Abstract
Active ion transport by basolateral Na-K-ATPase (Na pump) creates an Na+ gradient that drives fluid absorption across lung alveolar epithelium. The α1 and β1 subunits are the most highly expressed Na pump subunits in alveolar epithelial cells (AEC). The specific contribution of the β1 subunit and the relative contributions of alveolar epithelial type II (AT2) versus type I (AT1) cells to alveolar fluid clearance (AFC) were investigated using two cell type–specific mouse knockout lines in which the β1 subunit was knocked out in either AT1 cells or both AT1 and AT2 cells. AFC was markedly decreased in both knockout lines, revealing, we believe for the first time, that AT1 cells play a major role in AFC and providing insights into AEC-specific roles in alveolar homeostasis. AEC monolayers derived from knockout mice demonstrated decreased short-circuit current and active Na+ absorption, consistent with in vivo observations. Neither hyperoxia nor ventilator-induced lung injury increased wet-to-dry lung weight ratios in knockout lungs relative to control lungs. Knockout mice showed increases in Na pump β3 subunit expression and β2-adrenergic receptor expression. These results demonstrate a crucial role for the Na pump β1 subunit in alveolar ion and fluid transport and indicate that both AT1 and AT2 cells make major contributions to these processes and to AFC. Furthermore, they support the feasibility of a general approach to altering alveolar epithelial function in a cell-specific manner that allows direct insights into AT1 versus AT2 cell–specific roles in the lung.
Keywords: lung, ion transport, Na-K-ATPase, β1 subunit, β2-adrenergic receptor
Clinical Relevance
This study demonstrates an important role for the Na-K-ATPase β1 subunit in ion transport and alveolar fluid clearance and that AT1 cells contribute a major portion of alveolar fluid clearance in the lung. Compensatory mechanisms involving increased β3 subunit levels as well as β2-adrenergic receptor protein expression can be activated in response to β1 subunit deletion.
Lung alveolar epithelium, composed of alveolar epithelial type I (AT1) and type II (AT2) cells, forms a tight barrier that limits leakage of solutes and water from the interstitial and vascular compartments into alveolar air spaces. Regulation of alveolar fluid homeostasis is based on active ion transport across the alveolar epithelium (1). Polarized localization and function of the basolateral Na-K-ATPase (Na pump) and apical sodium channels (2) are of crucial importance in transepithelial ion transport and the accompanying fluid clearance across the alveolar epithelial barrier (3, 4).
AT2 cells, despite covering only ∼2.5% of the alveolar surface (5), have been assumed to contribute the major portion of transport activity in alveolar epithelium, on the basis of high channel and pump density in this cell type (6). However, AT1 cells express both epithelial sodium channel (ENaC) and Na-K-ATPase subunit proteins (7–9) and, in addition to covering a large surface area, have very high water permeability (10). It is therefore likely that AT1 cells make an important contribution to alveolar fluid clearance (AFC) (11, 12), although the relative contributions of AT1 and AT2 cells to ion transport and AFC in the lung are entirely unknown.
The Na pump catalyzes active transport of cytoplasmic Na+ in exchange for extracellular K+ at the basolateral cell surface (13, 14). Na-K-ATPase is a heterotrimer composed of one α, one β, and one γ subunit. The α subunit harbors the catalytic function of the Na pump, whereas the β subunit is important in the maturation of the structure and the function of the holoenzyme and for its transport and localization to the cell membrane (15). The function of the γ subunit is less clear, but experiments have demonstrated a modulatory role of Na pump activity and ion affinity that is tissue specific, on the basis of the differential expression of various γ subunits (FXYD proteins) in different tissues (16). The β1 subunit is highly expressed in both AT1 and AT2 cells (17), and Na pumps composed of α1 and β1 subunits are thought to be the predominant isozyme expressed in both cell types (18, 19), although expression of the α2 subunit in lung and AT1 cells has been reported (12). A number of in vivo studies based on Na pump β1 subunit overexpression in rodent lungs provide evidence to support an important role of this subunit in Na pump function and AFC, both at baseline and during lung injury (19–24). Expression of the β3 subunit has been demonstrated in rat lung (25), but the role of this subunit in the lung is largely unknown. Clinically, in patients with acute lung injury/acute respiratory distress syndrome, the capacity for higher levels of AFC is associated with better outcomes (26, 27).
The primary goals of this study were to elucidate the roles of Na pump β1 subunit in active transepithelial ion transport and AFC and to determine the relative contributions of AT1 versus AT2 cells to these processes. We generated two mouse lines with conditional knockout of the β1 subunit in either AT1 cells or in both AT1 and AT2 cells in mouse lung. This approach allowed us to directly assess the contribution of the β1 subunit in vivo and, we believe for the first time, to investigate specifically the roles of the two different types of alveolar epithelial cells (AEC) in alveolar function. Some of the results of these studies have been reported previously in abstract form (28–30).
Materials and Methods
Generation of Knockout Mice
Mice with a floxed allele of the Na pump β1 subunit gene (Atp1b1F/F) were generated (see online supplement). Briefly, Atp1b1F/F mice were crossed to Aqp5-cre mice (31) to generate Atp1b1Aqp5-cre mice deficient in the β1 subunit in AT1 cells, and to Sftpc-cre mice (32) to generate Atp1b1Sftpc-cre mice deficient in the β1 subunit in the entire alveolar epithelium (i.e., both AT1 and AT2 cells). All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Alveolar Fluid Clearance
Phosphate-buffered saline with 5% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) and 0.25 milligrams per milliliter BSA-Alexa Fluor 594 conjugate (Life Technologies, Carlsbad, CA) as tracer were instilled intratracheally into anesthetized mice. Alveolar fluid was aspirated after 30 minutes. AFC was calculated from fluorescence measured in instillate and aspirate.
In Vivo Lung Permeability
Permeability in vivo was calculated after jugular vein injection of 10 milligrams of fluorescein-BSA (Life Technologies) per kilogram of body weight from fluorescence measured 2 hours later in bronchoalveolar lavage fluid and serum.
Wet-to-Dry Lung Weight Ratios
Lungs were removed surgically, weighed, and then dried at 65°C for 48 hours. Dry weight was recorded and wet-to-dry lung weight ratios were calculated.
Isolation of AT2 Cells and Primary Culture of Mouse Alveolar Epithelial Cell Monolayers
AT2 cells were isolated from mouse lungs, and mouse alveolar epithelial cell monolayers (MAECM) were cultured as described previously (33).
Bioelectric Properties of MAECM
Transepithelial electrical resistance (RT) (kΩ·cm2) and spontaneous potential difference (PD) (mV) of MAECM were measured using a Millicell-ERS device (Millipore, Bedford, MA) on Day 6 after AT2 cells had transdifferentiated to an AT1 cell–like phenotype. Equivalent short-circuit current (IEQ) (μA/cm2) was calculated as PD/RT. PD and short-circuit current (ISC) (μA/cm2) were measured in modified Ussing chambers in the presence or absence of terbutaline, amiloride, and pimozide.
Unidirectional Flux of Na+
Unidirectional flux of Na+ was measured across MAECM at 37°C using 22NaCl (American Radiolabeled Chemicals, St. Louis, MO).
Western Analysis
Details regarding methods and antibodies used are provided in the online supplement.
Lung Histology
Standard methods as described in online supplement were used.
Antibody Staining
Staining methods and antibody information are described in the online supplement.
RNA Isolation, Reverse Transcription and Quantitative Polymerase Chain Reaction
Details regarding methods are provided in the online supplement. Primer sequences are listed in Table 1.
Table 1.
Quantitative Reverse Transcriptase–Polymerase Chain Reaction Primers
| Gene | GenBank Accession No. | Primer Sequence (5′ → 3′) | Product Size (bp) |
|---|---|---|---|
| Atp1a1 (Na+,K+-ATPase α1) | NM_144900 | TCAAGTCTTGGAGCTCGGAACT (FP) ACGTCTGCATCCCCACATG (RP) | 66 |
| Atp1a2 (Na+,K+-ATPase α2) | NM_178405 | TGAGCTGGGCCGAAAATACC (FP) GGGTCCATCTCTAGCCAGAAT (RP) | 85 |
| Atp1a3 (Na+,K+-ATPase α3) | BC034645 | TCTGCCCTGCTTAAGTGCATT (FP) TCCGTTCTCGCATCAGCTT (RP) | 61 |
| Atp1a4 (Na+,K+-ATPase α4) | NM_013734 | TCAGGAGTCTGTTCCCATAGCTAA (FP) GGAGAGCTGACTCGGAAGCA (RP) | 62 |
| Atp1b1 (Na+,K+-ATPase β1) | NM_009721 | TTCATCGGGACCATCCAAGT (FP) TCCTGGTATGTGGGCTTCAGT (RP) | 62 |
| Atp1b2 (Na+,K+-ATPase β2) | NM_013415 | TGCCCACACAATTTCCAACAT (FP) ACTCCCGACTCCTCTCTGTCTCT (RP) | 64 |
| Atp1b3 (Na+,K+-ATPase β3) | NM_007502 | GCCGAGTGGAAGCTGTTCAT (FP) GGTGCGCCCCAGAAACT (RP) | 56 |
| Atp1b4 (Na+,K+-ATPase β4) | NM_133690 | TGAAAATGACATTCGATCCATCA (FP) TAAGGGTAGTAGCGGAGGTCAAA (RP) | 69 |
| Polr2a | NM_009089 | GGCAAGGTCCCACAACCA (FP) ACAATTGATGTGTCCAGGTATGATG (RP) | 93 |
Definition of abbreviations: bp, base pair; FP, forward primer; RP, reverse primer.
Hyperoxia Exposure
Mice were housed in cages inside a hyperoxia chamber with 95% oxygen for 65 hours.
Ventilator-Induced Lung Injury
Anesthetized mice were ventilated with an Inspira ASV ventilator (Harvard Apparatus, Holliston, MA) under either noninjurious or injurious ventilation conditions (see online supplement).
Data Analysis
Data are shown as mean ± SEM. Unpaired Student’s t test was used for comparisons of two group means. Multiple (three or more) group means were analyzed by one-way analysis of variance with post hoc tests based on Student-Newman-Keuls approaches. P < 0.05 is considered statistically significant.
Results
Atp1b1 Gene Targeting and Generation/Verification of β1 Knockout Mice
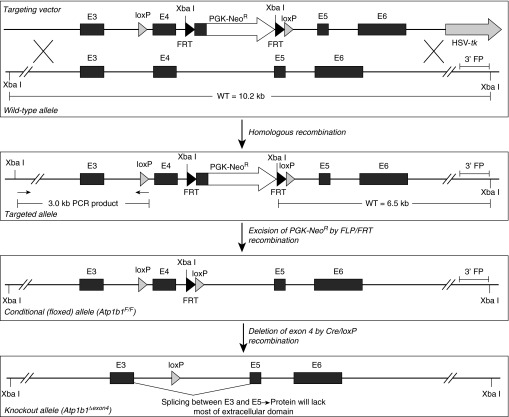
We generated a mouse line with a conditional (floxed) allele of the Atp1b1 gene (encoding the Na-K-ATPase β1 subunit) to enable cell-specific gene knockout by Cre/loxP recombination (Figure 1). Mouse Atp1b1 contains six exons; exon 1 codes for the cytoplasmic domain of the Na-K-ATPase β1 subunit protein, and the first part of exon 2 codes for the transmembrane domain. The remainder of the gene codes for the large extracellular domain that makes up 243 of the 305 amino acids in this protein. We flanked exon 4 of Atp1b1 with loxP sites to create a conditional allele of this gene (Atp1b1F/F) (Figure 1). Cre/loxP-mediated deletion of exon 4 results in a frame-shift mutation after splicing of exon 3 to exon 5. To elucidate the importance of the β1 subunit in adult mouse lung fluid homeostasis and its relative importance in AT1 versus AT2 cells, we generated two separate lines, one harboring a conditional β1 knockout specifically in AT1 cells using Aqp5-cre mice (31) and one in which β1 was inactivated in both AT1 and AT2 cells (alveolar epithelium-specific knockout) using Sftpc-cre mice (32). These two knockout lines are referred to as Atp1b1Aqp5-cre and Atp1b1Sftpc-cre, respectively. Knockout lines were analyzed to verify deletion of the Na pump β1 subunit gene (see online supplement). Genomic PCR (see Figure E1A in the online supplement) confirmed that the floxed Atp1b1 allele could be deleted correctly by Cre/loxP recombination. Western analysis (Figure E1B) confirmed that Na pump β1 protein was absent in AT2 cells isolated from Atp1b1Sftpc-cre knockout mice. We confirmed (Figure E1C) that Aqp5-cre activates green fluorescent protein (GFP) expression from a ROSAmT/mG reporter transgene specifically in AT1 cells in the alveolar epithelium and that efficiency of this Cre/loxP-mediated reporter activation is very high (>90% of AT1 cells) (31). Finally, we confirmed that Sftpc-cre activates GFP expression in the ROSAmT/mG reporter transgene in both AT1 and AT2 cells, although the GFP signal is considerably stronger in AT2 cells, in part reflecting the very thin AT1 cell architecture in vivo (Figure E1C).
Figure 1.
Gene targeting strategy. An Atp1b1 gene targeting vector was made by floxing exon 4, introducing a PGK-NeoR positive selection marker upstream of the 3′ loxP site, and placing an HSV-tk negative selection marker in a 3′-flanking position in the genomic fragment. The Atp1b1 gene targeting vector was introduced into W2 embryonic stem (ES) cells, and clones that had undergone homologous recombination were identified by Southern blot using a flanking probe (3′ FP) binding a 6.5-kb Xba I fragment from the targeted allele and a 10.2-kb fragment from the wild-type allele. Positive clones were then verified by PCR, with primers amplifying a 3.0-kb portion on the 5′ side of the targeted allele. After karyotyping of positive ES cell clones, chimeric mice were produced. Germline-transmitting chimeras were bred to FLPeR mice to remove the FRT-flanked PGK-NeoR selection marker, generating mice harboring the final floxed Atp1b1 allele (Atp1b1F/F). Cre/loxP-mediated deletion of exon 4 generates a deleted allele (Atp1b1Δexon4) with an out-of-frame mutation that disrupts the coding sequence in exons 5 and 6 (E5 and E6). FRT, flippase recognition target; PGK, phosphoglycerate kinase promoter; WT, wild type.
AFC Is Markedly Reduced in Mice Deficient in the Na Pump β1 Subunit
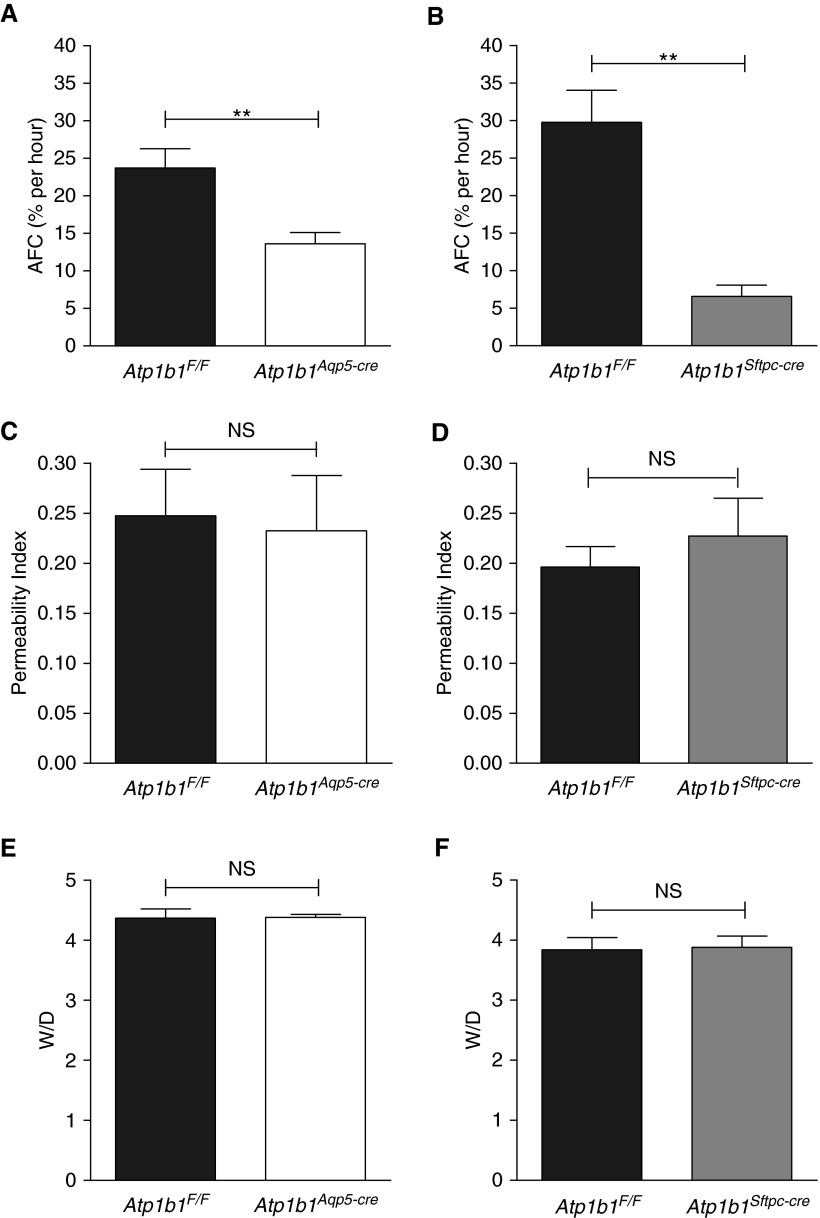
We measured AFC in the two β1 subunit knockout lines (Atp1b1Aqp5-cre and Atp1b1Sftpc-cre) and compared them to respective floxed litter mate control mice (Atp1b1F/F). AFC in Atp1b1Aqp5-cre knockout mice, deficient in the β1 subunit specifically in AT1 cells, demonstrated a highly significant (P = 0.006) reduction (43%) in AFC compared with Atp1b1F/F mice (Figure 2A). Atp1b1Sftpc-cre knockout mice, which lack the β1 subunit in both AT1 and AT2 cells, revealed even lower AFC, which was significantly (P = 0.002) reduced by 78% compared with control mice (Figure 2B). These data demonstrate that the Na pump β1 subunit is of crucial importance in AFC after fluid instillation and that both AT1 and AT2 cells make major contributions to AFC in the adult mouse lung. The portion of AFC attributable to AT1 and AT2 cells can be estimated as ∼55% (i.e., 43/78 = 0.55) and ∼45% (i.e., [78–43]/78 = 0.45), respectively, assuming that AT1/AT2 cell number ratios are similar in control and knockout mice on the basis of histological examination of lungs from floxed control mice and both knockout lines that showed no obvious morphologic differences (Figure E2). AFC in Atp1b1Aqp5-cre and Atp1b1Sftpc-cre knockout mice (57% and 22%, respectively, relative to floxed control mice) are likely based on active sodium transport because of residual Na pump activity.
Figure 2.
Alveolar fluid clearance (AFC), lung permeability, and wet-to-dry lung weight ratio (W/D) in β1 knockout mice. (A) AFC (% per h) in Atp1b1Aqp5-cre mice (13.6 ± 1.5) (n = 7) was significantly reduced compared with Atp1b1F/F mice (23.7 ± 2.6) (n = 7). (B) AFC (% per h) in Atp1b1Sftpc-cre mice (6.6 ± 1.5) (n = 4) was significantly reduced compared with Atp1b1F/F mice (29.8 ± 4.2) (n = 4). (C and D) There was no significant difference in in vivo permeability to fluorescein–bovine serum albumin between Atp1b1F/F (0.248 ± 0.047) (n = 6) and Atp1b1Aqp5-cre mice (0.232 ± 0.055) (n = 7) (C) or between Atp1b1F/F (0.196 ± 0.020) (n = 6) and Atp1b1Sftpc-cre mice (0.227 ± 0.038) (n = 6) (D). (E and F) At baseline, W/D of Atp1b1F/F (4.37 ± 0.15) (n = 8) versus Atp1b1Aqp5-cre (4.38 ± 0.05) (n = 12) mice (E) and of Atp1b1F/F (3.84 ± 0.08) (n = 6) versus Atp1b1Sftpc-cre (3.88 ± 0.08) (n = 6) mice (F) were not significantly different. **P < 0.01. NS, not significantly different from each other (P > 0.05).
Unchanged Lung Permeability and Absence of Lung Edema in β1 Knockout Mice
To investigate if β1 knockout lungs have altered paracellular permeability that might contribute to reductions in AFC, we injected fluorescein-BSA into the jugular vein of floxed and knockout mice. As shown in Figures 2C and 2D, lung permeability to fluorescein-BSA was not different in β1 knockout mice compared with floxed control mice. Consistent with these findings, lungs of both Atp1b1Aqp5-cre and Atp1b1Sftpc-cre mice had wet-to-dry lung weight ratios that were not different from their respective floxed control mice (Figures 2E and 2F), suggesting that, in both β1 subunit knockout lines, residual Na pump activity is sufficient to maintain lung fluid homeostasis.
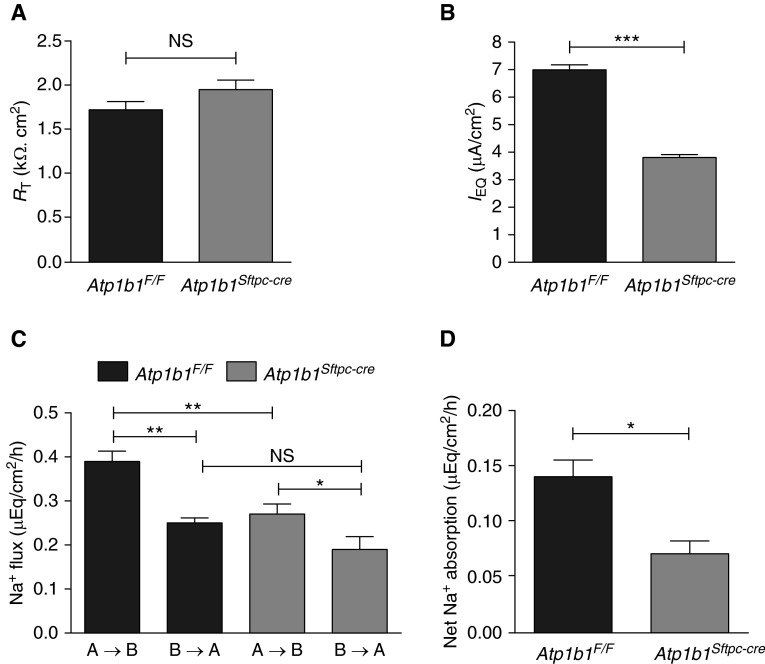
Decreased IEQ and Unchanged RT Across MAECM Deficient in β1 Subunit
Bioelectric properties (RT and IEQ) were evaluated in mouse alveolar epithelial cell monolayers (MAECM) prepared by cultivation of freshly isolated AT2 cells derived from Atp1b1F/F control and Atp1b1Sftpc-cre knockout mice. As shown in Figure 3A, RT of Atp1b1F/F control and Atp1b1Sftpc-cre knockout monolayers on Day 6 were not significantly different. In contrast, IEQ of Atp1b1Sftpc-cre knockout monolayers were significantly reduced compared with Atp1b1F/F control monolayers, consistent with results in vivo (Figure 3B) and supporting the observation that the Na pump β1 subunit plays a crucial role in active ion transport across MAECM.
Figure 3.
Bioelectric properties and Na+ absorption of β1 knockout mouse alveolar epithelial cell monolayers (MAECM). (A) Transepithelial electrical resistance (RT) across Atp1b1F/F MAECM (1.72 ± 0.09 kΩ·cm2) (n = 42) was not significantly different compared with Atp1b1Sftpc-cre MAECM (1.95 ± 0.11 kΩ·cm2) (n = 45). (B) Equivalent short-circuit current (IEQ) was significantly lower in Atp1b1Sftpc-cre MAECM (3.81 ± 0.11 μA/cm2) (n = 45) compared with Atp1b1F/F MAECM (7.00 ± 0.18 μA/cm2) IEQ (n = 42). (C) Unidirectional Na+ fluxes in the apical-to-basolateral (A→B) direction were significantly lower in Atp1b1Sftpc-cre MAECM, whereas fluxes in the basolateral-to-apical (B→A) direction were unchanged (n = 3). (D) Net Na+ absorption of Atp1b1F/F and Atp1b1Sftpc-cre MAECM (n = 3) were significantly different, with lower absorption in knockout monolayers. *P < 0.05; **P < 0.01; ***P < 0.001.
Decreased Na+ Absorption in MAECM Deficient in β1 Subunit
To more directly evaluate active ion transport across Atp1b1F/F control and Atp1b1Sftpc-cre knockout MAECM, unidirectional Na+ flux and net Na+ absorption were determined under zero electrical gradient (i.e., short-circuit) conditions in Ussing chambers. Figure 3C shows the unidirectional Na+ flux in each direction (i.e., apical to basolateral [A→B] and basolateral to apical [B→A]). In both control and knockout monolayers, unidirectional Na+ flux in the A→B direction was significantly greater than that in the B→A direction. Knockout monolayers had significantly lower unidirectional Na+ flux in the A→B direction (P < 0.01) and unchanged unidirectional Na+ flux in the B→A direction, compared with control monolayers. Baseline net Na+ absorption of knockout monolayers was significantly lower than that of control monolayers (Figure 3D). Net Na+ absorption in control and knockout monolayers was not significantly different from the observed active ion transport rate (i.e., ISC) during the Na+ flux measurements (data not shown), indicating that estimated net Na+ absorption accounts for most of the observed active ion transport across MAECM.
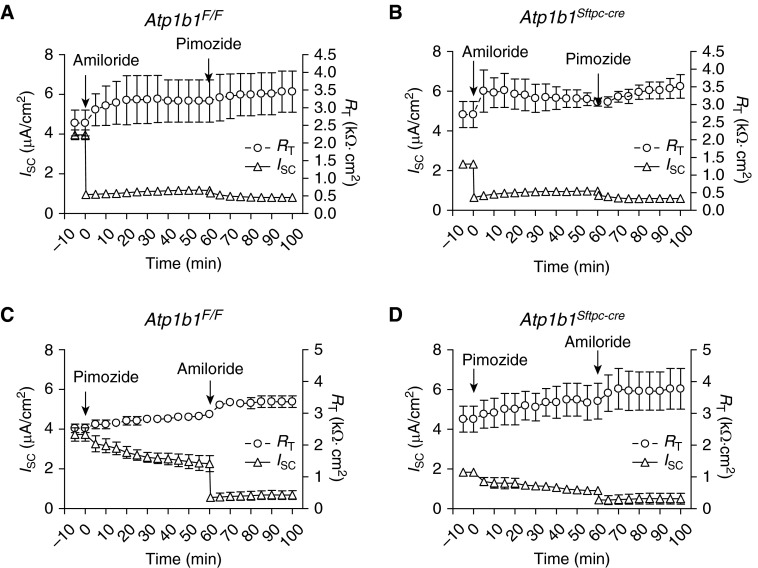
Sodium Channel Function Unaffected by β1 Knockout
AEC harbor apical amiloride-sensitive ENaC and pimozide-sensitive cyclic nucleotide-gated (CNG) nonselective cation channels (4). To determine if knockout of the Na pump β1 subunit had any effects on these ion channels, MAECM were treated with ENaC or CNG channel inhibitors, and bioelectric properties were evaluated. The addition of amiloride to apical fluid caused a rapid ∼70% decrease in ISC of both control and knockout MAECM (Figures 4A and 4B). Subsequent treatment with apical pimozide (10 μM) at 60 minutes led to a slight additional decrease in ISC of both monolayers. When the CNG inhibitor pimozide was added first, ISC of both control and knockout monolayers gradually decreased by ∼45% (Figures 4C and 4D). When amiloride (10 μM) was subsequently added to apical fluid, ISC of both monolayers rapidly decreased further by ∼35%. These data suggest that the function of apical sodium channels remains unchanged in β1 knockout MAECM.
Figure 4.
Short-circuit current (ISC) response to amiloride and pimozide. (A and B) At t = 0, amiloride (10 μM) was added to apical fluid of (A) Atp1b1F/F MAECM (n = 3) and (B) Atp1b1Sftpc-cre MAECM (n = 3). After 60 minutes, pimozide (10 μM) was added to apical fluid. (C and D) At t = 0, pimozide (10 μM) was added to apical fluid of (C) Atp1b1F/F MAECM (n = 3) and (D) Atp1b1Sftpc-cre MAECM (n = 3). After 60 minutes, amiloride (10 μM) was added to apical fluid.
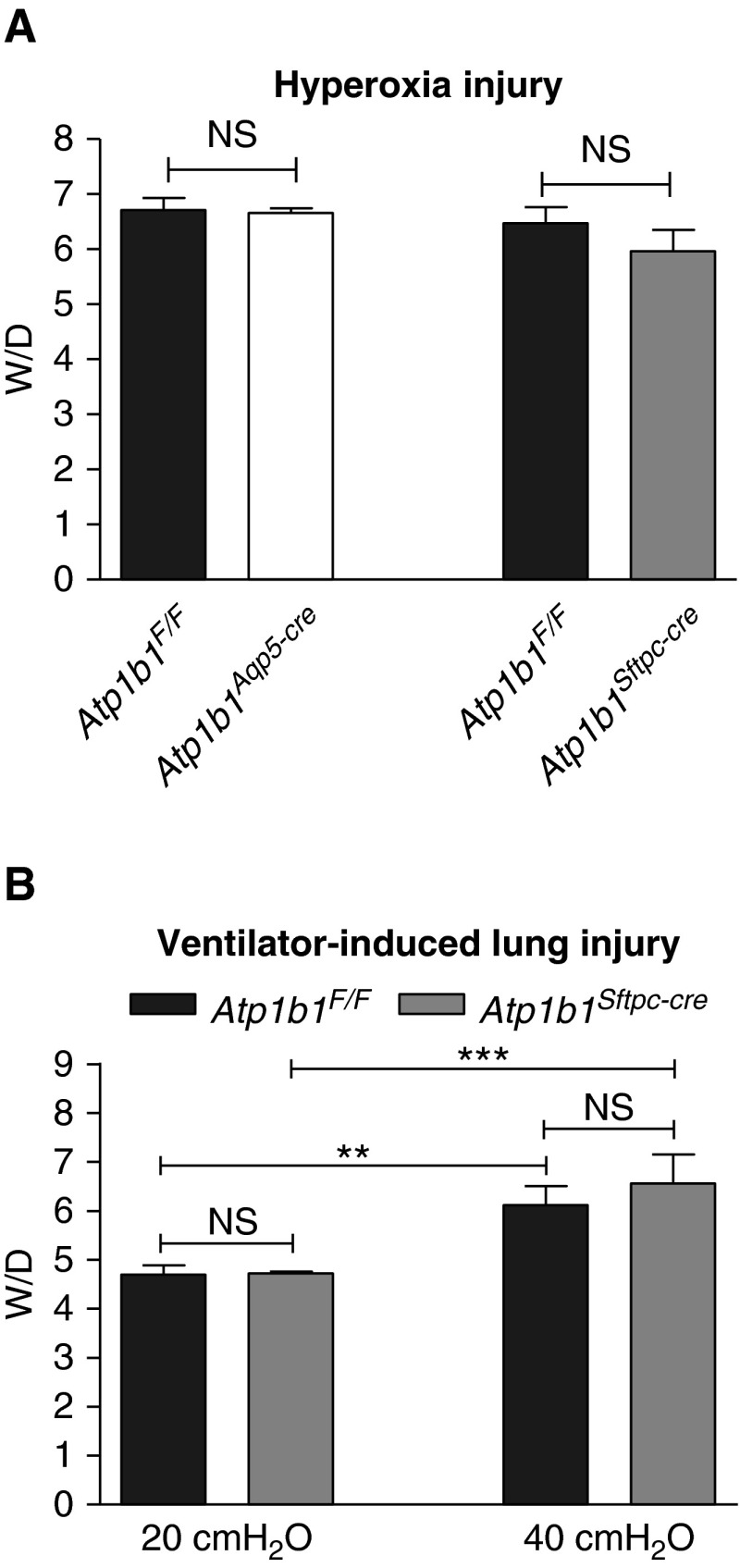
Wet-to-Dry Lung Weight Ratios after Hyperoxia or Ventilator-induced Lung Injury
Although residual AFC in both β1 knockout lines was sufficient to maintain a normal lung fluid balance in the unchallenged lung, we assessed whether or not decreased AFC in mice deficient in the β1 subunit affected their susceptibility to lung injury from hyperoxia or ventilator-induced lung injury (VILI). All animals developed increased wet-to-dry lung weight ratios after exposure to >95% oxygen for 65 hours (Figure 5A), but neither Atp1b1Aqp5-cre nor Atp1b1Sftpc-cre knockout mice exhibited ratios different from Atp1b1F/F control mice subjected to the same experimental conditions. Mice of both Atp1b1F/F and Atp1b1Sftpc-cre genotypes mechanically ventilated for 3 hours with a peak inspiratory pressure of 40 cm H2O and without positive-end expiratory pressure had significantly higher wet-to-dry lung weight ratios compared with mice ventilated under noninjurious conditions at 20 cm H2O, also without positive-end expiratory pressure (Figure 5B), but there was no significant difference in wet-to-dry weight ratios between control and knockout mice after VILI.
Figure 5.
W/D in mice exposed to hyperoxia or subjected to ventilator-induced lung injury (VILI). (A) W/D were not significantly different between floxed and knockout mice after hyperoxic injury (65 h in >95% oxygen). W/D in Atp1b1F/F and Atp1b1Aqp5-cre mice were 6.70 ± 0.22 versus 6.66 ± 0.09 (n = 3) and in Atp1b1F/F and Atp1b1Sftpc-cre mice were 6.47 ± 0.30 versus 5.96 ± 0.40 (n = 3). (B) No significant difference in W/D between genotypes was observed after either noninjurious (PIP = 20 cm H2O) or injurious (PIP = 40 cm H2O) ventilation. W/D in Atp1b1F/F and Atp1b1Sftpc-cre were 4.70 ± 0.11 versus 4.72 ± 0.02 (n = 3) after noninjurious ventilation and 6.12 ± 0.17 versus 6.57 ± 0.26 (n = 5) after VILI. PIP, peak inspiratory pressure. **P < 0.01; ***P < 0.001.
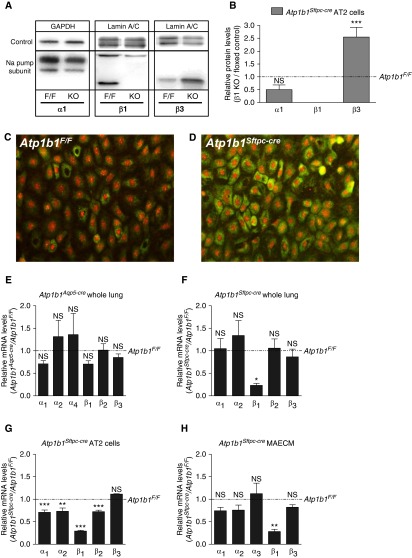
Increased Expression of Na Pump β3 Subunit Protein in Atp1b1Sftpc-cre Knockout Mice
Both Atp1b1Aqp5-cre and Atp1b1Sftpc-cre knockout mice had normal wet-to-dry lung weight ratios at baseline (Figures 2E and 2F), suggesting that residual Na-K-ATPase activity was sufficient to maintain fluid homeostasis. The Na pump β3 subunit is the second highest expressed β subunit in AEC. Western analysis revealed increased β3 protein expression in freshly isolated AT2 cells derived from Atp1b1Sftpc-cre mice (Figures 6A and 6B), and antibody staining of MAECM from knockout mice showed increased expression of β3 subunit protein compared with control MAECM (Figures 6C and 6D), although β3 subunit expression at the mRNA level in whole lung, freshly isolated AT2 cells, or MAECM did not change (Figures 6E–6H). Relative mRNA expression levels of other α and β subunit genes in whole lung of Atp1b1Aqp5-cre knockout mice were either unchanged or only moderately different from Atp1b1F/F control lungs, whereas in Atp1b1Sftpc-cre knockout lungs, a more substantial reduction in β1 subunit RNA levels was observed (Figure 6F), likely because the β1 gene is deleted in the entire epithelium. Similarly, Atp1b1Sftpc-cre knockout AT2 cells and MAECM demonstrated significantly lower expression of β1 subunit (Figures 6G and 6H). Expression of Na pump α1 subunit protein in AT2 cells obtained from Atp1b1Sftpc-cre knockout mice was unchanged, whereas β1 subunit protein was absent (Figure 6A). Increased expression of β3 subunit protein may contribute to residual AFC in Atp1b1Sftpc-cre knockout mice.
Figure 6.
Expression of Na pump subunits in sodium β1 knockout mice. (A) Representative Western blots reveal that Na pump α1 subunit was unchanged, β1 was undetectable, and β3 was increased in alveolar epithelial type II (AT2) cells from knockout mice. The upper band in β1 blot appears to be nonspecific. (B) Deletion of Na pump β1 subunit significantly increased β3 protein in AT2 cells isolated from Atp1b1Sftpc-cre (KO) compared with Atp1b1F/F (F/F) mice (n = 3). (C and D) Antibody staining of MAECM (Day 6) for Na pump β3 subunit in Atp1b1F/F cells (C) and Atp1b1Sftpc-cre cells (D) shows increased expression in MAECM generated from β1 knockout mice. (E–H) Relative mRNA expression of α and β subunit genes in whole lung from (E) Atp1b1Aqp5-cre and (F) Atp1b1Sftpc-cre mice (n = 4), and (G) isolated AT2 cells (n = 3) and (H) MAECM from Atp1b1Sftpc-cre mice (n = 3). Levels are relative to expression in Atp1b1F/F control lung, isolated AT2 cells, or MAECM. *P < 0.05, **P < 0.01, ***P < 0.001.
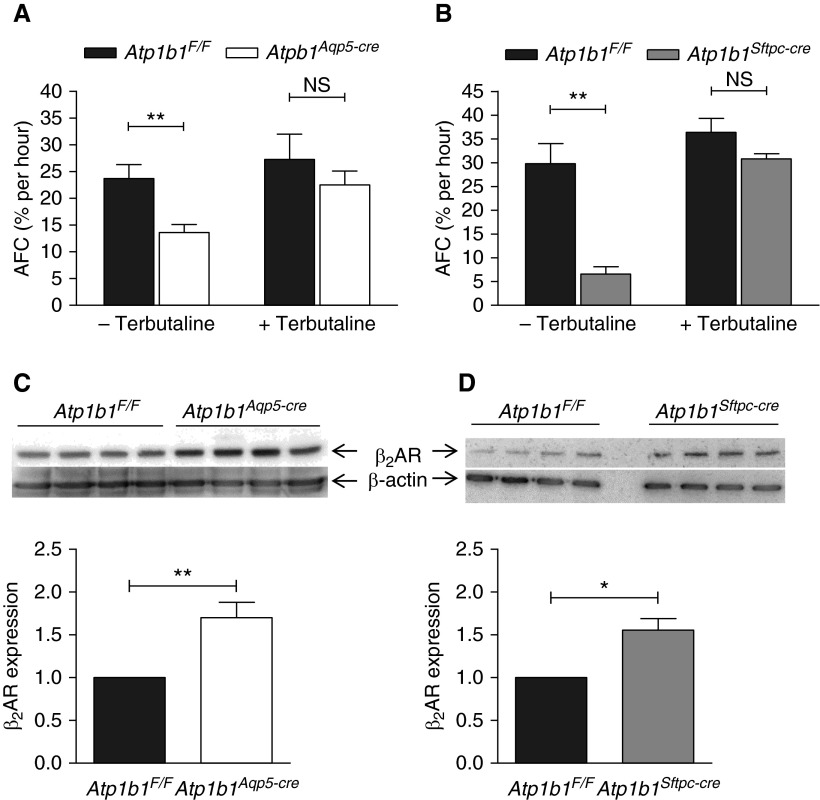
Increased β2-Adrenergic Responsiveness In Vivo in β1 Subunit Knockout Mice
When terbutaline was added to the instillate in AFC experiments, AFC increased markedly in both Atp1b1Aqp5-cre and Atp1b1Sftpc-cre knockout mice, reaching levels close to those measured in Atp1b1F/F control mice (+Terbutaline in Figures 7A and 7B). This was in contrast to AFC in the absence of terbutaline, which showed significantly lower AFC in both knockout mice (−Terbutaline in Figures 7A and 7B). To investigate the possibility that these observations could be caused by higher expression of the β2-adrenergic receptor (β2AR) in knockout mice, we performed Western analysis of the whole lung. As shown in Figures 7C and 7D, β2AR protein levels were significantly higher in the lungs of mice from both knockout lines compared with those of control mice, which may contribute to increased responsiveness to terbutaline stimulation and residual AFC in Atp1b1Aqp5-cre and Atp1b1Sftpc-cre knockout mice.
Figure 7.
AFC in the absence or presence of terbutaline and Western analysis of β2-adrenergic receptor (β2AR) expression in Na pump β1 knockout mice. (A) AFC measurements in the absence of terbutaline demonstrated lower AFC in Atp1b1Aqp5-cre versus control mice. In contrast, terbutaline-stimulated AFC in Atp1b1Aqp5-cre mice reached 22.5 ± 2.6% per hour (n = 4), which was not significantly different from Atp1b1F/F control mice (27.3 ± 4.7% per hour, n = 4). (B) AFC in the absence of terbutaline was significantly lower in Atp1b1Sftpc-cre versus control mice, whereas terbutaline-stimulated AFC in Atp1b1Sftpc-cre mice was 30.9 ± 1.0% per hour (n = 4), which was not significantly different from Atp1b1F/F control mice (36.4 ± 2.9% per hour, n = 4). (C and D) Western analysis of β2AR protein in whole lung in (C) Atp1b1Aqp5-cre and (D) Atp1b1Sftpc-cre mice shows increased expression in both knockout lines compared with Atp1b1F/F control mice. *P < 0.05; **P < 0.01.
Discussion
We generated and analyzed two mouse lines harboring knockout of the Na-K-ATPase β1 subunit, either in AT1 cells only (Atp1b1Aqp5-cre mice) or in both AT1 and AT2 cells (Atp1b1Sftpc-cre mice) in the lung. AFC was reduced by 78% in Atp1b1Sftpc-cre mice, providing evidence for an important role of the β1 subunit under conditions of excess alveolar fluid volume. AFC was also reduced substantially in Atp1b1Aqp5-cre mice (by 43%), indicating that AT1 cells are major contributors to active ion transport and associated fluid clearance (∼55% of overall AFC) in mouse lung. There were no changes in lung permeability in mice deficient in the Na pump β1 subunit. Despite lower active ion transport and decreased AFC, both knockout lines had normal wet-to-dry lung weight ratios at baseline, and no differences in wet-to-dry lung weight ratios were observed among genotypes after hyperoxia or VILI. Analysis of the bioelectric properties of cultured AEC monolayers demonstrated that AT1-like cells deficient in the Na pump β1 subunit featured significantly decreased active Na+ absorption and IEQ (and ISC), consistent with the observation that knockout mice were unable to clear fluid at the same rate as floxed control mice and demonstrating that the β1 subunit is crucial to AFC. Finally, in vitro analysis revealed that RT was unaffected in MAECM deficient in the β1 subunit, consistent with unchanged permeability in knockout lungs.
Cell-Specific Deletion of Atp1b1 and Effects on Na Pump Subunit Expression
In Figure 6E, which shows subunit expression levels in whole lung in AT1 cell–specific knockout mice, the decrease in β1 mRNA did not reach statistical significance. Given the fact that AT2 cells are present in larger numbers than are AT1 cells in the lung (∼2:1) (5), a relatively limited effect on whole lung β1 expression levels might be expected in AT1 cell–specific knockout lungs. It is also possible that the β1 expression level per cell differs between wild type AT1 and AT2 cells. If the level is higher in AT2 cells, this would also contribute to the apparent limited decrease in β1 mRNA in whole lungs of AT1 cell–specific knockout mice. A compensatory increase in β1 expression in AT2 cells in AT1 cell–specific knockout mice would have the same effect of masking the decreased mRNA level in AT1 cells when analyzing expression in whole lung. The decreased mRNA levels of the α1, α2, and β2 subunits in freshly isolated AT2 cells shown in Figure 6G were relatively small but nevertheless significant. At the protein level, we were able to detect α1, which did not change significantly (Figure 6A), whereas α2 and β2 proteins were undetectable. From other studies involving sodium pump subunits, it is known that changes in mRNA level are not always reflected at the protein level (e.g., β1 subunit is increased at the mRNA level in rat lung exposed to hyperoxia, whereas no corresponding increase in β1 protein was found [34]).
Relative Contributions of AT1 and AT2 Cells to Alveolar Ion and Water Transport
AT1 cell–specific β1 knockout mice showed ∼43% reduction in AFC, whereas animals lacking β1 in both AT1 and AT2 cells demonstrated ∼78% reduction in AFC. As noted above, these results indicate that a major portion (∼55%) of AFC in the lung is normally driven by AT1 cells, providing, we believe, the first direct evidence to support a major contribution of AT1 cells to AFC in the lung. Considering that the number of AT2 cells in the lung is greater than the number of AT1 cells (5), AFC contributed per AT1 cell is greater than that per AT2 cell. This finding contrasts with the earlier presumption that AT2 cells are responsible for most of the ion (and accompanying fluid) transport in the lung (6). However, because the total surface area of AT1 cells is much larger than that of AT2 cells (73-fold difference in rat lung [5]), the density of Na pumps per AT2 cell is likely much higher than that per AT1 cell. In these calculations of relative contribution to AFC from AT1 and AT2 cells, we assumed that β1 is efficiently and similarly knocked out in both AT2 and AT1 cells in the Atp1b1Sftpc-cre knockout line, which is likely because the Sftpc promoter driving the cre transgene starts to be expressed early during lung development (35) and the Atp1b1 gene will thus be deleted in progenitors of both AT2 and AT1 cells (36). Figure E1C (right panel) shows efficient Cre-mediated reporter activation, resulting in GFP expression in both AT1 and AT2 cells, although expression is considerably weaker in AT1 cells. Because the myristoylated GFP reporter protein encoded by the ROSAmT/mG transgene is inserted into the plasma membrane, an equal amount of myristoylated GFP expressed per cell in AT2 and AT1 cells would be expected to result in a weaker signal in AT1 cells given their considerably larger cell surface area. When comparing the two knockout lines and calculating the relative contributions of AT1 and AT2 cells, we have assumed equal knockout efficiency in AT1 cells in the two lines on the basis of efficient reporter activation in AT1 cells in both Aqp5cre;ROSAmT/mG and Sftpccre;ROSAmT/mG (Figure E1C, left and right panel, respectively).
Changes in Transport Properties of β1 Knockout MAECM
Isolated mouse AT2 cells cultured on polycarbonate filters transdifferentiate into AT1-like cells, forming functional monolayers with high RT that are suitable for characterization of ion transport and bioelectric properties (33). Given that Na pump β1 subunit is the most highly expressed β subunit in the lung, we hypothesized that knockout of Atp1b1 would result in decreased active Na+ transport. MAECM deficient in β1 subunit exhibited lower net Na+ absorption compared with MAECM from floxed mice. These results, demonstrating the importance of the Na pump β1 subunit for ion transport in AT1-like cells in culture, are consistent with our findings in vivo in Atp1b1Aqp5-cre mice in which deletion of β1 specifically in AT1 cells resulted in a major reduction in AFC. In experiments to evaluate responses in MAECM to an inhibitor of ENaC (amiloride) or CNG (pimozide) channels, we found proportional inhibition of active ion transport in knockout and control mice, suggesting that β1 subunit deficiency does not alter the relative contributions of these channels to transepithelial ion transport. However, because pimozide is known to affect D2-dopamine receptors, we cannot completely rule out the possibility that the observed pimozide effects on ISC might have been different between the genotypes. Increased expression of β3 subunit and β2AR in MAECM were not sufficient to restore ion transport. Although β1 subunits in neighboring cells have been reported to interact directly as adhesion molecules (37–39), RT was not decreased in β1 knockout MAECM. Although not included in this study, evaluation of the bioelectric properties of MAECM derived from AT2 cells of Atp1b1Aqp5-cre mice would show the effects of de novo β1 gene deletion. Thus, the β1 subunit gene would be deleted gradually during the process of AT2 cell transdifferentiation into AT1 cells, because transdifferentiated cells start to express Aqp5 and Cre. In both knockout models, deletion of the β1 subunit gene is dependent on the spatiotemporal expression profile of the promoter driving Cre expression. Sftpc-cre is expressed already in early lung progenitors in the developing embryo, whereas Aqp5-cre reaches appreciable expression levels perinatally. Because all studies were performed in adult mice, compensatory mechanisms may have developed in both knockout lines.
Response to Lung Injury in β1 Knockout Mice
We did not detect any differences in wet-to-dry lung weight ratios among genotypes after hyperoxia or VILI, although wet-to-dry lung weight ratios are relatively insensitive to small changes in alveolar fluid volume. Although AFC data would have been more useful, technical challenges prevented accurate measurement of AFC in injured lungs. The absence of higher wet-to-dry lung weight ratios after injury in β1 knockout mice versus control mice might be a result, in part, of a compensatory increase in expression of sodium pump β3 subunit and/or elevated expression of β2-adrenergic receptors. Expression of β3 subunit has been reported in normal rat lungs (25), but there have been no reports on its role in lung injury. Elevated expression of β2-adrenergic receptors in knockout mice correlated with increased responsiveness to terbutaline, supporting a potential role of adrenergic signaling as a compensatory mechanism in β1 knockout lungs. A higher increase (∼4-fold) in AFC in response to terbutaline in Atp1b1Sftpc-cre knockout mice is likely a reflection of increased adrenergic signaling in both AT1 and AT2 cells, because both cell types lack β1 in this knockout line. In Atp1b1Aqp5-cre knockout mice, however, β1 is missing only in AT1 cells, so the adrenergic response will be less pronounced (∼2-fold) since increased adrenergic signaling likely only takes place in AT1 cells. This reasoning is based on the assumption that increased adrenergic signaling is an intrinsic response in cells lacking the β1 protein and that increased adrenergic responsiveness is found only in these cells. We did not investigate expression levels of Na pump γ subunits in β1 knockout mice, although a recent report demonstrated expression of all seven FXYD genes (encoding γ subunits) in human lung at the mRNA level (40). This study also suggested that FXYD1 is a negative regulator of Na pump activity and that increased expression of FXYD1 in lungs from patients with ARDS may indicate a role for γ subunits in deficient ion transport and fluid clearance.
Conclusions
These findings from lung epithelial cell type–specific β1 subunit knockout mice demonstrate a major contribution of AT1 cells, greater than that of AT2 cells, to alveolar ion transport and AFC. Residual AFC in β1 subunit knockout mice, possibly caused, in part, by increased expression of β3 subunit and β2AR, appears sufficient to maintain lung fluid homeostasis at baseline. These studies demonstrate, we believe for the first time, that the roles of lung AEC can be addressed in an AT1 versus AT2 cell–specific manner in vivo, leading to improved understanding of the clinical implications pertaining to specific cell types in alveolar epithelium. Elucidation of the relative contributions of AT1 and AT2 cells to alveolar function/homeostasis may help lead to the development of new therapeutic approaches to lung disease.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank the USC Transgenic Core under the direction of Dr. Robert Maxson and Dr. Nancy Wu for fruitful collaborations to establish Atp1b1F/F mice; Dr. Gökhan Mutlu, Northwestern University, for generously sharing mouse AFC protocols and expertise; and Dr. Alicia McDonough, USC, for sharing rabbit polyclonal β3 antibodies. Histology and microscopy services were provided by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases (National Institutes of Health P30 DK048522 and S10 RR022508).
Footnotes
This work was supported by the Hastings Foundation, the Whittier Foundation, the American Heart Association (Grant-in-Aid No 12BGIA12060329 to P.F.), and the National Institutes of Health (R01ES017034 and U01HL108634 to E.D.C., R01HL056590 and R01HL095349 to P.M., and R37HL062569 and R01HL112638 to Z.B.).
Author Contributions: P.M., Z.B., and E.D.C. developed concepts and approach; P.F., Y.H.K., J.M.L., K.-J.K., and Z.B. conceived and designed experiments; P.F., Y.H.K., L.L.B., D.G., Y.J., and H.K. performed experiments; P.F. and Y.H.K. analyzed data; and P.F., Z.B., and E.D.C. prepared the manuscript. All authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0005OC on April 11, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthay MA, Clerici C, Saumon G. Invited review: active fluid clearance from the distal air spaces of the lung. J Appl Physiol (1985) 2002;93:1533–1541. doi: 10.1152/japplphysiol.01210.2001. [DOI] [PubMed] [Google Scholar]
- 2.Hummler E, Planès C. Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem. 2010;25:63–70. doi: 10.1159/000272051. [DOI] [PubMed] [Google Scholar]
- 3.Norlin A, Lu LN, Guggino SE, Matthay MA, Folkesson HG. Contribution of amiloride-insensitive pathways to alveolar fluid clearance in adult rats. J Appl Physiol (1985) 2001;90:1489–1496. doi: 10.1152/jappl.2001.90.4.1489. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson WJ, Benjamin AR, De Proost I, Orogo-Wenn MC, Yamazaki Y, Staub O, Morita T, Adriaensen D, Riccardi D, Walters DV, et al. Alveolar epithelial CNGA1 channels mediate cGMP-stimulated, amiloride-insensitive, lung liquid absorption. Pflugers Arch. 2011;462:267–279. doi: 10.1007/s00424-011-0971-0. [DOI] [PubMed] [Google Scholar]
- 5.Haies DM, Gil J, Weibel ER. Morphometric study of rat lung cells. I. Numerical and dimensional characteristics of parenchymal cell population. Am Rev Respir Dis. 1981;123:533–541. doi: 10.1164/arrd.1981.123.5.533. [DOI] [PubMed] [Google Scholar]
- 6.Schneeberger EE, McCarthy KM. Cytochemical localization of Na+-K+-ATPase in rat type II pneumocytes. J Appl Physiol (1985) 1986;60:1584–1589. doi: 10.1152/jappl.1986.60.5.1584. [DOI] [PubMed] [Google Scholar]
- 7.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L599–L608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med. 2001;163:1021–1029. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 10.Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci USA. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the α2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. Front Biosci (Landmark Ed) 2009;14:2130–2148. doi: 10.2741/3367. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 15.Geering K, Theulaz I, Verrey F, Häuptle MT, Rossier BC. A role for the β-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989;257:C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- 16.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens. 2008;17:526–532. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XL, Danto SI, Borok Z, Eber JT, Martín-Vasallo P, Lubman RL. Identification of Na+-K+-ATPase β-subunit in alveolar epithelial cells. Am J Physiol. 1997;272:L85–L94. doi: 10.1152/ajplung.1997.272.1.L85. [DOI] [PubMed] [Google Scholar]
- 18.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL. Na+-K+-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol. 1998;274:L149–L158. doi: 10.1152/ajplung.1998.274.1.L149. [DOI] [PubMed] [Google Scholar]
- 19.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, et al. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase β1 subunit gene. J Clin Invest. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi AV, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase β1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase β1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther. 2000;11:2231–2242. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- 22.Azzam ZS, Dumasius V, Saldias FJ, Adir Y, Sznajder JI, Factor P. Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation. 2002;105:497–501. doi: 10.1161/hc0402.102848. [DOI] [PubMed] [Google Scholar]
- 23.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med. 2003;168:1445–1448. doi: 10.1164/rccm.200207-702OC. [DOI] [PubMed] [Google Scholar]
- 24.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, Dean DA. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176:582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arystarkhova E, Sweadner KJ. Tissue-specific expression of the Na,K-ATPase β3 subunit. The presence of β3 in lung and liver addresses the problem of the missing subunit. J Biol Chem. 1997;272:22405–22408. doi: 10.1074/jbc.272.36.22405. [DOI] [PubMed] [Google Scholar]
- 26.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 27.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med. 2001;163:1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 28.Flodby P, Borok Z, Kim YH, Banfalvi A, Kim KJ, Crandall ED. AT1 cell-specific knockout of the sodium pump β1 subunit gene (Atp1b1) results in impaired alveolar fluid clearance. Am J Respir Crit Care Med. 2011;183:A4230. [Google Scholar]
- 29.Kim YH, Flodby P, DeMaio L, Kim KJ, Crandall ED, Borok Z. Decreased active ion transport across primary cultured alveolar epithelial cell monolayers generated from Na+,K+-ATPase β1-subunit knockout mice. Am J Respir Crit Care Med. 2011;183:A4231. [Google Scholar]
- 30.Flodby P, Borok Z, Gao D, Kim YH, Kim KJ, Crandall ED. Role of sodium pump β1 subunit in adult mouse lung alveolar fluid homeostasis. FASEB J. 2012;26:1069.6. [Google Scholar]
- 31.Flodby P, Borok Z, Banfalvi A, Zhou B, Gao D, Minoo P, Ann DK, Morrisey EE, Crandall ED. Directed expression of Cre in alveolar epithelial type 1 cells. Am J Respir Cell Mol Biol. 2010;43:173–178. doi: 10.1165/rcmb.2009-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 33.Demaio L, Tseng W, Balverde Z, Alvarez JR, Kim KJ, Kelley DG, Senior RM, Crandall ED, Borok Z. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1051–L1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter EP, Wangensteen OD, O’Grady SM, Ingbar DH. Effects of hyperoxia on type II cell Na-K-ATPase function and expression. Am J Physiol. 1997;272:L542–L551. doi: 10.1152/ajplung.1997.272.3.L542. [DOI] [PubMed] [Google Scholar]
- 35.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993;156:426–443. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- 36.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoshani L, Contreras RG, Roldán ML, Moreno J, Lázaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between β-subunits located in neighboring cells. Mol Biol Cell. 2005;16:1071–1081. doi: 10.1091/mbc.E04-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vagin O, Tokhtaeva E, Sachs G. The role of the β1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem. 2006;281:39573–39587. doi: 10.1074/jbc.M606507200. [DOI] [PubMed] [Google Scholar]
- 39.Vagin O, Dada LA, Tokhtaeva E, Sachs G. The Na-K-ATPase α₁β₁ heterodimer as a cell adhesion molecule in epithelia. Am J Physiol Cell Physiol. 2012;302:C1271–C1281. doi: 10.1152/ajpcell.00456.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wujak LA, Blume A, Baloğlu E, Wygrecka M, Wygowski J, Herold S, Mayer K, Vadász I, Besuch P, Mairbäurl H, et al. FXYD1 negatively regulates Na+/K+-ATPase activity in lung alveolar epithelial cells. Respir Physiol Neurobiol. 2016;220:54–61. doi: 10.1016/j.resp.2015.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.