Abstract
Respiratory viruses cause asthma exacerbations. Because eosinophils are the prominent leukocytes in the airways of 60–70% of patients with asthma, we evaluated the effects of eosinophils on a common respiratory virus, parainfluenza 1, in the lung. Eosinophils recruited to the airways of wild-type mice after ovalbumin sensitization and challenge significantly decreased parainfluenza virus RNA in the lungs 4 days after infection compared with nonsensitized animals. This antiviral effect was also seen in IL-5 transgenic mice with an abundance of airway eosinophils (NJ.1726) but was lost in transgenic eosinophil-deficient mice (PHIL) and in IL-5 transgenic mice crossed with eosinophil-deficient mice (NJ.1726-PHIL). Loss of the eosinophil granule protein eosinophil peroxidase, using eosinophil peroxidase–deficient transgenic mice, did not reduce eosinophils’ antiviral effect. Eosinophil antiviral mechanisms were also explored in vitro. Isolated human eosinophils significantly reduced parainfluenza virus titers. This effect did not involve degradation of viral RNA by eosinophil granule RNases. However, eosinophils treated with a nitric oxide synthase inhibitor lost their antiviral activity, suggesting eosinophils attenuate viral infectivity through production of nitric oxide. Consequently, eosinophil nitric oxide production was measured with an intracellular fluorescent probe. Eosinophils produced nitric oxide in response to virus and to a synthetic agonist of the virus-sensing innate immune receptor, Toll-like receptor (TLR) 7. IFNγ increased expression of eosinophil TLR7 and potentiated TLR7-induced nitric oxide production. These results suggest that eosinophils promote viral clearance in the lung and contribute to innate immune responses against respiratory virus infections in humans.
Keywords: eosinophil, parainfluenza virus, nitric oxide, asthma, Toll-like receptor 7
Clinical Relevance
Human and mouse eosinophils are antiviral against parainfluenza virus via the production of nitric oxide and by serving as a dead-end host for virus infection, but not through the production of eosinophil granule RNases or eosinophil peroxidase. Eosinophils may have an underappreciated antiviral role in respiratory tract infections in humans.
Eosinophils are found infiltrating the airways of approximately two-thirds of patients with asthma (1), and have been linked experimentally to airway hyperreactivity (2) and airway remodeling (3). Understandably, this has led to the assumption that eosinophils are solely maladaptive in asthma, and, therefore, many asthma treatments are designed to reduce eosinophilic inflammation (4–7). This strategy has produced variable results. Many patients with asthma remain poorly controlled despite maximal therapy (8). Exacerbations of asthma symptoms continue to cause significant morbidity, health care expenditures, and death (9, 10). Moreover, prevention and treatment for the most common cause of asthma exacerbations, respiratory virus infections, are virtually nonexistent (11). These deficiencies highlight our limited understanding of eosinophil biology, and have prompted studies attempting to define the role of eosinophils during respiratory virus infections in asthma. For example, IL-5 transgenic mice with elevated systemic eosinophils had lower titers of respiratory syncytial virus (RSV) compared with wild-type mice (12), whereas IL-5 transgenic mice with airway eosinophilia had lower titers of pneumonia virus in mice compared with controls (13). Similarly, we found that eosinophils recruited to the airways after ovalbumin sensitization were associated with less parainfluenza 1 virus in the lungs of guinea pigs, although those experiments were not designed to establish a causal role for eosinophils (14). Collectively, these studies suggest that eosinophils contribute to antiviral immunity in the lungs.
Eosinophils detect invading viruses via several mechanisms, including Toll-like receptor (TLR)–ligand interactions (15). TLR7 binds single-stranded RNA that is a common genomic motif for many respiratory viruses (16). TLR7 ligation triggers myeloid differentiation primary response 88 (MyD88) adaptor protein–dependent signaling that promotes an eosinophil antiviral response (12). However, the mediators responsible for eosinophils’ antiviral activity are under debate. Eosinophils release a variety of granule proteins after activation that may be antiviral. Eosinophil cationic protein and eosinophil-derived neurotoxin are members of the RNase A superfamily, and are proposed to have a virucidal effect by degrading viral RNA genomes (13, 17, 18). Eosinophil peroxidase is also released from eosinophil granules and contributes to the generation of antimicrobial reactive oxygen species (19). Whether eosinophils target all viruses with these proteins or by unique mechanisms requires further study.
Parainfluenza virus is one of several viruses known to cause asthma exacerbations (20). Parainfluenza is detected in the airways of up to 18% of adults during acute asthma exacerbations (21). Its prevalence is similar to influenza and coronavirus, and significantly greater than RSV in adults. Given that an estimated 16 million acute care visits for asthma exacerbations occur annually (22), parainfluenza-induced exacerbations represent a considerable burden of disease, yet the interaction between parainfluenza virus and eosinophils is not clearly defined.
In this study, we investigated the hypothesis that eosinophils inhibit parainfluenza virus infection in the lungs. The experiments described herein evaluated the effect of eosinophils on parainfluenza virus clearance in vivo and assessed the role of potential antiviral mediators. We also examined the effects of IFNγ on eosinophils’ antiviral responses as a potential mechanism for augmenting eosinophil-mediated antiviral activity.
Materials and Methods
Animals
Mice expressing IL-5 in airway epithelium (NJ.1726) (23), eosinophil-deficient mice (PHIL) (24), and eosinophil peroxidase–deficient mice (25) were maintained by backcrossing on a C57BL/6 background. Animals were handled in accordance with the U.S. Animal Welfare Act. Protocols were approved by Institutional Animal Care and Use Committees of Oregon Health & Science University (Portland, OR) and the Mayo Clinic (Scottsdale, AZ).
Virus Propagation
Parainfluenza virus (Sendai virus; ATCC, Manassas, VA) was grown in rhesus monkey kidney (RMK) cell monolayers, purified by sucrose density centrifugation, and titered in RMK cells (26) (see the online Materials and Methods). Titers were expressed as multiples of the 50% tissue culture infectious dose (TCID50; amount of virus required to infect 50% of RMK monolayers).
In Vivo Sensitization, Challenge, and Infection
Female mice aged 6–8 weeks were sensitized by intraperitoneal injection with 0.04 μg and 0.2 μg ovalbumin (Sigma, St. Louis, MO) with alum on Days 1 and 14, respectively. On Days 24, 26, and 28, mice were anesthetized with ketamine (45 mg/kg) and xylazine (8 mg/kg), and challenged with intratracheal 2% ovalbumin. Mice were infected with parainfluenza (2.8 × 104 TCID50 Units) intranasally on Day 27. On Day 31, lungs were lavaged and homogenized (see the online Materials and Methods).
Quantification of Viral RNA Content in Lung
Parainfluenza matrix protein RNA in lung homogenates was amplified by real-time RT-PCR and normalized to 18S. Relative RNA expression was converted to absolute RNA replicates using a parainfluenza RNA standard curve (see the online Materials and Methods).
Human Eosinophil Isolation
Eosinophils were isolated to greater than 99% purity and greater than 99% viability from blood of healthy human volunteers by density centrifugation using Ficoll-Paque Plus (Sigma) and negative selection (see the online Materials and Methods). The protocol was approved by the Institutional Review Board. Subjects provided written informed consent.
Effect of Eosinophils on Virus Infectivity
Human eosinophils were incubated overnight with or without IFNγ (300 U/ml). Medium was removed and parainfluenza (1 × 105 TCID50) was added for 2 hours. Some cultures were pretreated with nitric oxide synthase inhibitor, Nω-Nitro-L-arginine methyl ester hydrochloride ([l-NAME]; 100 μM; Sigma) 30 minutes before inoculation. Viral infectivity was quantified in RMK cells by hemadsorption assay. Infectious titers are expressed as TCID50 U/ml of culture supernatant.
Nitric Oxide Measurement
Human eosinophils were loaded with a nitric oxide–detecting fluorescent probe, (Strem, Newburyport, MA), and exposed to parainfluenza or synthetic TLR7 agonist, see the online Materials and Methods. Fluorescence was measured 60 minutes later by plate reader.
Effect of Eosinophils on Viral Replicates
Human eosinophils were inoculated with parainfluenza (1 × 105 TCID50) for 2 hours, washed to remove unbound virus, and maintained in fresh medium for 72 hours. Viral RNA in culture supernatants was quantified every 24 hours by real-time RT-PCR (see the online Materials and Methods).
TLR7 Expression
Eosinophil TLR7 expression was evaluated by real-time RT-PCR and immunofluorescence (see the online Materials and Methods).
Statistical Analyses
Data were compared using one-way ANOVA with Bonferroni’s post hoc test. Data are presented as mean (±SEM). A P value less than 0.05 was considered significant.
Results
Ovalbumin Sensitization and Challenge Augments Parainfluenza Virus Clearance in the Lungs of Wild-Type Mice
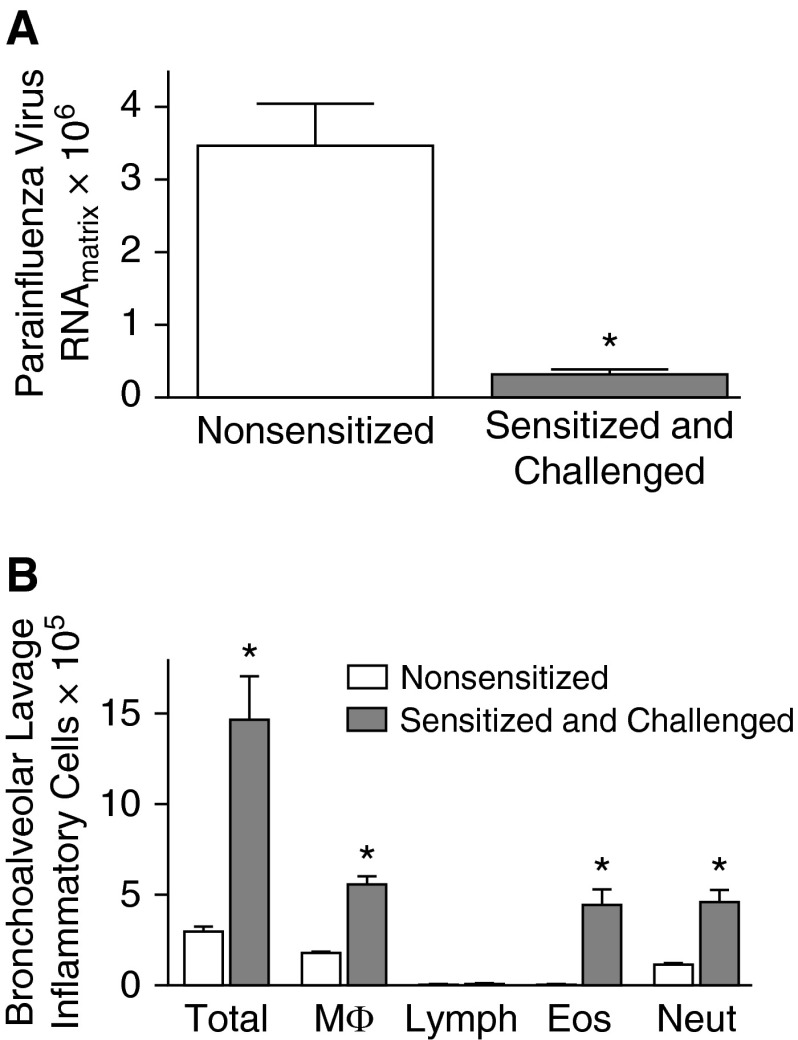
C57BL/6 mice, sensitized and challenged with ovalbumin, had significantly reduced (by ∼90%) parainfluenza virus RNA in the lungs 4 days after infection compared with nonsensitized animals (Figure 1A). Ovalbumin sensitization and challenge caused a substantial increase in eosinophils in bronchoalveolar lavage fluid, as well as macrophages and neutrophils (Figure 1B).
Figure 1.
Ovalbumin sensitization and challenge augments clearance of parainfluenza virus infection in the lungs in vivo. (A) C57BL/6 mice were sensitized (intraperitoneal, Days 1 and 14) and challenged (intratracheal, Days 24, 26, and 28) with ovalbumin, and infected with parainfluenza virus (intranasal, Day 27). Parainfluenza virus RNA was quantified by real-time RT-PCR 4 days after infection. Sensitized and challenged mice had significantly reduced parainfluenza RNA in the lung compared with nonsensitized, parainfluenza-infected control mice. (B) Ovalbumin sensitization and challenge increased total inflammatory cells, macrophages (MΦ), eosinophils (Eos), and neutrophils (Neut) in bronchoalveolar lavage fluid compared with nonsensitized controls. There were no differences in lymphocytes (Lymph) between groups (n = 5). *P < 0.05 compared with nonsensitized, parainfluenza-infected control mice. Data are presented as means (±SEM).
Eosinophils Promote Parainfluenza Virus Clearance in the Lung In Vivo
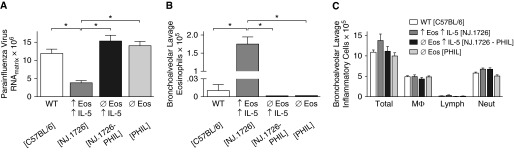
NJ.1726 mice (↑Eos ↑IL-5) constitutively express IL-5 in the pulmonary epithelium and have elevated numbers of eosinophils in the lungs (23). At 4 days after infection, parainfluenza RNA was significantly decreased in the lungs of NJ.1726 mice compared with wild-type littermate controls (Figure 2A). NJ.1726 IL-5 transgenic mice were crossed with PHIL mice (ØEos), which are congenitally devoid of eosinophils, to differentiate the effect of eosinophils from IL-5. Parainfluenza RNA content in the lungs of IL-5–expressing eosinophil-deficient NJ.1726-PHIL mice (ØEos ↑IL-5) was similar to that in infected wild-type mice, indicating that eosinophils, not IL-5, were mediating the antiviral effect. Similarly, eosinophil-deficient PHIL mice alone had parainfluenza RNA levels comparable to infected wild-type mice (Figure 2A). Bronchoalveolar lavage confirmed a significant increase in eosinophils in the airways from NJ.1726 mice compared with wild-type littermate controls, and an absence of eosinophils in PHIL and NJ.1726-PHIL mice (Figure 2B). There were no significant differences in the total leukocytes or other inflammatory cell subsets in bronchoalveolar lavage between groups (Figure 2C).
Figure 2.
Eosinophils promote clearance of parainfluenza virus in the murine lung in vivo. Wild-type (WT) control mice (C57BL/6), transgenic mice with high lung eosinophils due to overexpression of IL-5 by a lung-specific promoter (NJ.1726; ↑Eos ↑IL-5), transgenic mice with high lung IL-5 but devoid of eosinophils (NJ.1726-PHIL; ØEos ↑IL-5), and eosinophil-deficient mice (PHIL transgenic mice; ØEos) were infected with parainfluenza virus (intranasal). (A) Parainfluenza RNA content in the lung was quantified by real-time RT-PCR 4 days after infection. NJ.1726 mice (↑Eos ↑IL-5) had significantly reduced parainfluenza RNA compared with control mice (WT). NJ.1726-PHIL mice (ØEos ↑IL-5) and PHIL mice (ØEos) had parainfluenza RNA levels comparable to WT. (B) Bronchoalveolar lavage from parainfluenza-infected NJ.1726 mice (↑Eos ↑IL-5) contained significantly more eosinophils compared with all other groups. No eosinophils were detected in the bronchoalveolar lavage in PHIL (ØEos) or NJ.1726-PHIL mice (ØEos ↑IL-5). (C) There were no differences in other inflammatory cell types between groups (n = 7–10). *P < 0.05. Data are presented as means (±SEM).
Eosinophil Peroxidase Is Not Required for Eosinophils’ Antiviral Activity In Vivo
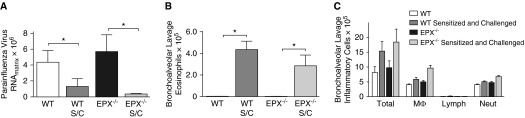
Eosinophil peroxidase is a granule protein released by eosinophils. Wild-type and transgenic eosinophil peroxidase–deficient mice were sensitized and challenged with ovalbumin, and infected with parainfluenza virus. Parainfluenza virus RNA was quantified from homogenized lung by real-time RT-PCR 4 days after infection. Ovalbumin sensitization and challenge was associated with significantly reduced parainfluenza RNA in both wild-type and eosinophil peroxidase–deficient mice compared with nonsensitized controls (Figure 3A), indicating that the eosinophil-mediated antiviral effect was not dependent on the release of eosinophil peroxidase. Sensitized and challenged wild-type and eosinophil peroxidase–deficient mice had significantly elevated airway eosinophils relative to nonsensitized wild-type and eosinophil peroxidase–deficient mice (Figures 3B and 3C).
Figure 3.
Eosinophil peroxidase is not required for eosinophils’ antiviral effect in vivo. (A) WT mice and eosinophil peroxidase knockout mice (EPX−/−) were sensitized and challenged (S/C) with ovalbumin and infected with parainfluenza virus. Viral RNA was quantified by real-time RT-PCR 4 days after infection. Sensitized and challenged WT (WT S/C) and EPX−/− mice (EPX−/− S/C) had significantly reduced parainfluenza RNA in the lung compared with nonsensitized, parainfluenza-infected control mice. (B) Ovalbumin sensitization and challenge increased eosinophils in the bronchoalveolar lavage fluid in WT and EPX−/− mice compared with nonsensitized controls. (C) There were no differences in other inflammatory cell types between groups (n = 4–5). *P < 0.05. Data are presented as means (±SEM).
Eosinophils Infected with Parainfluenza Produce Noninfectious Viral Progeny
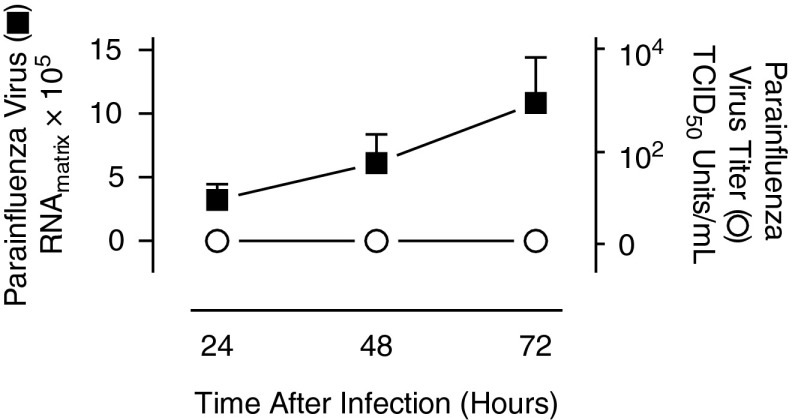
Isolated human eosinophils were inoculated with parainfluenza for 2 hours, then washed to remove unbound virus and cultured in fresh medium for 72 hours to evaluate whether parainfluenza infects eosinophils directly and, if so, whether eosinophils support production of viral progeny. Parainfluenza RNA, quantified by real-time RT-PCR from culture supernatants, progressively increased over 72 hours (Figure 4, left axis), indicating that the virus is capable of infecting eosinophils and producing viral transcripts. However, despite replication, the parainfluenza virus titers, as determined by hemadsorption assay, remained undetectable at these time points (Figure 4, right axis), indicating that eosinophils do not support production of infectious virus.
Figure 4.
Parainfluenza-infected eosinophils produce viral progeny that are not infectious. Human eosinophils were isolated from the peripheral blood and infected with parainfluenza virus for 2 hours, washed to remove unbound virus, and maintained in culture for 72 hours. Parainfluenza RNA from eosinophil supernatants was quantified by real-time RT-PCR every 24 hours (solid square, left axis). Concurrently, infectious parainfluenza virus titers were assessed by hemadsorption assay using rhesus monkey kidney cells and expressed as 50% tissue culture infectious dose (TCID50) U/ml (open circle, right axis) (n = 4). Data are presented as means (±SEM).
Eosinophil Granule RNases Do Not Mediate an Antiviral Effect
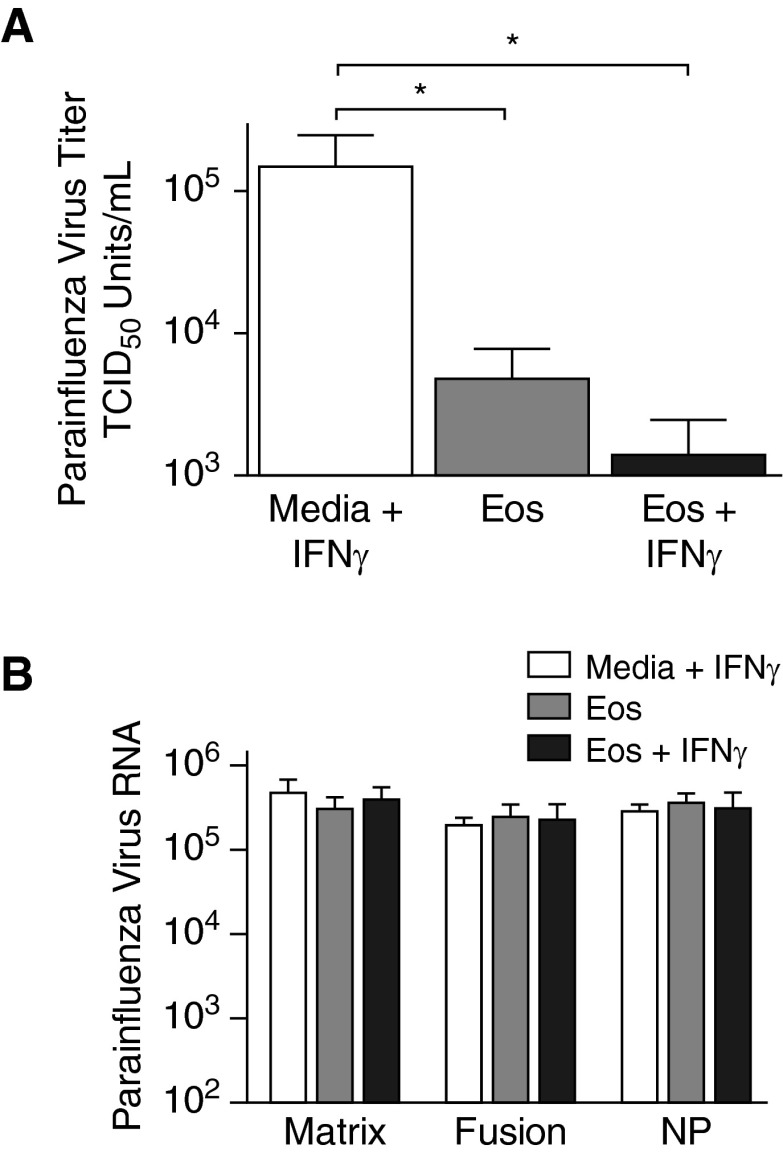
The levels of RNA encoding three parainfluenza virus structural proteins (i.e., matrix protein, fusion protein, and nucleoprotein) were compared 2 hours after inoculation between virus-infected eosinophil cultures and cultures with virus-inoculated media to determine if the eosinophil-mediated antiviral effect resulted from the release of eosinophil granule RNases. Concurrently, parainfluenza virus titers were assessed at this time point in RMK cells by hemadsorption assay. Eosinophils significantly decreased parainfluenza virus titers compared with virus-inoculated cultures without eosinophils, showing that eosinophils reduce parainfluenza virus infectivity (Figure 5A). A further reduction in virus titers was seen when eosinophils were pretreated with IFNγ. However, the quantities of RNA for all three parainfluenza virus structural proteins were similar between cultures containing virus-infected eosinophils and virus-inoculated cultures devoid of eosinophils (Figure 5B), demonstrating that reduced infectivity was not due to the degradation of the viral RNA genome by the eosinophil granule RNases.
Figure 5.
Eosinophils’ antiviral activity against parainfluenza does not involve granule RNases. Human eosinophils were isolated from peripheral blood and infected with parainfluenza virus. (A) Viral infectivity was assessed 2 hours after inoculation by hemadsorption assay and expressed as viral TCID50 U/ml. Parainfluenza infectivity was nearly undetectable in the supernatants of cultured eosinophils (Eos) and eosinophils pretreated with IFNγ (Eos + IFNγ) compared with parainfluenza virus cultured in media without eosinophils (Media + IFNγ). (B) RNA encoding parainfluenza virus structural proteins, matrix, fusion, and nucleoprotein (NP) was quantified in eosinophil supernatants by real-time RT-PCR 2 hours after inoculation. No differences were detected between any of the three genes, suggesting that the reduction in parainfluenza infectivity was not due to viral RNA degradation by eosinophil granule RNases (n = 4–7). *P < 0.05. Data are presented as means (±SEM).
Eosinophil-Derived Nitric Oxide Reduces Parainfluenza Virus Infectivity
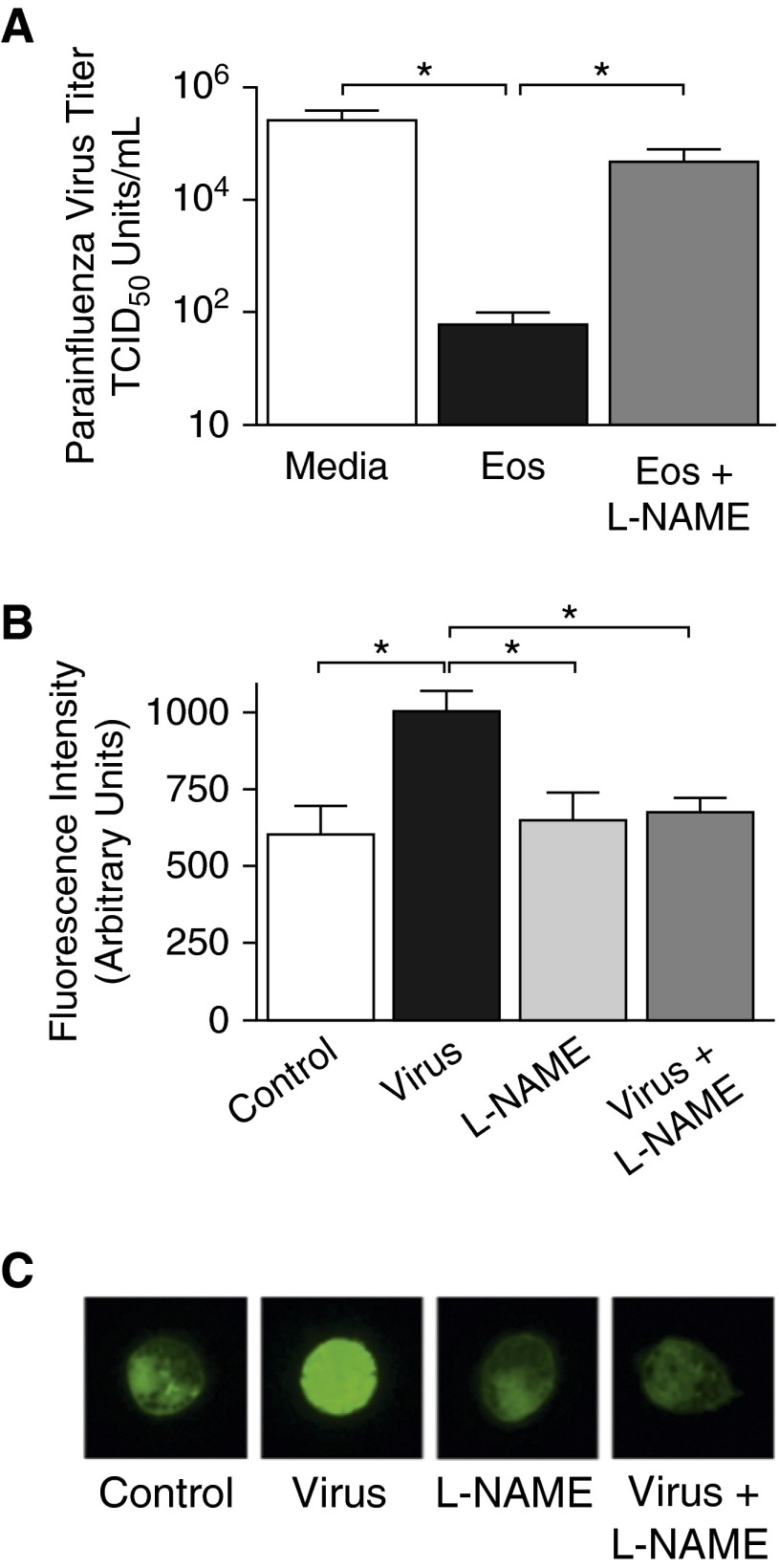
Parainfluenza virus was incubated in the presence of isolated human eosinophils for 2 hours and then viral infectivity was quantified by titering in RMK cells (via hemadsorption assay). As shown in Figure 6A, eosinophils significantly decreased parainfluenza virus titers compared with parainfluenza incubated in media without eosinophils. Eosinophils were cultured with the nitric oxide synthase inhibitor, l-NAME, to determine if the eosinophil-mediated antiviral effect was due to nitric oxide production. Eosinophils treated with l-NAME lost their ability to reduce virus titers, indicating that, upon exposure to virus, eosinophils attenuate viral infectivity through the production of nitric oxide (Figure 6A). The quantity of nitric oxide produced by eosinophils was evaluated with a copper-conjugated intracellular nitric oxide–detecting fluorescent probe 1 hour after addition of virus. Eosinophils exposed to parainfluenza virus had significantly increased fluorescence, indicating that eosinophils produce nitric oxide in response to parainfluenza infection. This increased fluorescence was blocked by l-NAME, confirming that nitric oxide was the mediator responsible (Figures 6B and 6C).
Figure 6.
Eosinophil-derived nitric oxide reduces parainfluenza virus infectivity. Human eosinophils isolated from peripheral blood were cultured overnight with IFNγ. (A) Eosinophils were treated with the nitric oxide synthase inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (l-NAME; 100 μM) or vehicle for 30 minutes, then infected with parainfluenza virus. Virus infectivity was quantified 2 hours after infection by hemadsorption assay and expressed as viral TCID50 U/ml. Parainfluenza virus infectivity was significantly decreased in the presence of eosinophils. Eosinophils treated with l-NAME lost their antiviral activity (n = 4). (B) Nitric oxide production was assessed using an intracellular nitric oxide–detecting fluorescent probe. Eosinophils loaded with the probe were treated with l-NAME or vehicle and infected with parainfluenza for 1 hour. Eosinophil fluorescence increased significantly in response to parainfluenza infection, indicating an increase in nitric oxide production by eosinophils. l-NAME treatment prevented parainfluenza-induced nitric oxide production by eosinophils (n = 4). (C) Representative images of eosinophils loaded with nitric oxide–detecting fluorophore. Magnification, ×100. *P < 0.05. Data are presented as means (±SEM).
IFNγ Increases Eosinophil TLR7 Expression and Nitric Oxide Production
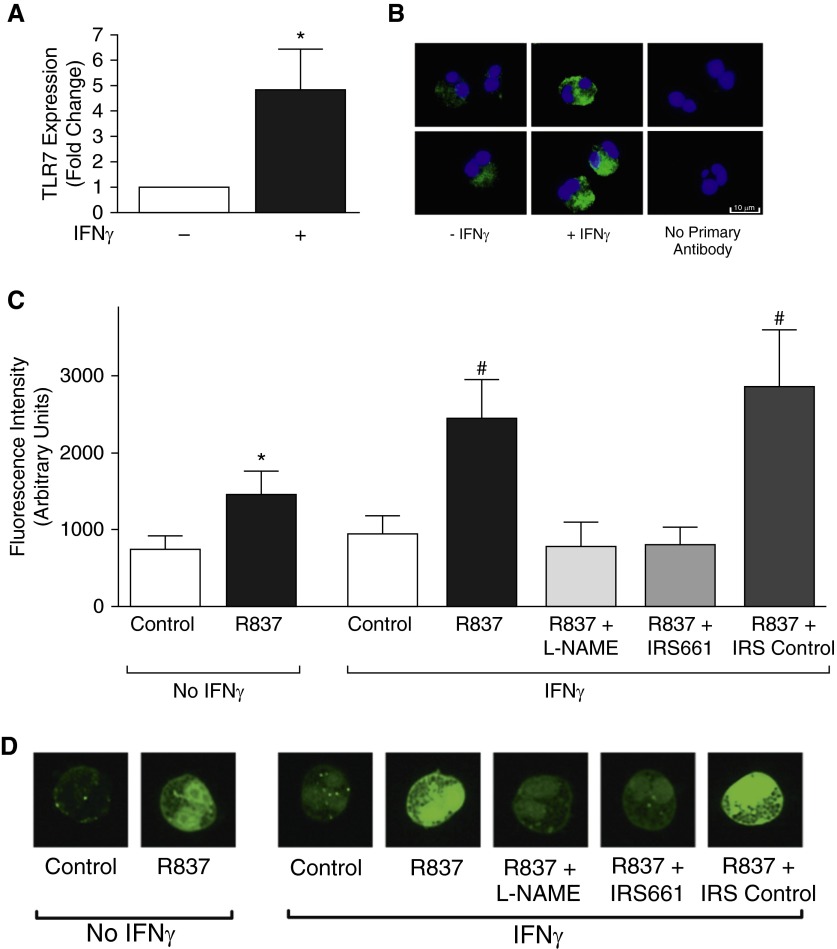
Eosinophils were cultured with or without IFNγ overnight. IFNγ increased eosinophil TLR7 expression relative to unstimulated eosinophils, both quantitatively by real-time RT-PCR (Figure 7A) and qualitatively by immunostaining (Figure 7B). Eosinophils’ response to TLR7 agonist was also evaluated with a nitric oxide–detecting fluorescent probe. Eosinophils treated for 1 hour with the synthetic TLR7 agonist, R837, had significantly increased fluorescence compared with vehicle-treated controls, indicating that TLR7 agonist induces nitric oxide production (Figures 7C and 7D). R837-mediated nitric oxide production was potentiated in eosinophils exposed to IFNγ. l-NAME and the oligonucleotide TLR7 antagonist, IRS661, but not a control oligonucleotide, blocked R837-induced nitric oxide production.
Figure 7.
IFNγ up-regulates Toll-like receptor 7 (TLR7) expression and potentiates TLR7-induced nitric oxide production in eosinophils. Human eosinophils were isolated from peripheral blood and grown in culture. (A) TLR7 expression was quantified by real-time RT-PCR from eosinophils treated with or without IFNγ. TLR7 RNA was significantly increased in eosinophils treated with IFNγ compared with untreated eosinophils (n = 5). (B) Eosinophils were immunostained with antibodies against TLR7 (green), and nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). TLR7 was expressed in unstimulated (−IFNγ) and IFNγ stimulated (+IFNγ) eosinophils. Images obtained with an LSM780 confocal microscope (numerical aperture 1.4; Zeiss, Thornwood, NY). (C) Eosinophils were loaded with an intracellular nitric oxide–detecting fluorescent probe and stimulated with synthetic TLR7 agonist R837. Eosinophil fluorescence increased in response to R837. IFNγ pretreatment potentiated the R837-induced increase in fluorescence. R837-induced fluorescence was blocked by l-NAME and by the oligonucleotide TLR7 antagonist IRS661, but not by a control oligonucleotide (IRS control) (n = 6). (D) Representative images of eosinophils loaded with nitric oxide–detecting fluorophore. Magnification, ×100. *P < 0.05 compared with no IFNγ control. #P < 0.05 compared with all other groups, except between IFNγ-treated R837 and R837 + IRS control. Data are presented as means (±SEM).
Discussion
Our results demonstrate a significant antiviral effect of eosinophils on parainfluenza virus infection in mouse airways in vivo and in isolated human eosinophils in vitro. Eosinophils appear to mediate their antiviral effects via both passive and proactive mechanisms. Specifically, our data show that eosinophils are a “dead-end” cellular host for parainfluenza, failing to propagate infectious viral progeny after infection. Thus, elevated eosinophil numbers in the lung may passively disrupt airway infection by acting as a cellular decoy and/or “sink,” interfering with the progressive expansion of viral numbers during infection. Our data also show that eosinophils proactively mediated antiviral activities through the production of nitric oxide and up-regulation of TLR7. In contrast, we did not find evidence that eosinophils directly attenuate parainfluenza through the release of eosinophil peroxidase or by degrading the viral RNA genome through the release of granule RNases.
These findings expand on prior eosinophil studies (12–14, 17, 18, 27) by establishing an antiviral role for eosinophils against parainfluenza virus, while suggesting that key differences exist in the eosinophils’ antiviral mechanisms against different viruses. Previous studies proposed that eosinophils inhibit RSV and pneumonia virus in mice, in part via the degradation of viral RNA genomes by eosinophils’ granule RNases, eosinophil cationic protein, and eosinophil-derived neurotoxin (13, 17, 18). However, we found that eosinophils did not alter the quantity of viral RNA transcripts for three key parainfluenza virus structural proteins (i.e., matrix, fusion, and nucleoprotein), despite causing a substantial reduction in parainfluenza infectivity, titered in RMK cells by hemadsorption assay. This suggests that eosinophils do not inhibit parainfluenza through RNase activity on the viral genome. Importantly, our methods differed from prior studies by quantifying both viral RNA and infectivity compared with only assessing viral infectivity after treating infected eosinophils with RNase inhibitors. Concurrent measurement of viral RNA levels and infectivity led to the additional finding that, although human eosinophils infected with parainfluenza produce viral RNA, these viral progeny are not infectious. This phenomenon, termed an “abortive” infection, has been described for many viruses, including RSV (28) and coronavirus (29) in macrophages, and influenza A in neutrophils (30), as well as for parainfluenza virus in Madin-Darby bovine kidney cells (31) and chick embryo fibroblasts (32). Evidence suggests that specific virus and host cell characteristics impact productive versus abortive outcomes during virus infections (33), but those characteristics require further investigation.
Eosinophils also inhibit parainfluenza through the production of nitric oxide. Previously, systemic suppression of nitric oxide production using an inhibitor of nitric oxide synthase delayed clearance of RSV in wild-type mice and in IL-5 transgenic animals with systemic eosinophilia in vivo (12, 34). Given the widespread expression of nitric oxide synthases, however, these studies were unable to distinguish the specific role of eosinophil-derived nitric oxide, which our study has elucidated. The ability of eosinophils to produce nitric oxide has been suspected for some time, given the presence of both constitutive and inducible nitric oxide synthase isoforms in eosinophils (35), but detecting nitric oxide has historically been challenging due to its short half-life and rapid diffusion across biologic membranes (36). These issues were overcome by using a copper-conjugated intracellularly retained fluorescent probe. We found that eosinophils generate nitric oxide in response to virus infection, and in response to stimulation with synthetic TLR7 agonist. IFNγ potentiated TLR7-mediated nitric oxide production in eosinophils, likely in part through the up-regulation of TLR7 expression. Thus, our results show that antiviral cytokine signaling augments eosinophils’ viral detection and their antiviral nitric oxide response.
Nitric oxide mediates antiviral effects via the production of a variety of reactive nitrogen products that inhibit viral proteases (37), and alter host cell function through nitrosylation of tyrosine and thiol groups (38). The consequences of these nitrogen species are diverse, ranging from inhibition of virus protein and RNA synthesis to potentiation of inflammation and injury to the respiratory tract (39). Nitric oxide also regulates many other physiologic processes, including eosinophil migration (40) and survival (41, 42), airway epithelial cell ciliary motility (43), and airway caliber through airway smooth muscle relaxation (44). Eosinophil-derived nitric oxide may affect multiple functions in the airway beyond what our experiments were designed to assess.
Eosinophils’ attenuation of parainfluenza virus infection was not dependent on the release of eosinophil peroxidase. Eosinophil peroxidase is a granule protein that, together with the respiratory burst from activated eosinophils, generates potent reactive oxygen species that have been suggested to contribute to host immune defense (45). For example, eosinophil peroxidase has been shown to have virucidal effects against human immunodeficiency virus in vitro (46). However, the role of eosinophil peroxidase in vivo as a host defense mechanism remains unclear. Eosinophil peroxidase deficiency had no effect in an experimental model of helminthic parasite infections in mice (47), and its deficiency has also been detected in humans without manifesting an apparent phenotype (48). Our data further suggest that eosinophil peroxidase has no role in eosinophils’ antiviral activity against parainfluenza.
Importantly, our experiments differentiate the effects of eosinophils’ versus IL-5. Although NJ.1726 IL-5 transgenic mice (↑Eos ↑IL-5) with an abundance of airway eosinophils had reduced parainfluenza virus in the lung, NJ.1726-PHIL mice (ØEos ↑IL-5) with elevated IL-5 in the absence of eosinophils did not. This is pertinent given the presence of IL-5 receptors on leukocytes other than eosinophils, including macrophages and neutrophils (49), which could have contributed to the antiviral effect in vivo. Furthermore, our data demonstrate that, although an induced airway eosinophilia promoted viral clearance, clearance of virus was similar for eosinophil-deficient mice relative to wild-type control animals. This suggests that the presence of a specific airway eosinophilia mediates one or more unique antiviral mechanisms not occurring at homeostatic baseline, and underlines the need for better characterization of asthma phenotypes when evaluating respiratory virus infections in humans.
Human studies have historically been confounded by the heterogeneity of asthma phenotypes (50), the need for invasive bronchoscopic testing, the immune effects of corticosteroid treatment, differences in virus detection methods (i.e., PCR, ELISA, culture) and the variety of respiratory viruses that cause asthma exacerbations (20). Recent trials that studied exacerbation rates in well characterized steroid-resistant eosinophilic subjects with asthma treated with mepolizumab, a monoclonal antibody against IL-5, found no difference in the incidence of respiratory tract infections (4, 5). However, airway infections were rare events, and were diagnosed clinically without objective evidence of the presence of an infecting virus. Furthermore, these trials did not quantify airway and lung parenchymal eosinophils, nor did they characterize the eosinophils’ activation state after treatment. Nonetheless, future studies that define the interaction between eosinophils and viruses may lead to novel treatment targets for eosinophilic asthma and for respiratory virus infections in humans.
The results of our study paradoxically suggest that eosinophils have beneficial antiviral effects during respiratory virus infections despite considerable evidence linking viruses to asthma exacerbations (20, 21). However, we cannot conclude that eosinophils’ antiviral effects result in fewer exacerbations. On the contrary, it is possible, and perhaps likely, that eosinophils’ ability to detect and respond to viruses promotes an exuberant, and ultimately detrimental, airway inflammatory response in subjects with asthma. Although our study did not specifically address airway physiology, Adamko and colleagues (14) found that eosinophils mediate virus-induced airway hyperreactivity in sensitized guinea pigs, and were associated with lower viral titers, yet lower viral titers did not improve airway hyperreactivity. Thus, the negative effects of activated eosinophils’ in the airway may mask any positive impact from fewer viral transcripts. Consequently, as therapies become progressively more targeted against pulmonary eosinophils, there will be an even greater need for understanding the complexity of eosinophil biology in the lungs.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health (HL124165, HL113023, AR61567, HL121254, HL71795).
Author Contributions: All authors contributed to the design of the study, analysis of the data, drafting of the manuscript, and have approved the final draft.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0405OC on April 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J. 2009;3:198–206. doi: 10.1111/j.1752-699X.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 2.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol (1985) 1991;71:2255–2261. doi: 10.1152/jappl.1991.71.6.2255. [DOI] [PubMed] [Google Scholar]
- 3.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681–686. doi: 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 5.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 6.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 7.Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46:622–639. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, Suruki RY, Kawatkar AA. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4:120–129.e3. doi: 10.1016/j.jaip.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 9.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braman SS. The global burden of asthma. Chest. 2006;130(1) Suppl:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 11.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 13.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, M2 muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 16.Diebold SS. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008;60:813–823. doi: 10.1016/j.addr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil–derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 18.Domachowske JB, Dyer KD, Adams AG, Leto TL, Rosenberg HF. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–3363. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, et al. Viruses and bacteria in acute asthma exacerbations—a GA² LEN-DARE systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Asthma Faststats. 2015 [updated 2016 Feb 10; accessed 2016 Feb 14]. Available from: www.cdc.gov/nchs/fastats/asthma.htm.
- 23.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 25.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 26.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 27.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franke-Ullmann G, Pförtner C, Walter P, Steinmüller C, Lohmann-Matthes ML, Kobzik L, Freihorst J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–280. [PubMed] [Google Scholar]
- 29.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassidy LF, Lyles DS, Abramson JS. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. J Clin Microbiol. 1988;26:1267–1270. doi: 10.1128/jcm.26.7.1267-1270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao T, Ryan KW. Host range restriction of parainfluenza virus growth occurs at the level of virus genome replication. Virology. 1996;220:69–77. doi: 10.1006/viro.1996.0287. [DOI] [PubMed] [Google Scholar]
- 32.Blair CD, Brennan PJ. Effect of Sendai virus infection on lipid metabolism in chick embryo fibroblasts. J Virol. 1972;9:813–822. doi: 10.1128/jvi.9.5.813-822.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, Mason RJ, Chan MC. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85:6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark JM, Khan AM, Chiappetta CL, Xue H, Alcorn JL, Colasurdo GN. Immune and functional role of nitric oxide in a mouse model of respiratory syncytial virus infection. J Infect Dis. 2005;191:387–395. doi: 10.1086/427241. [DOI] [PubMed] [Google Scholar]
- 35.Zanardo RC, Costa E, Ferreira HH, Antunes E, Martins AR, Murad F, De Nucci G. Pharmacological and immunohistochemical evidence for a functional nitric oxide synthase system in rat peritoneal eosinophils. Proc Natl Acad Sci USA. 1997;94:14111–14114. doi: 10.1073/pnas.94.25.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saura M, Zaragoza C, McMillan A, Quick RA, Hohenadl C, Lowenstein JM, Lowenstein CJ. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide–mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Zheng S, Dweik RA, Erzurum SC. Role of epithelial nitric oxide in airway viral infection. Free Radic Biol Med. 2006;41:19–28. doi: 10.1016/j.freeradbiomed.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomazzi SM, Ferreira HH, Conran N, De Nucci G, Antunes E. Role of nitric oxide on in vitro human eosinophil migration. Biochem Pharmacol. 2001;62:1417–1421. doi: 10.1016/s0006-2952(01)00782-1. [DOI] [PubMed] [Google Scholar]
- 41.Beauvais F, Michel L, Dubertret L. The nitric oxide donors, azide and hydroxylamine, inhibit the programmed cell death of cytokine-deprived human eosinophils. FEBS Lett. 1995;361:229–232. doi: 10.1016/0014-5793(95)00188-f. [DOI] [PubMed] [Google Scholar]
- 42.Beauvais F, Joly F. Effects of nitric oxide on the eosinophil survival in vitro: a role for nitrosyl-heme. FEBS Lett. 1999;443:37–40. doi: 10.1016/s0014-5793(98)01673-1. [DOI] [PubMed] [Google Scholar]
- 43.Jiao J, Wang H, Lou W, Jin S, Fan E, Li Y, Han D, Zhang L. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp Cell Res. 2011;317:2548–2553. doi: 10.1016/j.yexcr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Belvisi MG, Stretton D, Barnes PJ. Nitric oxide as an endogenous modulator of cholinergic neurotransmission in guinea-pig airways. Eur J Pharmacol. 1991;198:219–221. doi: 10.1016/0014-2999(91)90626-2. [DOI] [PubMed] [Google Scholar]
- 45.Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klebanoff SJ, Coombs RW. Virucidal effect of stimulated eosinophils on human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1996;12:25–29. doi: 10.1089/aid.1996.12.25. [DOI] [PubMed] [Google Scholar]
- 47.Ramalingam T, Porte P, Lee J, Rajan TV. Eosinophils, but not eosinophil peroxidase or major basic protein, are important for host protection in experimental Brugia pahangi infection. Infect Immun. 2005;73:8442–8443. doi: 10.1128/IAI.73.12.8442-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zabucchi G, Soranzo MR, Menegazzi R, Vecchio M, Knowles A, Piccinini C, Spessotto P, Patriarca P. Eosinophil peroxidase deficiency: morphological and immunocytochemical studies of the eosinophil-specific granules. Blood. 1992;80:2903–2910. [PubMed] [Google Scholar]
- 49.Linch SN, Danielson ET, Kelly AM, Tamakawa RA, Lee JJ, Gold JA. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am J Respir Crit Care Med. 2012;186:246–254. doi: 10.1164/rccm.201201-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26:710–715. doi: 10.1016/j.pupt.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.