of HDM-Induced Airways Inflammation in Glrx1−/− Mice
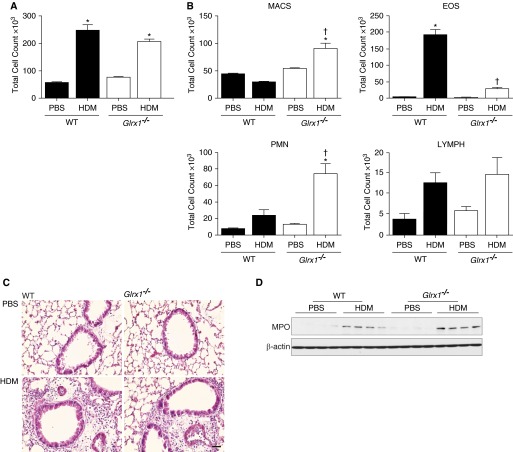
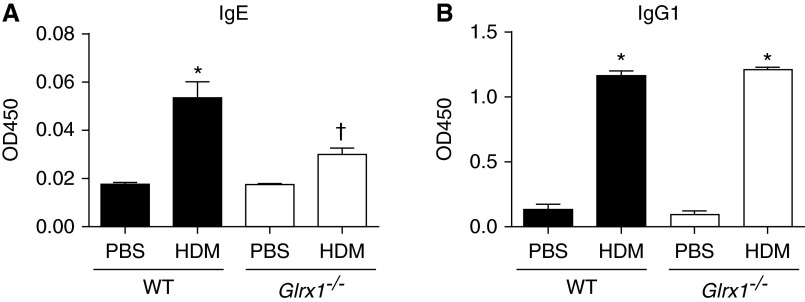
The extent of HDM-induced inflammation was assessed by total and differential counts of cells collected by BAL and by evaluation of histopathology. In response to HDM, overall BAL cell counts were elevated to a similar extent in both WT and Glrx1−/− mice compared with PBS controls (Figures 2A and 2C). However, in HDM-challenged Glrx1−/− mice, significantly more neutrophils were present in the BAL fluid compared with WT littermates (Figure 2B). Stronger increases in myeloperoxidase expression were also observed in the lung tissues of HDM-exposed Glrx1−/− mice compared with HDM-exposed WT animals (Figure 2D). In contrast to the increases in neutrophils, airway eosinophils were strongly decreased in Glrx1−/− mice compared with WT mice exposed to HDM (Figure 2B). Total macrophages were also elevated in HDM-challenged Glrx1−/− mice compared with WT groups, whereas lymphocytes were comparable between both groups (Figure 2B). HDM resulted in similar increases in serum IgG1 levels in WT and Glrx1−/− Balb/cJ mice compared with respective PBS controls (Figure 3B). In contrast, serum IgE was significantly attenuated in Glrx1−/− mice compared with HDM-exposed WT mice (Figure 3A). Overall, these findings suggest that, although the overall magnitude of airways inflammation is similar, the nature of the inflammatory response to HDM is influenced by the Glrx1–protein PSSG redox axis.
Figure 2.
Evaluation of airway and tissue inflammation in WT and Glrx1−/− mice after 15 challenges of HDM. (A) Total and (B) differential cell counts in bronchoalveolar lavage from WT and Glrx1−/− mice after exposure to PBS or HDM. Total cell numbers were determined by Advia, and data are expressed as means (± SEM) (eight or nine mice per group). *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM. (C) Histopathological evaluation of tissue inflammation via hematoxylin and eosin staining from PBS- or HDM-exposed mice (scale bar, 50 μm). (D) Immunoblot for myeloperoxidase in homogenized lung tissue. β-Actin is shown as a loading control. Immunoblots are representative of three independent experiments; WT PBS (n = 4), Glrx1−/− PBS (n = 4), WT HDM (n = 4), and Glrx1−/− HDM (n = 4). EOS, eosinophils; LYMPH, lymphocytes; MACS, macrophages; MPO, myeloperoxidase; PMN, polymorphonuclear neutrophils.
Figure 3.
Assessment of HDM-induced serum IgG1 and IgE in WT and Glrx1−/− mice. (A) Assessment of total HDM-specific IgE and (B) IgG1 in the serum in WT and Glrx1−/− mice. Serum Ig levels were measured by ELISA. Shown are corrected optical density at 450 nm (OD450) values. Data are expressed as means (± SEM) of six mice per group. *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM.
Ablation of Glrx1 Attenuates Th2 Cytokine Expression and Enhances IL-17A and IFN-γ Expression in the Lung after HDM Challenge
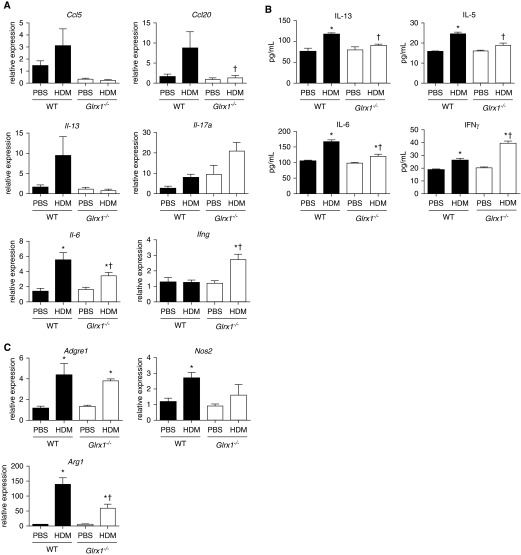
To further investigate the extent of HDM-induced allergic airway inflammation in WT and Glrx1−/− mice, we evaluated mRNA expression of different cytokines in whole-lung homogenates. mRNA expression of Ccl20, Ccl5 (regulated upon activation, normal T cell expressed and secreted), and Il-13 was increased in the lungs of WT mice after repeated HDM challenge; however, these increases were not apparent in Glrx1−/− mice (Figure 4A). Although the type 2–associated cytokines, IL-13, IL-5, and IL-6, were increased in the lungs of WT mice in response to HDM challenge, production of these cytokines was significantly attenuated in Glrx1−/− mice (Figure 4B). Non–type 2 responses, such as IL-17A, -E, or -F production, as well as neutrophil recruitment, have been reported in the lungs of patients with asthma, particularly those with severe or corticosteroid-resistant asthma (13). In association with increased neutrophils recovered from the BAL fluid of Glrx1−/− mice, mRNA expression of Il-17a was also significantly elevated in response to HDM exposure, compared with WT mice (Figure 4A). IL-17A content in the lung was undetectable in both WT and Glrx1−/− mice 72 hours after being killed (data not shown). Interestingly, mRNA expression and levels of the Th1 cytokine, IFN-γ, were markedly increased in lung tissues from HDM-exposed Glrx1−/− mice compared with WT counterparts (Figures 4A and 4B). Despite increases in macrophage numbers in BAL of HDM-exposed Glrx1−/− mice, mRNA expression of the macrophage marker, F4/80 (Adgre1), in lung tissues was similarly elevated in HDM-exposed WT and Glrx1−/− mice. In contrast, expression of nitric oxide synthase 2 (Nos2) and arginase-1 (Arg1), markers of M1 and M2 macrophage activation, respectively, were both decreased in HDM-exposed Glrx1−/− mice compared with respective WT groups (Figure 4C). Collectively, these findings suggest that HDM-induced immune responses are markedly different between WT and Glrx1−/− mice.
Figure 4.
Analysis of HDM-induced cytokine mRNA expression and protein levels. (A) mRNA expression of Ccl5 (regulated upon activation, normal T cell expressed and secreted), Ccl20, Il-13, Il-17a, Il-6, and Ifng from whole-lung homogenates of WT and Glrx1−/− mice was analyzed by real-time RT-PCR. Results were normalized to the housekeeping gene cyclophilin. Data are expressed as fold increases in expression compared with WT PBS controls. Data are expressed as means (± SEM) of 6 mice per group. *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM. (B) IL-13, IL-5, IL-6, and IFN-γ levels were measured in whole lung homogenates by ELISA. Data are expressed as means (± SEM) of six mice per group. *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM. (C) mRNA expression of Adgre (F4/80), nitric oxide synthase 2 (Nos2), and Arginase-1 (Arg1) from whole-lung homogenates of WT and Glrx1−/− mice was analyzed by real-time RT-PCR. Results were normalized to the housekeeping gene cyclophilin. Data are expressed as fold increases in expression compared with WT PBS controls. *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM.
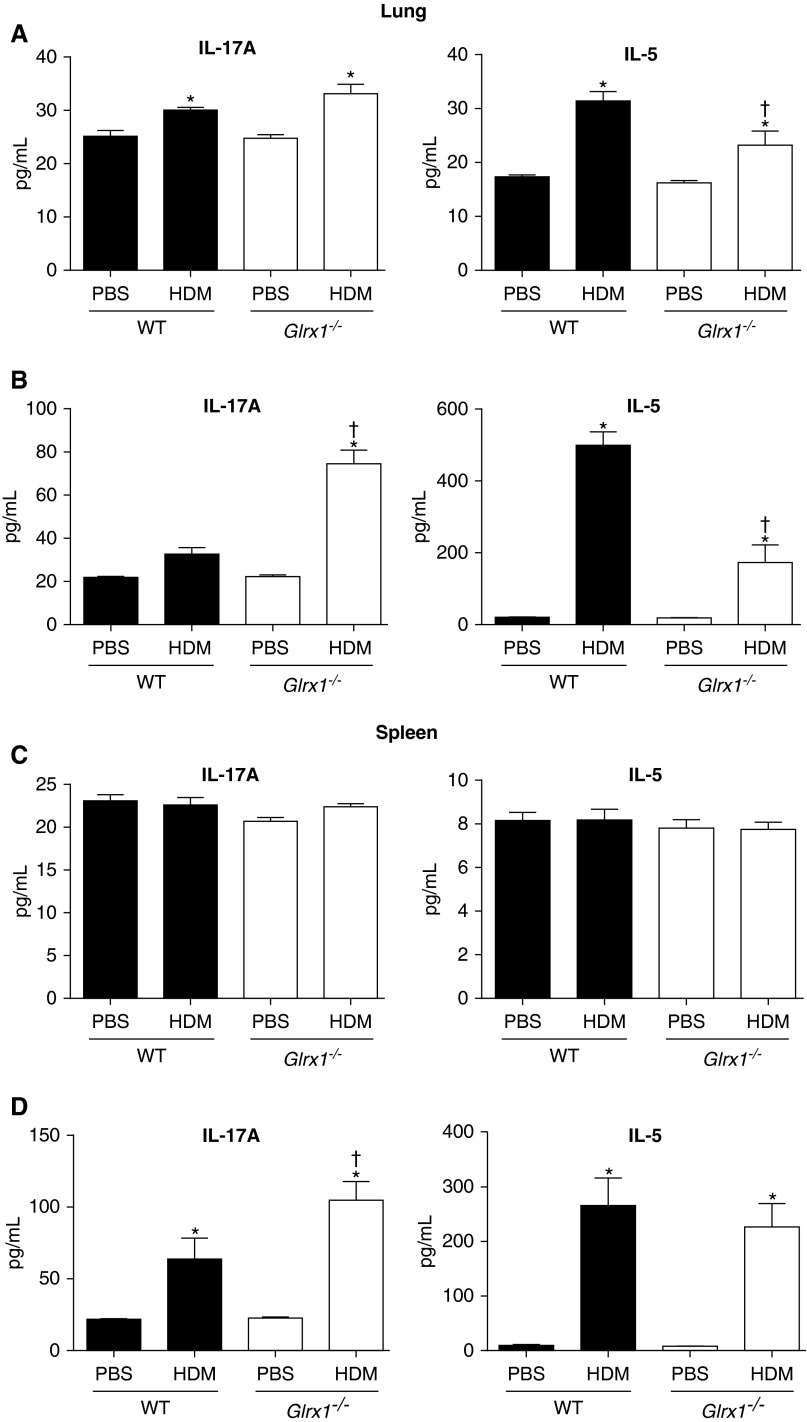
To further dissect these apparent differences, single-cell suspensions were prepared from lungs or spleens for assessment of spontaneous production of IL-5 or IL-17A (Figures 5A and 5C), or after restimulation of cells with HDM in vitro (Figures 5B and 5D). Cells prepared from the lungs (Figure 5A), but not the spleens (Figure 5C), of HDM-challenged WT and Glrx1−/− mice spontaneously produced significantly increased amounts of IL-17A and IL-5, compared with the PBS groups. Compared with WT counterparts, increases in spontaneous IL-5 production were significantly attenuated, whereas increases in IL-17A production tended to be enhanced in lung single-cell suspensions prepared from HDM-challenged Glrx1−/− mice (Figure 5A). In response to restimulation of cells with HDM in vitro, IL-5 production was further increased in the lung (Figure 5B) and spleen (Figure 5D) cells from both WT and Glrx1−/− HDM-exposed mice. However, the overall increases in IL-5 in response to HDM restimulation of lung cells were significantly attenuated in Glrx1−/− cells compared with respective WT counterparts (Figure 5B). HDM restimulation augmented IL-17A production in splenocytes, but not lung cells, prepared from WT mice. In contrast, after restimulation of Glrx1−/− lung cells and splenocytes with HDM, IL-17A production was significantly increased compared with WT counterparts (Figures 5A–5D, left panels). Collectively, these results demonstrate that absence of Glrx1 results in enhanced IL-17A and IFN-γ production and airway macrophages and neutrophils, yet dampens type 2 responses, manifested by decreases in eosinophils, IL-5, IL-6, and IL-13 expression, and decreases in HDM-induced production of IL-5.
Figure 5.
Assessment of HDM-stimulated Il-5 and Il-17A cytokine in single-cell suspensions prepared from lung tissue or spleens isolated from WT or Glrx1−/− mice exposed to PBS or HDM. Media were collected from single–lung cell (A and B) or spleen suspensions (C and D) from unstimulated (top) and cells restimulated (bottom) for 96 hours with 15 μg/ml of HDM. IL-17A and IL-5 production was quantified by ELISA. Data are expressed as means (± SEM; six mice per group) and are representative of three independent experiments. *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM.
Assessment of HDM-Induced Airway Hyperresponsiveness and Mucus Metaplasia in Glrx1−/− Mice
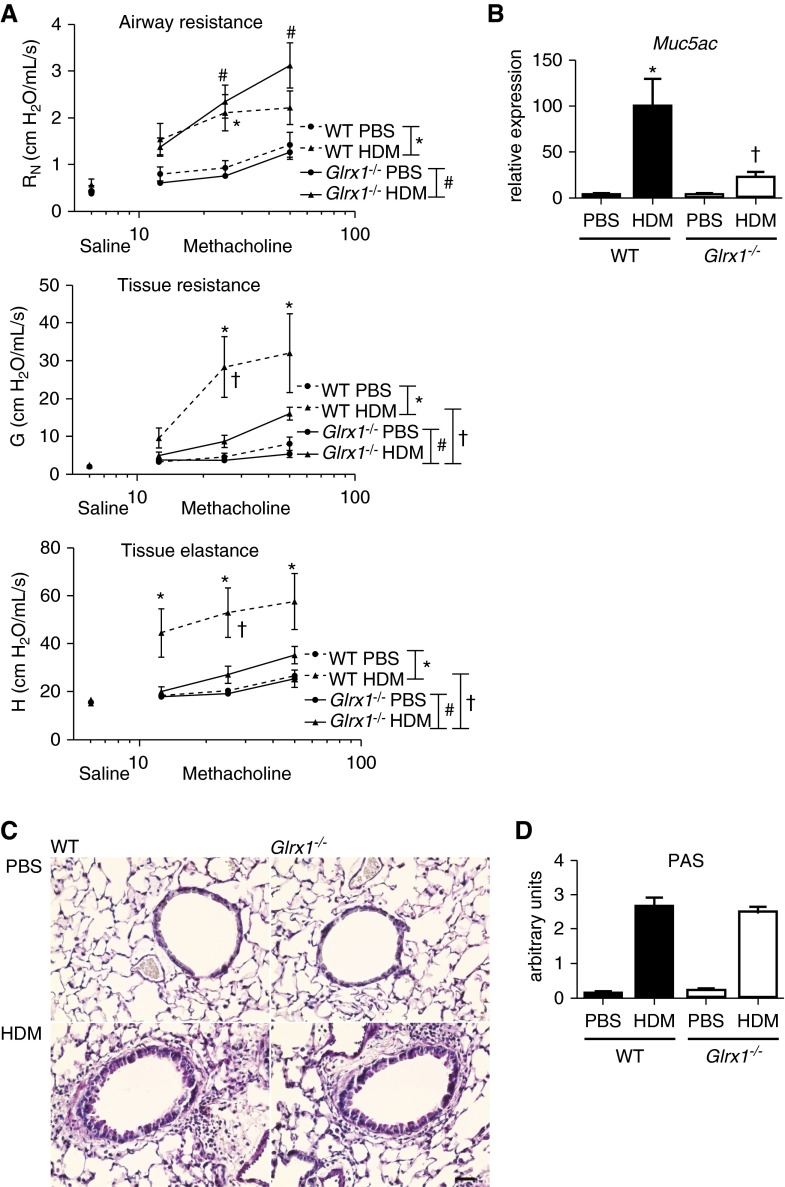
To address the effects of ablation of Glrx1 on HDM-induced alterations in respiratory mechanics, a forced oscillation technique was used to evaluate responsiveness to ascending doses of methacholine. As expected, airway Newtonian resistance (RN), tissue resistance, and tissue elastance were significantly elevated in HDM-challenged WT mice as compared with PBS controls (Figure 6A). Although RN was similarly elevated in Glrx1−/− animals, increases in tissue resistance and tissue elastance parameters, representing the peripheral airways, were significantly attenuated toward control values (Figure 6A). Characteristic features of allergic airways disease include excess mucus production and goblet cell hyperplasia (11, 14), which have been shown to contribute to airway closure and hyperresponsiveness (15, 16). Analysis of the mucin gene, mucin-5AC (Muc5ac), revealed attenuated mRNA expression in the lungs of HDM-exposed Glrx−/− mice in comparison to WT mice (Figure 6B). However, periodic acid Schiff staining revealed comparable HDM-induced mucus metaplasia in lung tissues from WT and Glrx1−/− mice (Figures 6C and 6D).
Figure 6.
Evaluation of alterations in respiratory mechanics and mucus metaplasia in WT and Glrx1−/− mice in response to 15 challenges of 50 μg of HDM. (A) Assessment of airway hyperresponsiveness (AHR) via a forced oscillation technique in WT and Glrx1−/− mice. Changes in respiratory mechanics were analyzed 72 hours after the final instillation of either PBS or HDM. Ascending doses of methacholine were administered to determine Newtonian resistance (RN), tissue resistance (G), and tissue elastance (H). Data are expressed as means (± SEM; six to roughly eight mice per group). For AHR data, a three-way ANOVA was used with an additional term for methacholine dose. */#P < 0.05 compared with PBS controls; †P < 0.05 compared with WT mice challenged with HDM. (B) Evaluation of mucin-5AC (Muc5ac) gene expression in homogenized lung tissue. mRNA was analyzed by real-time RT-PCR, and results were normalized to the housekeeping gene cyclophilin and are expressed as fold increases in expression compared with WT PBS controls. Data are expressed as means (± SEM; six mice per group). *P < 0.05 (ANOVA) compared with PBS controls; †P < 0.05 (ANOVA) compared with WT mice challenged with HDM. (C) Periodic acid–Schiff (PAS) staining of airway mucus in WT or Glrx1−/− mice exposed to HDM or PBS (scale bar, 50 μm). (D) Quantification of airway mucus staining (PAS) intensity was determined by scoring of slides by two blinded investigators. Data are expressed as means (± SEM) from six to eight mice per group. *P < 0.05 (Kruskal–Wallis) compared with respective PBS control groups. †P < 0.05 (Kruskal–Wallis) compared with WT HDM groups.
Discussion
The maintenance of redox homeostasis is crucial for appropriate cellular function. It is widely accepted that imbalances in the production and removal of oxidants are associated with chronic inflammatory disorders, such as asthma. Glutathione is the predominant endogenous antioxidant in mammalian cells and constitutes an essential redox buffer. Emerging studies suggest that the protein, PSSG, represents a mechanism whereby oxidants exert their (patho)physiological effects (17). Notably, the existence of Glrxs, which catalyze deglutathionylation reactions, have given credence to the (patho)physiological role of PSSG. Glrxs have been implicated in a number of diseases, including allergic airways disease (17, 18). Airways (sputum) of patients with asthma displayed increased GLRX1 and PSSG compared with patients without asthma. GLRX1 protein levels were specifically enhanced in eosinophilic and paucigranulocytic, but not in neutrophilic, patients with asthma, whereas PSSG levels were decreased only in eosinophilic and neutrophilic patients with asthma compared with healthy control subjects. These findings suggest that GLRX1 and PSSG correlate with inflammatory phenotypes in asthma (5). Interestingly, a significant negative correlation between GLRX1 and lung function was also observed, suggesting an association between decreased GLRX1, increased PSSG, and improved lung function (5). Our laboratory has previously demonstrated that the systemic loss of Glrx1 resulted in enhanced resolution of airway hyperresponsiveness (AHR) and mucus metaplasia in OVA-induced allergic airways disease (6). Results from the present study demonstrate that Glrx1 is increased in response to repeated HDM exposure (Figure 1B), along with increases in PSSG reactivity. Increases in HDM-stimulated PSSG were elevated in Glrx1−/− mice compared with WT animals (Figure 1C), consistent with the role of Glrx1 in catalysis of deglutathionylation reactions in physiological settings. A number of possibilities exist for the lack of complete reversal of PSSG by Glrx1, and include lack of targeting of Glrx1 to sites of PSSG formation, or potential inactivation of the Glrx1 enzyme. Future studies will be needed to address these possibilities.
In the present study, we also demonstrated that the extent of overall airway inflammation was increased comparably in both WT and Glrx1−/− mice after repeated intranasal HDM administration (Figures 2A and 2C). However, clear differences in the type of infiltrating cells were apparent between the two strains (Figure 2B). Infiltrating cells in the lungs of WT mice were predominantly eosinophils and lymphocytes, and, to a lesser extent, neutrophils. Conversely, neutrophils and macrophages were significantly elevated in Glrx1−/− mice compared with WT mice (Figures 2B and 2D). These findings complement the aforementioned study in patients with asthma wherein GLRX1 protein levels were enhanced in eosinophilic, but not in neutrophilic, patients with asthma (5). The mechanism for increases in lung neutrophils in lung tissues from Glrx1−/− mice remains unclear. Interestingly, a recent study demonstrated that dynamic actin PSSG in neutrophils is regulated by Glrx1, and overexpression of Glrx1 impaired neutrophil migration (19). It is not clear whether altered PSSG occurred in neutrophils and affected cellular function in the lungs of Glrx1-deficient animals, thereby contributing to HDM-induced allergic responses and altered respiratory mechanics. Despite increases in airway macrophages in HDM-exposed Glrx1−/− mice (Figure 2B), mRNA expression of the macrophage marker, F4/80 (Adgre) in lung tissues was similar in WT and Glrx1−/− mice, whereas Nos2 and Arg1 mRNA, markers for M1 and M2 macrophage polarization, were both decreased in Glrx1−/− mice (Figure 4C). We have previously demonstrated notable PSSG and Glrx1 reactivity in macrophages, and that Glrx1−/− macrophages display an immature phenotype (20). It is therefore plausible that the Glrx1–PSSG axis plays a role in the altered phenotype of HDM-induced allergic airways disease by controlling the function of macrophages. Further studies will be required to dissect the role of the Glrx1/PSSG redox module in regulating neutrophil and macrophage recruitment and function in settings of allergic asthma.
In addition to significantly increased airway neutrophils and macrophages, and decreased eosinophils in Glrx1−/− mice, a potential shift away from the type 2 responses as manifested by decreases in IL-4, IL-5, IL-6, and IL-13, was apparent in lungs of Glrx1−/− mice. Type 2 inflammation mediators produced by activated CD4+ T cells (21) stimulate increased IgE production in B cells (22). WT mice demonstrated significantly elevated serum IgE in response to HDM (Figure 3A) in addition to increased IL-5 and IL-13 content within the lung (Figure 4B). Conversely, HDM-specific serum IgE (Figure 3A), as well as IL-5, IL-6, and IL-13 (Figure 4B), were significantly attenuated in Glrx1−/− mice. Consistent with increased neutrophilia, Il-17a mRNA expression was significantly increased after HDM exposure in Glrx1−/− mice compared with WT mice (Figure 4A). Moreover, restimulation of single–lung cell suspensions with HDM revealed enhanced IL-17A and dampened IL-5 production from HDM-challenged Glrx1−/− mice as compared with WT littermates (Figure 5A), responses that appeared blunted in single-cell suspensions generated from the spleens (Figure 5B), indicating that T cell activation and/or polarization might have preferentially taken place in the lung. Indeed, recent studies have shown that naive T cells migrate to the peripheral tissues, including the lung (23). Furthermore, CD4+ T cell proliferation and Th17 differentiation was shown to occur primarily in the lung and not the draining lymph nodes after bleomycin-mediated lung injury (24), suggesting that similar events might be operative in the present study. However, evaluation of Th2 and Th17 lineage–specific transcription factors, GATA binding protein 3 (GATA3) and RAR-related orphan receptor γ-t (RORγt), from whole-lung homogenates did not reveal any considerable differences between WT and Glrx1−/− HDM-challenged mice (data not shown). In vitro polarization of naive T cells isolated from WT and Glrx1−/− mice also failed to reveal significant differences in the capacity to differentiate toward Th1, Th2, and Th17 lineages (data not shown). Further analysis of cytokines promoting Th17 differentiation and stabilization, such as transforming growth factor-β, IL-1β, and IL-23 (25) and studies that address the potential contribution of innate innate lymphoid cells (26) may help reveal how Glrx1 and the protein-thiol redox environment influences the nature of innate and adaptive immune responses to allergens.
In the present study, we also demonstrated increases in IFN-γ in HDM-exposed Glrx1−/− mice compared with WT mice, suggestive of a bias toward Th1 polarization in the absence of Glrx1. It is noteworthy that a high IFN-γ, low secretory leukocyte protease inhibitor phenotype has been recently linked to severe steroid-resistant asthma in humans and a mouse model of steroid-resistant airways disease. In that study, IFN-γ was linked to increases in AHR, but not airway inflammation (27). It is therefore possible that Glrx1 affects HDM-induced allergic airways disease by regulating Th1 polarization or effector function, a scenario that also will require additional studies.
Our group has previously demonstrated enhanced resolution of AHR in Glrx1−/− mice after the OVA protocol, which corresponded to significant decreases in mucus metaplasia compared with WT mice (6). In response to HDM, similar modulation of AHR was observed in Glrx1−/− mice (Figure 6A), with increases in RN being apparent, whereas tissue stiffness (damping) and elastance were significantly decreased compared with WT littermates. Thus, despite the differences in the antigens used, the route of sensitization, and inflammatory cell and cytokine milieu, absence of Glrx1 appears to affect AHR in a similar manner in both models. Despite the prior demonstration that mucus metaplasia was decreased in Glrx1−/− mice using the OVA model, the present study showed comparable mucus metaplasia in WT and Glrx1−/− mice subjected to HDM (Figures 6C and 6D), despite the observed decreased Muc5ac mRNA expression in Glrx1−/− mice compared with WT mice (Figure 6B). The apparent discrepancy between these findings is likely attributable to the timing of evaluation of lung tissues after the final challenge with antigen, which differed. Of interest in this regard is the recent demonstration that airway mucins are targets for oxidation, manifested by disulfide-based cross-links that increase mucin elasticity in association with pathological mucus gel formation (28). It is not known at this time whether mucins are targets for PSSG, and whether this attenuates cross-linking. Such a scenario could explain attenuation of AHR in Glrx1−/− mice, despite the apparent similar extent of overall mucus metaplasia. Previous studies have suggested that inflammation-triggered airway smooth muscle contraction alone is not sufficient for airway hyperreactivity to methacholine, and that noncontractile factors, such as mucus, are essential for AHR (29–31).
Current pathological and physiological evidence suggests a role for the peripheral airways and parenchyma in the production of Th2 cytokines and airflow obstruction in patients with asthma (32). Increased numbers of IL-4– and IL-5–expressing cells were found within the small airways of resected lung specimens from patients with asthma, and the expression of Il-5 mRNA was increased predominantly in the small airways (33). Regardless of HDM restimulation, Glrx1−/− mice exhibited significantly decreased IL-5 protein content within the lung as compared with HDM-exposed WT mice (Figures 5A and 5B). These results suggest that Glrx1 may be involved in inflammatory processes in small airways, and may regulate peripheral AHR.
The exact cells and/or tissue compartments wherein Glrx1/PSSG function to regulate allergic inflammation and AHR will require additional studies that incorporate in situ analysis of PSSG and cell-specific ablation of Glrx1. Similarly, identification of the proteins targeted via PSSG in HDM-induced lung disease and patients with asthma will also require additional analyses that incorporate Glrx1-selective cysteine derivatization and detection via mass spectrometry (34), which was beyond the scope of the present study. The identification of a number of proteins that are known targets for PSSG (4), coupled with the emerging biological role of protein PSSG in (patho)biological processes (18), offer the potential for the development of targeted protein-thiol–based therapeutics to alter the profile of allergic inflammation.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants R01 HL085464 and HL060014 (Y.M.W.J.-H.), and P30 GM103532.
Author Contributions: Conception and design—S.M.H., X.Q., and Y.M.W.J.-H.; execution and analysis of experiments—S.M.H., X.Q., D.G.C., S.B.C., K.G.L., R.S., J.L.A., M.J.R., M.A., N.D., S.A., Y.-S.H., and M.E.P.; interpretation of data—S.M.H., X.Q., J.D.N., D.H.M., J.T.J., D.J.T., L.K.A.L., V.A., C.G.I., E.F.M.W., N.L.R., A.E.D., A.v.d.V., M.E.P., and Y.M.W.J.-H.; drafting the manuscript—S.M.H., X.Q., and Y.M.W.J.-H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0401OC on April 1, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 2.Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. Oxidants positively or negatively regulate nuclear factor κB in a context-dependent manner. J Biol Chem. 2010;285:15746–15752. doi: 10.1074/jbc.M110.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadeem A, Masood A, Siddiqui N. Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008;2:215–235. doi: 10.1177/1753465808094971. [DOI] [PubMed] [Google Scholar]
- 4.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- 5.Kuipers I, Louis R, Manise M, Dentener MA, Irvin CG, Janssen-Heininger YM, Brightling CE, Wouters EF, Reynaert NL. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur Respir J. 2013;41:469–472. doi: 10.1183/09031936.00115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman SM, Tully JE, Lahue KG, Anathy V, Nolin JD, Guala AS, van der Velden JL, Ho Y-S, Aliyeva M, Daphtary N, et al. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2012;303:L528–L538. doi: 10.1152/ajplung.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory LG, Lloyd CM. Orchestrating house dust mite–associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman SM, Nolin JD, Tully JE, Lahue KG, Aliyeva M, Daphtary N, Lundblad LKA, Chapman DG, Ho Y-S, Irvin CG, et al. Genetic ablation of glutaredoxin-1 in a model of house dust mite-induced allergic asthma modulates airway neutrophilia, IL-17A and AHR [abstract] Am J Respir Crit Care Med. 2014;189:A2240. [Google Scholar]
- 9.Hoffman SM, Nolin JD, Tully JE, Lahue KG, Chapman DG, Aliyeva M, Daphtary N, Lundblad LKA, Abdalla S, Ather JL, et al. Ablation of the thiol transferase glutaredoxin-1 augments protein S-glutathionylation and modulates type 2 inflammatory responses and IL-17 in a house dust mite model of allergic airway disease in mice [abstract] Ann Am Thorac Soc. 2016;13:S97. doi: 10.1513/AnnalsATS.201510-656MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho Y-S, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riesenfeld E, Allen GB, Bates JH, Poynter ME, Wu M, Aimiand S, Lundblad LK. The temporal evolution of airways hyperresponsiveness and inflammation. J Allergy Ther. 2012;1:1–7. doi: 10.4172/2155-6121.S1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor-κB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 16.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman S, Nolin J, McMillan D, Wouters E, Janssen-Heininger Y, Reynaert N. Thiol redox chemistry: role of protein cysteine oxidation and altered redox homeostasis in allergic inflammation and asthma. J Cell Biochem. 2015;116:884–892. doi: 10.1002/jcb.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, et al. Reactive oxygen species–induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37:1037–1049. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aesif SW, Anathy V, Kuipers I, Guala AS, Reiss JN, Ho YS, Janssen-Heininger YM. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol. 2011;44:491–499. doi: 10.1165/rcmb.2009-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb DC, Cai Y, Matthaei KI, Foster PS. Comparative roles of IL-4, IL-13, and IL-4Rα in dendritic cell maturation and CD4+ Th2 cell function. J Immunol. 2007;178:219–227. doi: 10.4049/jimmunol.178.1.219. [DOI] [PubMed] [Google Scholar]
- 22.Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011;12:114. doi: 10.1186/1465-9921-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cose S, Brammer C, Khanna KM, Masopust D, Lefrançois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 24.Oh K, Park H-B, Byoun O-J, Shin D-M, Jeong EM, Kim YW, Kim YS, Melino G, Kim I-G, Lee D-S. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011;208:1707–1719. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 27.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, Ghosh S, Erzurum SC, Willard B, Hazen SL, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol (1985) 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 32.Tulic MK, Christodoulopoulos P, Hamid Q. Small airway inflammation in asthma. Respir Res. 2001;2:333–339. doi: 10.1186/rr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minshall EM, Hogg JC, Hamid QA. Cytokine mRNA expression in asthma is not restricted to the large airways. J Allergy Clin Immunol. 1998;101:386–390. doi: 10.1016/s0091-6749(98)70252-0. [DOI] [PubMed] [Google Scholar]
- 34.Guo J, Gaffrey MJ, Su D, Liu T, Camp DG, II, Smith RD, Qian W-J. Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat Protoc. 2014;9:64–75. doi: 10.1038/nprot.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.