Abstract
Purpose: To report the 12-month results of the MAJESTIC clinical study of the self-expanding Eluvia paclitaxel-eluting stent in the treatment of femoropopliteal lesions. Methods: The prospective, single-arm, multicenter trial (clinicaltrials.gov identifier NCT01820637) enrolled 57 patients (mean age 69±9 years; 47 men) with chronic lower limb ischemia referable to de novo or restenotic lesions in the native superficial femoral and/or proximal popliteal arteries. A third of the patients had diabetes. Mean lesion length was 70.8±28.1 mm, and diameter stenosis was 86.3%±16.2%; 26 (46%) lesions were occluded. Primary patency was defined as duplex ultrasound peak systolic velocity ratio ≤2.5 and the absence of target lesion revascularization (TLR) or bypass. Major adverse events (MAEs) included all-cause death through 1 month and target limb major amputation and TLR through 12 months. Results: All 57 patients had a single Eluvia stent implanted, employing pre- and postdilation in 93% (53/57) and 95% (54/57) of cases, respectively. Technical success was 97% (55/57; 2 failures due to residual stenosis >30%). At 12 months, primary patency was 96% (49/51) and the MAE rate was 4% (2/53); both MAEs were TLRs. No stent fractures were identified. There were no major amputations. One death occurred 368 days postprocedure, unrelated to the device or procedure. Improvements in the Rutherford category were sustained through 1 year, with 81% (43/53) exhibiting no symptoms (category 0) and 13% (7/53) presenting with mild claudication (category 1). Mean ABI improved from 0.73±0.22 at baseline to 1.02±0.20 at 12 months. Conclusion: MAJESTIC results showed that patients whose femoropopliteal arteries were treated with the Eluvia drug-eluting stent sustained high patency and low MAE rates through 12 months.
Keywords: claudication, drug-eluting stent, paclitaxel, peripheral artery disease, popliteal artery, restenosis, superficial femoral artery, target lesion revascularization
Introduction
New stent designs and drug-eluting technologies are intended to improve outcomes following femoropopliteal treatment for peripheral artery disease. Long-term patency following bare metal stenting (BMS) is encouraging but remains unsatisfactory, with reported 1-year primary patency peaking at ~80%.1–9 Likewise, target lesion revascularization (TLR) rates for BMS also show room for improvement, with 1-year rates averaging ~13% in recent clinical trials.2-9
Current approaches to prevent restenosis and thereby reduce reintervention rates include applying an antirestenotic agent, such as paclitaxel, to the vessel wall by means of a drug-coated balloon or stent. Use of a paclitaxel-coated stent has been shown to improve patency over BMS in TransAtlantic Inter-Society Consensus (TASC) II A and B femoropopliteal lesions.10,11 Paclitaxel, which arrests the cell cycle in the G2/M phase, interrupts arterial smooth muscle cell proliferation and migration, as well as extracellular matrix formation.12,13 These later steps of the restenosis cascade that are inhibited by paclitaxel are not initiated until several weeks or months following an angioplasty or stenting procedure,14,15 suggesting that paclitaxel’s antirestenotic effects could be enhanced if its presence were sustained in the arterial wall over a longer period of time.
The Eluvia Drug-Eluting Vascular Stent System (Boston Scientific, Marlborough, MA, USA) was designed to elute paclitaxel over time owing to a biocompatible fluoropolymer coating. The MAJESTIC clinical study was designed to evaluate the performance of the Eluvia drug-eluting stent for treating stenotic lesions in the femoropopliteal segment.
Methods
Study Design
MAJESTIC is a prospective, single-arm, multinational clinical study of the Eluvia stent system for treating femoropopliteal lesions (clinicaltrials.gov identifier NCT01820637). Adult patients with chronic, symptomatic lower limb ischemia, defined as Rutherford category16 2, 3, or 4, and stenotic (≥70% by visual angiographic assessment), restenotic (from non-drug-coated balloon angioplasty only), or occlusive lesion(s) ≥30 mm and ≤110 mm in length located in the native superficial femoral artery (SFA) or proximal popliteal artery were eligible to participate (Table 1).
Table 1.
Inclusion and Exclusion Criteria for MAJESTIC.
| Inclusion criteria |
| • Age 18 years or older |
| • Signed consent form |
| • Chronic, symptomatic lower limb ischemia defined as Rutherford categories 2–4 |
| • Stenotic, restenotic (from angioplasty only; previous treatment with drug-coated balloon is not allowed) or occlusive lesion(s) located in the native SFA or PPA |
| • Degree of stenosis ≥70% by visual angiographic assessment |
| • Reference vessel diameter ≥4 and ≤6 mm |
| • Total lesion length (or series of lesions) ≥30 mm and ≤110 mm |
| • Target lesion located at least 3 cm above the inferior edge of the femur |
| • Patent infrapopliteal and popliteal artery, ie, single vessel runoff or better with at least 1 of 3 vessels patent (<50% stenosis) to the ankle or foot |
| Exclusion criteria |
| • Target vessel with in-stent restenosis |
| • Prior surgery of the SFA/PPA in the target limb to treat atherosclerotic disease |
| • Use of atherectomy, laser, or other debulking devices in the SFA/PPA during the index procedure |
| • History of major amputation in the target limb |
| • Life expectancy <12 months due to other medical comorbid condition(s) |
| • Known hypersensitivity or contraindication to contrast dye that, in the opinion of the investigator, cannot be adequately premedicated |
| • Known hypersensitivity/allergy to the trial stent system or protocol-related therapies |
| • Platelet count <100,000 or >600,000 mm3 |
| • Concomitant renal failure with a serum creatinine >2.3 mg/dL |
| • Receiving dialysis or immunosuppressant therapy |
| • History of myocardial infarction or stroke within 6 months prior to enrollment |
| • Unstable angina pectoris at the time of enrollment |
| • Pregnant and/or breastfeeding |
| • Current participation in another investigational drug or device clinical study that has not completed the primary endpoint at the time of enrollment |
| • Septicemia at the time of the index procedure |
| • Presence of other hemodynamically significant outflow lesions requiring intervention within 30 days of the index procedure |
| • Presence of aneurysm in the target vessel |
| • Presence of iliac artery aneurysm(s) |
| • Acute ischemia and/or acute thrombosis of the SFA/PPA |
| • Persistent intraluminal thrombus at the proposed target lesion post thrombolysis |
| • Perforated vessel as evidenced by extravasation of contrast media |
| • Heavily calcified lesions resistant to PTA |
Abbreviations: PPA, proximal popliteal artery; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery.
The study was conducted in accordance with ISO 14155:2011 (2nd edition; 2011-02-01), Clinical Investigation of Medical Devices for Human Subjects–Good Clinical Practice, and ethical principles that have their origins in the Declaration of Helsinki. The institutional ethics committees/research boards for participating sites approved the study protocol, and all patients were required to provide informed consent.
Eluvia Stent
The self-expanding nitinol Eluvia stent is based on the Innova (Boston Scientific) stent platform, which was designed to provide the flexibility, radial strength, and fracture resistance needed for the SFA. The design, which has closed cells on the ends and open cells in the middle, is also intended to facilitate uniform drug delivery both circumferentially and along the artery length. The active layer of the stent’s dual-layer coating includes the fluoropolymer PVDF-HFP [poly(vinylidene fluoride-co-hexafluoropropylene)], which is the coating polymer on the Promus Element coronary stent (Boston Scientific),17,18 and the antiproliferative agent paclitaxel at a nominal concentration of 0.167 µg/mm2.19 The biocompatible polymer17 did not inhibit endothelialization or promote thrombus formation in preclinical models of coronary or peripheral stenting.18,20 Paclitaxel stabilizes microtubules and inhibits neointimal formation by preventing arterial smooth muscle cell proliferation and migration.12,13 It also inhibits extracellular matrix formation, which is excessive in restenosis.13
Stents available for use in the study had diameters of 6 or 7 mm and lengths of 40, 80, or 120 mm, with 75- or 130-cm delivery systems. The triaxial stent delivery system is compatible with 6-F sheaths and 0.035-inch (0.89-mm) guidewires.
Interventional Procedures
Anticoagulant and antiplatelet medications were to be prescribed consistent with the center’s clinical practice. At a minimum, at least 75 mg acetylsalicylic acid (ASA) and 75 mg clopidogrel were to be administered daily for 60 days following the procedure; ASA use was recommended to continue through the 3-year follow-up period.
Diagnostic angiography of the target vessel was performed to confirm eligibility (site assessment). Pre- and postdilation of the target lesion were recommended in the study protocol. Use of atherectomy or other debulking devices in the target vessel during the index procedure was an exclusion criterion (Table 1). A primary stenting strategy with full coverage of the target lesion was recommended. Tandem lesions could be treated during the index procedure if they could be covered with a single stent. Iliac artery lesions could be treated during the index procedure provided that treatment was successfully completed without clinical sequelae prior to treatment of the target lesion.
Preinterventional Examination and Follow-up
Ankle-brachial index (ABI) and Rutherford classification were assessed prior to treatment. Follow-up clinical visits completed at 1, 9, and 12 months included Rutherford classification, ABI measurement, and duplex ultrasound measurements. Radiographs taken at the 12-month visit were sent to an x-ray core laboratory (Vascore, Boston, MA, USA) to assess stent integrity; potential fractures were then subject to verification by the angiographic core laboratory (Beth Israel Deaconess Medical Center, Boston, MA, USA) based on comparison with procedural angiograms.
Outcome Measures and Endpoints
The primary efficacy outcome was 9-month primary patency defined as core laboratory–adjudicated duplex peak systolic velocity ratio (PSVR) ≤2.5 in the absence of TLR, bypass of the target lesion, or major amputation of the target limb. Primary patency at 9 months was compared with a literature-derived performance goal of 75%, which was determined based on past performance of bare metal and drug-eluting stents.1,2,7,21-25
The major adverse event (MAE) endpoint was defined as all causes of death through 1 month, target limb major amputation through 9 months, and TLR through 9 months. Efficacy (ie, patency by duplex) and safety (eg, MAE) assessments were also performed at 12 months (±30 day follow-up visit window) to determine the continuous durability and safety of the device.
Patient Population
Between August 2013 and March 2014, a total of 57 patients (mean age 69±9 years; 47 men) recruited from 13 centers in Europe, Australia, and New Zealand had Eluvia stents implanted in their femoropopliteal arteries. Baseline patient and lesion characteristics are summarized in Table 2. Most patients had a history of smoking (50, 88%), and 20 (35%) had diabetes. Mean lesion length was 70.8±28.1 mm; 26 (46%) lesions were occluded, and 37 (65%) had severe calcification as assessed by the angiographic core laboratory.
Table 2.
Baseline Patient and Lesion Characteristics.a
| Patient characteristics (n=57) | |
|---|---|
| Age, y | 69.3±9.3 |
| Men | 47/57 (82) |
| Race/ethnicity | |
| Caucasian | 54 (95) |
| Asian | 1 (2) |
| Other | 2 (3) |
| Medical history | |
| Current diabetes mellitus | 20 (35) |
| Hyperlipidemia requiring medication | 36 (63) |
| Hypertension requiring medication | 42 (74) |
| Chronic obstructive pulmonary disease | 6 (10) |
| Coronary artery disease | 22 (39) |
| Myocardial infarction | 9 (16) |
| Congestive heart failure | 3 (5) |
| History of smoking | 50 (88) |
| Lesionsb (n=57) | |
| SFA segment | |
| Proximal | 1 (2) |
| Mid | 34 (60) |
| Distal | 44 (77) |
| PPA involvement | 5 (9) |
| Length, mm | 70.8±28.1 |
| Diameter stenosis, % | 86.3±16.2 |
| Reference vessel diameter, mm | 5.2±0.8 |
| TASC II classificationc | |
| A | 25 (44) |
| B | 26 (46) |
| C | 6 (10) |
| D | 0 (0) |
| Calcification | |
| None/mild | 12 (21) |
| Moderate | 8 (14) |
| Severe | 37 (65) |
| Total occlusion | 26 (46) |
| Ulcerated lesiond | 4 (7) |
| Vessels patent to foot | |
| 0 | 3 (5) |
| 1 | 16 (28) |
| 2 | 18 (32) |
| 3 | 13 (23) |
Abbreviations: PPA, proximal popliteal artery; SFA, superficial femoral artery; TASC, TransAtlantic Inter-Society Consensus.
Continuous data are presented as mean ± standard deviation; categorical data are shown as counts (percentages).
Values as determined by the angiographic core lab, unless otherwise indicated.
Site assessment.
Lesions had an appearance of ulceration on angiography.
Statistical Analysis
Patient and lesion characteristics and clinical outcome measures are summarized with descriptive statistics. Continuous variables are presented as mean ± standard deviation; categorical variables are expressed as frequencies and percentages.
A sample size of 57 patients was enrolled to provide statistical power >80% to assess the primary patency performance goal of 75%, justified by expected primary patency of 90%, a performance margin of 15%, a 1-sided alpha of 5%, and 20% attrition rate. The 9-month primary patency was defined as a success if the lower bound of the exact 95% confidence interval (CI) was greater than the prespecified performance goal of 75%. Kaplan-Meier estimates were plotted for primary patency through 12 months, and standard errors were calculated using the Society for Vascular Surgery standard.16 All statistical analyses were performed with the SAS for Windows software (version 9.3 or higher; SAS Institute Inc, Cary, NC, USA).
Results
Procedure Outcomes
All 57 patients had a single Eluvia stent implanted, employing pre- and postdilation in 93% (53/57) and 95% (54/57) of cases, respectively. Technical success was 97% (55/57), that is, lesion crossed and dilated with residual angiographic stenosis ≤30%. Mean postprocedure in-stent diameter stenosis was 8.5%±16.1%. The 2 technical failures were due to residual stenosis >30% (ie, 38% and 40%). Pre- and postdilation were completed in both cases, with no reported device deployment issues, stretching, additional stent bailout, or thrombus seen during the index procedure. One patient also had a non-Eluvia stent implanted to treat an SFA lesion proximal to the target lesion.
Efficacy and Safety
Primary patency at 9 months was 94% (51/54; 95% CI 86.3% to 98.5%). The lower 1-sided 95% confidence bound exceeded the performance goal of 75%; thus the primary endpoint was met. The primary patency observed at 12 months was 96% (49/51; 95% CI 86.5% to 99.5%). The Kaplan-Meier estimate of primary patency through 1 year was 96.4% (Figure 1). The 3 patency failures at 9 months included 1 patient with PSVR >2.5; however, duplex did not indicate any issues at 12 months, and because the patient had no TLR, the treated lesion was no longer considered a patency failure. The other 2 failures were TLRs that occurred prior to the 9-month follow-up in 2 male claudicants without diabetes. A 49-year-old previous smoker with hypertension had an occluded 60-mm right mid-distal SFA lesion (reference vessel diameter 5 mm) treated with a 6×80-mm Eluvia stent postdilated with a 6×20-mm balloon, leaving a 10% residual stenosis. He developed sudden ischemic symptoms ~5 months later. Angiography revealed severe stenosis proximal to the study stent, with thrombotic occlusion distal to the lesion extending into the stent, likely due to progression of the underlying atherosclerotic disease. This was the only patient to have stent thrombosis within 12 months of the initial procedure. Two covered stents (totaling 300 mm in length) were placed. A third 6×20-mm BMS was added due to thrombus on the proximal edge of the second covered stent, and a fourth 6×80-mm BMS was implanted to treat a popliteal outflow lesion. This complex case resulted in 0% final stenosis with “good flow through stented regions,” and the patient was discharged on warfarin.
Figure 1.
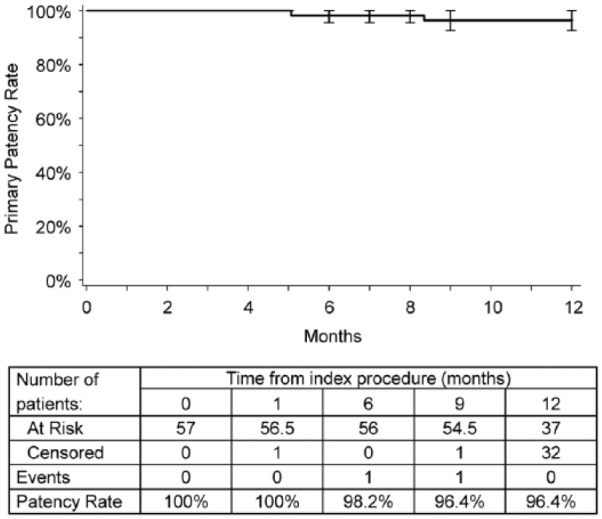
Kaplan-Meier estimate of primary patency. Intervals are end-inclusive; event rate and standard error estimates are for the interval end. Standard errors were ≤3% at each time point.
Approximately 2 months later, the patient presented with sudden onset calf pain; angiography revealed thrombosis throughout the previously revascularized segment. The patient’s international normalized ratio (INR) was 1.7. Symptoms resolved following thrombolysis, and computed tomography angiography confirmed no residual thrombus. The patient was discharged on clopidogrel and warfarin. Approximately 2 weeks later, the patient suffered a workplace-related pelvic fracture, and ~2 weeks after that the patient was again admitted for sudden-onset resting calf pain; his INR was 2.2. Thrombotic occlusion was identified, and the patient underwent femoropopliteal bypass surgery. The patient was discharged on antiplatelet therapy (without warfarin) and continues to be monitored.
The second TLR was performed on a 51-year-old smoker. The 50-mm-long target lesion was in the left mid-SFA (reference vessel diameter 5.5 mm), with 90% stenosis and moderate calcification at baseline. In the index procedure, predilation was performed with a 4×40-mm balloon, followed by deployment of a 6×80-mm Eluvia stent and postdilation with a 5-mm balloon; residual stenosis was 0. The patient developed symptoms consistent with Rutherford category 2 about 8 months postprocedure. Antiplatelet therapy had been continuous. Imaging revealed a stenosis at the proximal end of the stent near the ostium. Balloon dilation was performed outside the study stent, but 50% residual stenosis remained, and a 5×80-mm BMS was placed proximally. The stent was postdilated with no residual stenosis. No thrombus was observed.
No additional patients underwent TLRs from 9 to 12 months, and there were no major amputations. One death occurred 368 days postprocedure, unrelated to the device or procedure. The composite MAE rate through 12 months was 4% (2/53).
Antiplatelet Therapy
Prior to the index procedure, 95% (54/57) of patients received anticoagulation therapy. Dual antiplatelet therapy for the first 60 days postprocedure was required by the study protocol. During these first 60 days, 91% (51/56) of patients reported use of ASA, 68% (38/56) used clopidogrel, and 5% (3/56) used warfarin. At 12 months, 94% (50/53) continued with ASA, 36% (19/53) were on clopidogrel, 4% (2/53) were on warfarin, and 6% (3/53) used “other” antiplatelet therapy.
Clinical Outcomes
As shown in Figure 2, most patients exhibited moderate to severe claudication (Rutherford category 2 or 3) at baseline. By 1 month, 96% (54/56) had improved by 1 or more categories; none exhibited a worsening in level. Improvements were sustained through 1 year (Figure 2), with 81% (43/53) exhibiting no symptoms (category 0), and 13% (7/53) presenting with mild claudication (category 1). Mean ABI improved from 0.73±0.22 at baseline to 0.98±0.15 at 1 month and was 1.02±0.20 at 12 months. At 12 months, 88% (45/51) of patients had an ABI increase of at least 0.1 compared with baseline or had reached an ABI of at least 0.9. Through 12 months, no stent fractures were identified by angiographic core laboratory analysis.
Figure 2.
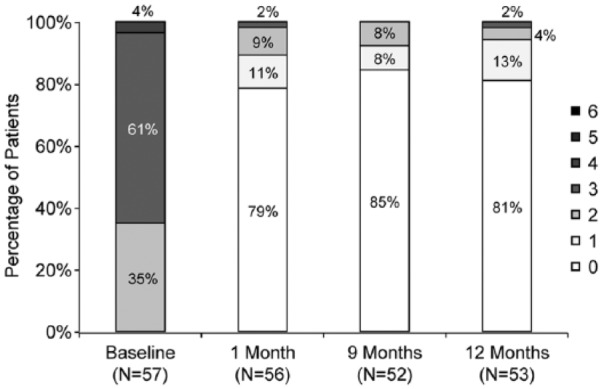
The shift to lower Rutherford categories (less severe symptoms) persisted through 12 months.
Discussion
Twelve-month results from the MAJESTIC study of the Eluvia stent system demonstrate a high patency rate (96%), accompanied by a low MAE rate (4%) driven by TLR events in 2 patients. These encouraging results were achieved despite the presence of some challenging lesion characteristics, such as severe calcification in 65% of lesions, occlusions in nearly half the lesions, distal SFA/proximal popliteal involvement in >75%, and a relatively long mean lesion length (70.8 mm) compared with other trials of femoropopliteal treatments.10,26
MAJESTIC met its primary efficacy endpoint with 9-month primary patency exceeding a prespecified performance goal, which was based on published results from studies of BMS1,2,7,21,22,24,25 and sirolimus-coated23 stents. The performance goal provides a historical benchmark but may not be representative of current femoropopliteal treatment options. Whereas the sirolimus-coated stent did not reduce the restenosis rate compared with BMS in the SIROCCO study,23 and an everolimus-eluting stent provided 12-month primary patency of 68% in a single-arm trial (STRIDES),27 more recent studies of paclitaxel-coated stents in femoropopliteal arteries demonstrate patency and safety advantages over BMS.10,11 Clinical studies of the paclitaxel-coated Zilver PTX stent have shown 12-month Kaplan-Meier primary patency estimates of 83.1% for primary stenting and 89.9% for provisional stenting in a randomized setting10 and 86.2% in a single-arm study.28 The clinically driven TLR rate was 9.5% for primary stenting in both studies.10,28 One-year data from a registry of 690 patients treated with Zilver PTX showed a restenosis rate of 37% and major adverse limb events associated with 22% of lesions.29 Although comparisons with other studies are limited due to differences in study designs, outcome definitions, and patient and lesion characteristics, MAJESTIC results provide further support for the efficacy and safety of paclitaxel-eluting stents to treat femoropopliteal lesions and suggest that sustained paclitaxel elution may contribute to a low restenosis rate.
Kaplan-Meier curves from previous studies of drug-coated stents illustrate a pattern of restenosis typified by notable declines in patency and TLR-free rates in the period from 9 through 12 months.10,23,27 Although Kaplan-Meier analyses are limited by the visit-based nature of patency data collection and the timing of interventions, the plateau observed in MAJESTIC to date suggests that Eluvia may be inhibiting restenosis during this susceptible period.
The high patency results observed in the MAJESTIC study might be due to the extended drug release profile of the Eluvia stent. Paclitaxel release from the Eluvia stent was shown to continue through at least 180 days in a preclinical porcine iliofemoral model, with potentially therapeutic levels continuing to be present in arterial tissue.19 This prolonged paclitaxel elution is made possible by the PVDF-HFP coating. The polymer has a history of clinical safety in coronary applications17 and did not inhibit endothelialization or promote thrombus formation in preclinical models.18,20
Limitations
Limitations of the MAJESTIC study include its single arm, nonrandomized design, and relatively small sample size. A larger, randomized study is needed to verify the results observed in this first-in-human trial. Although the study sample included patients with comorbid medical conditions and lesion characteristics known to adversely influence outcomes following femoropopliteal stenting, the average lesion length was relatively short compared with those seen in typical practice, and results may not generalize to the heterogeneous clinical population.
Conclusion
The results of the MAJESTIC clinical study showed that patients whose femoropopliteal arteries were treated with the Eluvia stent sustained a high patency rate with clinical improvement and a low MAE rate through 12 months.
Acknowledgments
The authors thank the following Boston Scientific employees for their assistance: Lieve Cornelis and Teri Takle-Flach for clinical program management; H. Terry Liao, PhD, for statistical analysis; and Elizabeth J. Davis, PhD, for medical writing.
Appendix
MAJESTIC investigators: Britta Vogel, Universitätsklinikum Heidelberg, Germany; Marc Bosiers, AZ Sint-Blasius, Dendermonde, Belgium; Steve Dubenec, Royal Prince Alfred Hospital, Camperdown, Australia; Stuart Hawkins, Middlemore Hospital, Otahuhu, New Zealand; Andrew Holden, Auckland City Hospital, Auckland, New Zealand; Koen Keirse, Regionaal Ziekenhuis Heilig Hart Tienen, Belgium; Christian Löwe, Medical University Vienna, Allgemeines Krankenhaus (AKH), Vienna, Austria; Stefan Müller-Hülsbeck, Ev. Luth. Diakonissenanstalt Flensburg, Germany; Dierk Scheinert, Universität Leipzig, Germany; Herman Schroë, Ziekenhuis Oost Limburg, Genk, Belgium; Ramon Varcoe, Prince of Wales Hospital, Randwick, Australia; Thodur Vasudevan, Braemar Hospital, Hamilton, New Zealand; Thomas Zeller, Universitäts-Herzzentrum Freiburg Bad Krozingen GmbH, Bad Krozingen, Germany.
Footnotes
Authors’ Note: Preliminary findings from the MAJESTIC study were presented at the Charing Cross Symposium, April 28–May 1, 2015, London, UK; CIRSE, September 26–30, 2015, Lisbon, Spain; and VIVA 2015, November 2, 2015, Las Vegas, NV, USA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stefan Müller-Hülsbeck serves as a consultant for Boston Scientific Corporation (BSC) and has received consulting fees, speaker honoraria, and support for accommodation and traveling when presenting BSC-related data. Thomas Zeller serves as a consultant for Boston Scientific, Cook, Medtronic, W.L. Gore, Veryan, Spectranetics, Trireme, and Terumo and has received consulting fees, speaker honoraria, and support for accommodation and traveling from these companies. Herman Schroë serves as a consultant for Boston Scientific and has received consulting fees, speaker honoraria, and support for accommodation and traveling when presenting BSC-related data. Juan Diaz-Cartelle is an employee of and owns stock in Boston Scientific Corporation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Boston Scientific Corporation.
References
- 1. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. [DOI] [PubMed] [Google Scholar]
- 2. Krankenberg H, Schluter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the Femoral Artery Stenting Trial (FAST). Circulation. 2007;116:285–292. [DOI] [PubMed] [Google Scholar]
- 3. Bosiers M, Deloose K, Callaert J, et al. 4-French-compatible endovascular material is safe and effective in the treatment of femoropopliteal occlusive disease: results of the 4-EVER trial. J Endovasc Ther. 2013;20:746–756. [DOI] [PubMed] [Google Scholar]
- 4. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 5. IDEV. Supera Peripheral Stent System SSED (SUPERB). 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120020b.pdf.
- 6. Laird JR, Jain A, Zeller T, et al. Nitinol stent implantation in the superficial femoral artery and proximal popliteal artery: twelve-month results from the Complete SE multicenter trial. J Endovasc Ther. 2014;21:202–212. [DOI] [PubMed] [Google Scholar]
- 7. Bosiers M, Torsello G, Gissler HM, et al. Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I study. J Endovasc Ther. 2009;16:261–269. [DOI] [PubMed] [Google Scholar]
- 8. Cordis. SMART CONTROL SSED (STROLL). 2012. http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120002b.pdf.
- 9. Matsumura JS, Yamanouchi D, Goldstein JA, et al. The United States study for evaluating endovascular treatments of lesions in the superficial femoral artery and proximal popliteal by using the Protege Everflex nitinol stent system II (DURABILITY II). J Vasc Surg. 2013;58:73–83e1. [DOI] [PubMed] [Google Scholar]
- 10. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 11. Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013;61:2417–2427. [DOI] [PubMed] [Google Scholar]
- 12. Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. [DOI] [PubMed] [Google Scholar]
- 13. Wiskirchen J, Schober W, Schart N, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Invest Radiol. 2004;39:565–571. [DOI] [PubMed] [Google Scholar]
- 14. Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. [DOI] [PubMed] [Google Scholar]
- 15. Forrester JS, Fishbein M, Helfant R, et al. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991;17:758–769. [DOI] [PubMed] [Google Scholar]
- 16. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 17. Stone GW, Teirstein PS, Meredith IT, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57:1700–1708. [DOI] [PubMed] [Google Scholar]
- 18. Wilson GJ, Huibregtse BA, Stejskal EA, et al. Vascular response to a third generation everolimus-eluting stent. EuroIntervention. 2010;6:512–519. [DOI] [PubMed] [Google Scholar]
- 19. Hou D, Huibregtse BA, Eppihimer MJ, et al. Fluorocopolymer-coated nitinol self-expanding low dose paclitaxel-eluting stent: pharmacokinetics and vascular biology responses in porcine iliofemoral model. EuroIntervention. 2016. In press. [DOI] [PubMed] [Google Scholar]
- 20. Chin-Quee SL, Hsu SH, Nguyen-Ehrenreich KL, et al. Endothelial cell recovery, acute thrombogenicity, and monocyte adhesion and activation on fluorinated copolymer and phosphorylcholine polymer stent coatings. Biomaterials. 2010;31:648–657. [DOI] [PubMed] [Google Scholar]
- 21. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276. [DOI] [PubMed] [Google Scholar]
- 22. Mewissen MW. Self-expanding nitinol stents in the femoropopliteal segment: technique and mid-term results. Tech Vasc Interv Radiol. 2004;7:2–5. [DOI] [PubMed] [Google Scholar]
- 23. Duda SH, Bosiers M, Lammer J, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710. [DOI] [PubMed] [Google Scholar]
- 24. Dearing DD, Patel KR, Compoginis JM, et al. Primary stenting of the superficial femoral and popliteal artery. J Vasc Surg. 2009;50:542–547. [DOI] [PubMed] [Google Scholar]
- 25. Dick P, Wallner H, Sabeti S, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74:1090–1095. [DOI] [PubMed] [Google Scholar]
- 26. Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 27. Lammer J, Bosiers M, Zeller T, et al. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg. 2011;54:394–401. [DOI] [PubMed] [Google Scholar]
- 28. Dake MD, Scheinert D, Tepe G, et al. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J Endovasc Ther. 2011;18:613–623. [DOI] [PubMed] [Google Scholar]
- 29. Iida O, Takahara M, Soga Y, et al. One-year results of the ZEPHYR (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery) registry: predictors of restenosis. JACC Cardiovasc Interv. 2015;8:1105–1112. [DOI] [PubMed] [Google Scholar]


