Abstract
Background
Previous literature suggests that a higher ratio of palmitic acid (PA)/oleic acid (OA) in the diet induces inflammation, which may result in deficient brain insulin signaling, and, secondarily, impaired physical activity, sleep efficiency, and cognitive functioning.
Objective
We hypothesized that lowering the typical dietary PA/OA would affect the activation of relevant brain networks during a working memory task and would also lower secretion of pro-inflammatory cytokines.
Design
In 12 female subjects participating in a randomized, cross-over trial comparing 3-week high PA diet (HPA) and low PA and a high OA diet (HOA), we evaluated functional magnetic resonance imaging (fMRI) using an N-back test of working memory, cytokine secretion by lipopolysaccharide(LPS)-stimulated peripheral blood mononuclear cells (PBMC), and plasma cytokine concentrations.
Results
Brain activation during the HPA diet compared to the HOA diet was increased in regions of the basal ganglia including the caudate and putamen (p<0.005). In addition, compared to the HOA diet, during the HPA diet, the plasma concentrations of IL-6 (p= 0.04) and IL-1β (p= 0.05) were higher, and there was a higher secretion of IL-18 (p=0.015) and a trend for higher IL-1β secretion (p=.066) from LPS-stimulated PBMCs.
Conclusions
The HPA diet resulted in increased brain activation in the basal ganglia compared to the HOA diet as well as increased secretion of pro-inflammatory cytokines. These data provide evidence that short-term (2 week) diet interventions impact brain network activation during a working memory task and that these effects are reversible since the order of the study diets was randomized. These data are consistent with the hypothesis that lowering the dietary PA content via substitution with OA also could affect cognition.
Keywords: palmitic acid, oleic acid, functional magnetic resonance imaging, cytokines, brain activation
1. Introduction
Cognition comprises a number of brain processes including memory, attention, problem-solving, and decision-making. Neuroscientists and physicians as well as lay people are interested in environmental factors that might enhance or impair cognition at any age, including diet, physical exercise, and sleep efficiency. Variations in dietary content of the saturated fatty acid (SFA), palmitic acid (PA; C16:0) and the monounsaturated FA (MUFA), oleic acid (OA; C18:1 n-9 or ω-9) have been linked to alterations in cognitive function in humans1,2.
Specifically, Samieri et al.1 reported the results of a sub-study conducted as part of a double-blind, placebo-controlled factorial trial of low dose aspirin and vitamin E supplements for the primary prevention of cardiovascular disease and cancer in women (Women’s Health Study). Subjects aged ≥65 years underwent initial cognitive testing and then again at two follow-up points at approximately two-year intervals1. Based on the trajectory of change in cognitive function, global cognition and verbal memory were enhanced as a function of the MUFA/SFA ratio, particularly when comparing the extreme quintiles, respectively 0.9 and 1.31.
In our previously published studies, we reported the results of a diet history obtained from our young adult volunteers in two separate cohorts during screening. Our subjects’ habitual” intake resulted in a MUFA/SFA ratio of 0.83 (Cohort 1) and 1.08 (Cohort 2)3, similar to the lowest three quintiles of the Samieri study1. In two separate trials, we used the same, cross-over study paradigm in which we markedly lowered the PA intake by substituting OA, resulting in two highly contrasting MUFA/SFA ratios, one, the “HPA diet”, (0.88) similar to the lowest quintile in the study by Samieri et al.1 and to our subjects’ “habitual diet” and a low PA/high OA diet (“HOA”) with a ratio (10.1)3,4 much higher than those in the fifth quintile of participants in the Women’s Health Study. In these two distinct cohorts of young adults, we have studied the effects of lowering the PA intake on a number of outcome variables including insulin sensitivity, inflammation, physical activity, mood, muscle gene expression, and blood lipid profiles3–6. Notably, lowering the dietary PA/OA ratio increased physical activity and lowered mood disturbance 3.
Prior studies also have shown links between MUFA/SFA changes and behavioral and cognitive outcomes. Sartorius et al.7 showed that a high SFA diet in mice as well as acute intraventricular injection of PA decreased activation of insulin signaling in the brain, decreased locomotor activity in response to acute intraventricular injection of insulin, and disrupted normal wakefulness and sleep behavior compared to a high MUFA diet. Blocking the activity of interleukin (IL)-6 in mice fed the high SFA diet enhanced physical activity8. Hanson et al.2 found that feeding older adults a single meal high in both SFA and high glycemic load carbohydrates improved and impaired cognition acutely, respectively, in those with or without cognitive impairment. Other studies suggest that a high SFA diet adversely affects the hippocampus and memory in rats, possibly via induction of inflammatory pathways9. It is relevant to specifically emphasize our findings that lowering the habitual dietary PA/OA ratio (same as raising the MUFA/SFA ratio) was associated with lower circulating concentrations of IL-6 and tumor necrosis factor-α (TNFα) and lower secretion of IL-1β, IL-18, and TNFα by lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells (PBMCs)4,5.
In view of evidence that shifts in the dietary MUFA/SFA ratio affect cognition in the general population1 and our own data relating to the reversible effects of this ratio on physical activity behavior and mood3, we hypothesized that diets high or low in PA would differentially activate brain networks associated with working memory using functional magnetic resonance imaging (fMRI) as well as affect systemic inflammation.
2. Material and Methods
2.1 Subjects, screening, and design
This study was approved by the University of Vermont (UVM) Institutional Review Board (IRB). The clinical aspects were managed at the UVM Clinical Research Center (CRC) and the imaging was completed at the UVM MRI Center for Biomedical Imaging. The subjects were derived from a sub-set of young adults participating in a randomized, double-masked, cross-over study of lean and obese adults in order to determine how dietary PA intake affected PA oxidation, insulin sensitivity, and inflammatory signaling (“parent protocol”), but our priorities for recruitment of women for the larger study necessitated our studying only women with respect to this sub-study using fMRI5,10. Supplementary Figure 1 depicts the consort diagram for this sub-study. Twelve, healthy, lean or obese, but non-diabetic women aged 18 – 40 years were recruited (age range: 20–36 years, mean ± SEM = 26.5±1.3 years; body mass index >18<25, n=7, or >30, n=5). Exclusion criteria were similar to our previous studies5,10,11.
As previously reported6, we used two dietary history techniques to assess our subjects’ habitual intake. In the cohort of subjects reported here, the habitual intake was as follows (% kcal): protein, 15.8; carbohydrate, 49.7; total fat, 36.6; saturated fat, 13.6; and monounsaturated fat, 11.8 (MUFA/SFA ratio, 0.93) respectively. The SFA intake is higher than is usually recommended for optimal cardiovascular health12. After screening to rule out relevant health problems, all subjects ingested a low fat/low-PA, baseline/control diet for seven days (Protein, 19.7 % kcal; Carbohydrate, 51.6% kcal; Fat, 28.4% kcal; PA, 5.3% kcal; OA, 15.9% kcal)5,10. This diet was patterned after the Therapeutic Lifestyles Diet12. Then, the subject participated in a cross-over study of two, 3-week experimental, low glycemic load diets, administered in random order: a diet high in PA (HPA; Fat, 40.4% kcal; PA, 16.0% kcal; OA,16.2% kcal; linoleic acid, 5.0% kcal; MUFA/SFA = 0.88); a diet low in PA and high in OA (HOA; Fat, 40.1% kcal; PA, 2.4% kcal; OA, 28.8% kcal; linoleic acid, 6.4% kcal; MUFA/SFA = 10.1)(based on analysis at Covance Laboratories, Madison, WI)5,10. For all 3 diets, FA composition was varied by adding oil blends to the six precisely formulated meals comprising the control diet (breakfast, lunch, and dinner, for two days) and nine meals comprising the experimental diets (meals for 3 days). The foods, including chicken and turkey (the only sources of meat) were all very low in fat. Thus, FA were mainly provided by vegetable oil blends appropriate to each diet (Natural Oils International, Inc., Simi Valley, California). The HPA and HOA diets otherwise contained the exact same foods with a three-day rotating menu. These oils, at room temperature, were mixed with food that had been warmed; thus, these oils were not used for cooking. The oil blend for the control diet consisted of palm oil (36.9%), high oleic sunflower oil (19.3%), and hazelnut oil (43.8%). The HPA oil blend consisted of palm oil (89%), peanut oil (6.75%), and virgin olive oil (4.25%), and the HOA “blend” consisted only of hazelnut oil. The HOA and HPA diets had identical, low glycemic loads (10.7, average of the three days of menus)3.
All food and drink, except water, were provided by the CRC, and body weight remained stable throughout the study since we adjusted the energy intake as required to maintain a constant body weight over the 8 weeks of the study4. The subjects reported to the CRC in the morning, Monday – Friday, during each of the Control and Experimental Diet periods. The subjects ate their breakfast there on those days and were given instructions regarding convenient ways to add the oils to food items on each of the menus. In addition, the subjects received advice and support regarding the requirements of the study during these visits to the CRC3,4,6. Subjects also were given instructions to use spatulas, provided by the CRC, to help scrape all oil from its container. Each day, the subjects completed and signed a questionnaire attesting to or commenting about their having eaten all the food (and food oil) and to not having consumed any food or drink, except water, not on the menu. In addition, all food and oil containers were inspected each day to be sure all food and oil were consumed. Any food or dietary oil that was left over in the containers was weighed, and the data used to construct a modified food intake for that day. Generally, we have only encountered occasional failure to eat all the food each day. In one previous study4, we found that the average number of days (out of 56) when some food was returned during the HPA and HOA diets was 1.33 and 1.67, and the average daily consumption of the oil for the HPA and HOA diets as a percentage of total oil administered was 99.9% and 99.2% (127.8 and 127.6 g/d).
The primary outcome was the blood oxygen level dependent measure from the fMRI during a working memory task. fMRI studies were completed in the fasted state, on day 16 of each experimental diet (after 15 days of diet). On day 8 of the Control/baseline diet and on the 22nd day of each experimental diet (HPA and HOA), blood was collected in the fasted state for measurement of cytokines in plasma and from LPS-stimulated PBMCs.
2.2 fMRI working memory task and analysis
All subjects were imaged on a Philips Achieva 3.0 Tesla MRI. fMRI was performed using EpiBOLD (echoplanar blood oxygenation level dependent) imaging using a single-shot sequence (TR 2500 ms, TE 35 ms, flip angle 90 degrees, 1 NSA for 197 volumes). Resolution was 2.5 mm × 2.8 mm × 4 mm. Thirty-four contiguous slices 4 mm thick with no gap were obtained in the axial oblique plane parallel to the AC-PC plane using a FOV of 240 mm and a matrix size of 128 × 96. Field map correction for magnetic inhomogeneities was accomplished by acquiring images with offset TE at the end of the functional series. fMRI acquisition and preprocessing procedures were similar to our prior studies 11.
The fMRI task was a visually presented verbal N-back used to probe working memory circuitry. Participants saw a string of consonants (except L, W, and Y), presented in upper case letters, one every three seconds. Four conditions were presented: 0-back, 1-back, 2-back, and 3-back. The 0-back control condition had a minimal working memory load; participants were asked to decide if the current letter matched a single target letter that was specified before the epoch began. In the 1-, 2-, and 3-back conditions, participants indicated whether the current letter on the screen matched a letter that was either 1, 2 or 3 back in the sequence.
The 0-, 1-, 2-, and 3-back conditions were repeated three times in a counterbalanced order such that the same condition was not repeated two times in a row. In this block design task, participants responded to nine items in each block that took 27 seconds. A rest break followed with a plus sign (+) fixation for 12 seconds. The total time of the task was 8 minutes 12 seconds. Participants practiced the N-back task before the scanning session to ensure that they understood task instructions.
Statistical analyses involved deriving one mean image per individual for the contrast of interest in the activation task (e.g., 2-back minus 0-back) after accounting for the hemodynamic response function. These contrast images were then used for the second level paired t-test to examine diet effects on brain functioning. To correct for multiple comparisons, we used a gray matter mask generated from the current data and the cluster-level statistical threshold estimator from Brain Voyager QX to estimate a minimum cluster size threshold based on the approach of Forman et al.13 that estimated a minimum cluster size of 12 voxels in functional space (3×3×3) at α=0.005.
2.3 Metabolic assays
The FA composition of serum phospholipids (phosphatidylcholine, phosphatidylethanolamine, and cardiolipin) was analyzed by flame ionization detector gas chromatography4,5.
2.4 Measurement of cytokines in plasma and secreted by PBMCs
Plasma cytokines were measured from all 12 subjects5; cytokine secretion by PBMCs was measured on only 11/12 subjects because of technical issues5.
2.5 Data analysis
This study employed a two-treatment, two-period, two-sequence cross-over design. Diet effects were analyzed using a repeated measures analysis of variance, including sequence, period, and treatment effects, with the baseline value as a covariate, when available4,5.
3. Results
3.1 Working memory-related brain activation and performance
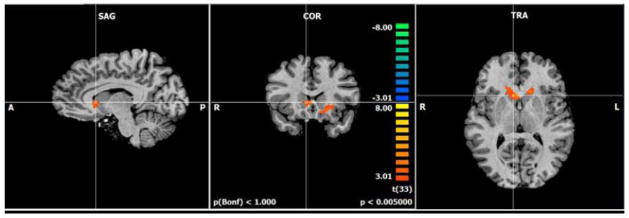
The working memory task showed the expected 14 bilateral frontal, parietal, cerebellar, anterior cingulate, and basal ganglia network activation. There was activation for the 2-back minus 0-back contrast during the HPA diet compared to the HOA diet in the right caudate nucleus and left putamen in the basal ganglia (Figure 1; Table 1).
Figure 1.
Greater brain activation was found after the HPA diet compared to the HOA diet for the 2-back minus 0-back conditions (p<.005; n=12) in the right caudate and left putamen during fMRI scanning. Orange colors represent activation that is greater for HPA diet compared to the HOA diet on the 2-back condition compared to the 0-back condition. Hash marks are centered on the right caudate head. The fMRI contrast images were analyzed with standard second level repeated measures ANOVA in Brain Voyager using diet as a within-subjects factor. To correct for multiple comparisons, we used a gray matter mask generated from the current data. We then used the cluster-level statistical threshold estimator from Brain Voyager QX to estimate a minimum cluster size threshold. Abbreviations used: fMRI, functional magnetic resonance; HOA, high oleic acid diet; HPA, high palmitic acid diet
Table 1.
Effects of HPA diet compared to the HOA diet for the 2-back minus 0-back contrast including Talairach coordinates, cluster size (mm3), region descriptions (Brodmann’s areas, BA), t values and uncorrected voxel-level p values.
| Contrast | Coordinates | Cluster Extent | Region Description | t value | p value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 2-back – 0-back | |||||||
| HPA-HOA | 5 | 16 | 6 | 605 | Right caudate head | 5.14 | <0.001 |
| −25 | 37 | 9 | 1574 | Left putamen | 4.42 | <0.001 | |
3.2 FA composition of serum phospholipids
During the HPA diet, the PA/OA ratio was 67–69% higher in serum phosphatidylcholine (p<0.0001), phosphatidylethanolamine (p=0.005), and cardiolipin (p<0.0001) compared to the HOA diet (Figure 2). These data provide evidence that the diets were ingested as intended and had the anticipated effects on cellular lipids 3,4,6.
Figure 2.
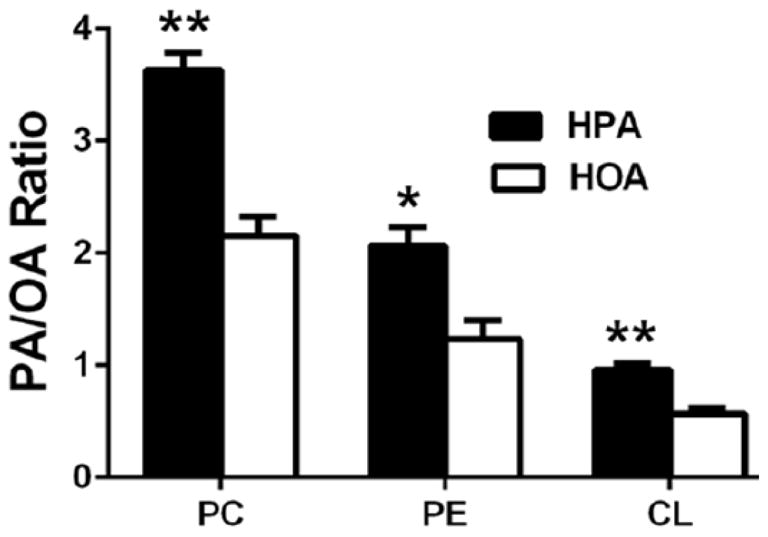
The HPA diet was associated with higher PA/OA ratios in serum phosphatidylcholine (PC), phosphatidylethanolamine (PE), and cardiolipin (CL). Blood samples were collected from overnight-fasted subjects at the end of the baseline diet and each experimental diet (HPA, HOA). The fatty acid content of serum PC, PE, and CL was measured using thin layer chromatography followed by gas chromatography (see Methods). Results are mean ± SEM (n=12). * p = 0.005; ** p ≤ 0.0001 for diet effects.
3.3 Secretion of cytokines by LPS-stimulated PBMCs and plasma cytokine concentration
Compared to the HOA diet, during the HPA diet, there was a higher secretion of IL-18 (p=0.015) and a trend for higher IL-1β secretion (p=.066) from LPS-stimulated PBMCs, consistent with enhanced activation of the Nucleotide Oligomerization Domain (NOD)-Like Receptor Protein (NLRP3) inflammasome (Figure 3A). The HPA diet also was associated with higher plasma concentrations of IL-6 (p=0.04) and IL-1β (p=0.05), indicative of activation of both toll-like receptor-4 (TLR4) and the NLRP3 inflammasome in vivo (Figure 3B). The plasma concentration of TNFα trended upward (36%) during HPA (p=0.09; Figure 3B). However, we observed no statistically significant correlations between diet-change in plasma concentration of cytokines or PBMC secretion of cytokines and respective diet-change in activation of those brain networks responsive to the working memory task.
Figure 3.
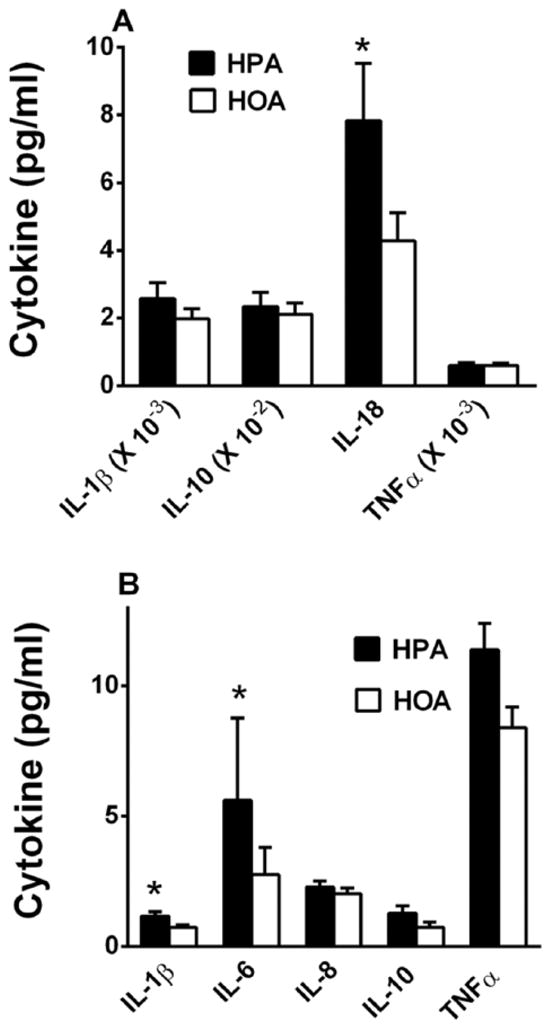
A higher PA/OA ratio in the diet enhanced LPS-stimulated cytokine production by PBMCs and increased plasma concentrations of pro-inflammatory cytokines. A. LPS-stimulated cytokine production by PBMCs after HPA and HOA diets (n=11). PBMCs were collected from overnight-fasted subjects at the end of the baseline diet and each experimental diet (HPA, HOA) and stimulated in vitro for 24 hr. with 1 ng/ml lipopolysaccharide. Secreted cytokines were measured by BioPlex or ELISA (see Methods). In order to display all the cytokines in the same graph, actual cytokine concentrations were multiplied by the respective correction factors, shown on the abscissa. IL-1β trended upward during the HPA diet (p=0.066). B. Plasma concentrations of pro-inflammatory cytokines after the HPA and HOA diets (n=12). Results are mean ± SEM. * p<0.05 for diet effects.
4. Discussion
This study is the first to examine effects of varying the dietary PA/OA ratio on human brain functioning. Specifically, working memory-related brain activation was increased in the caudate and putamen of the basal ganglia after two weeks of a diet high in PA compared to a diet low in PA and high in OA. Additionally, we confirmed previous data5 suggesting that the HPA diet resulted in relatively increased secretion of some cytokines modulated by TLR4 and the NLRP3 inflammasome.
While it is recognized that glucose and amino acids alter brain function (e.g.15,16), our study provides an important indication that another class of macronutrients, FAs, appear to alter brain activation during a cognitive stimulus. These data add to an emerging concept that, as with other sources of dietary energy, FAs, with their own unique chemical properties, affect neuronal activity, perhaps via changes in inflammatory signaling2,7,8,17. Thus, in future considerations of the health effects of various food sources of dietary FAs, brain effects may need to be taken into account.
The brain activation data showed differences between the HPA and HOA diets in the caudate and putamen during a working memory task. Working memory involves the active maintenance, manipulation, and updating of information in memory over a short period of time 18. The striatum, which includes the caudate and putamen, has been shown to be involved in the updating of information in working memory 19,20. Additionally, the striatum is involved in reward responses (e.g., 21), normal eating behaviors 22, and voluntary motor control 23. The current study showed that a high PA diet increased activation in the striatum and this effect was reversible with a diet low in PA and high in OA. The mechanisms by which these FA interventions affected brain functioning, aside from the possible link to inflammatory signaling, remain to be determined.
One prior study employing resting-state MRI (no functional task) found that 12 weeks of a high saturated FA diet resulted in decreased intrinsic resting brain activity in the hippocampus and inferior parietal cortex, but there was no change in resting brain activation in subjects fed a diet enriched with monounsaturated FAs 7. It is difficult to compare the results of a resting state study with a task-based study like the one described here, and further studies are needed to understand the influence of FAs on brain functioning.
The present study and two of our previous studies suggest that the HPA diet relatively increases inflammation and specifically IL-1β and IL-6 secretion 4,5. Animal studies suggest that inflammation also might be a mechanism for altering normal brain function, where neuronal integrity is preserved 24. Blocking TLR4 or the use of a neutralizing IL-6 antibody enhanced insulin signaling in the brain and improved brain function, including enhanced sleep efficiency and locomotion8; this observation could be relevant to our present findings as well our previous observation that the HPA diet was associated with higher circulating concentration of IL-64 and with lower physical activity3.
It is possible that brain-derived neurotrophic factor (BDNF) is at the nexus where a high dietary PA/OA ratio, enhanced systemic inflammation, and alterations in brain function converge. BDNF is among a group of neurotrophins which support synaptic plasticity and is required for hippocampus-mediated learning, as well as acting as both a neurotransmitter and neuromodulator that affects the pre-synaptic release of other neurotransmitters25. Molteni et al.26 showed dietary fatty acid effects on brain BDNF. They fed a high sugar and high SFA diet to rats; compared to controls, brain mRNA expression of BDNF was reduced and learning was impaired within two months of the diet. The brain protein level of BDNF was also lower, when measured after 6 months of diet26. BDNF signals partially via the insulin receptor substrate-1, phosphatidylinositol 3-kinase, and Akt pathway, similar to insulin27, but there also is evidence that intracerebroventricular infusion of insulin increased BDNF protein level in the hippocampus of young (4 months) but not older (24 months) rats28 and that insulin signaling is required for normal BDNF transport and hippocampal synaptogenesis 29.
There are obvious limitations to inferences about brain function in humans that can be drawn from measurements of inflammation originating in the peripheral blood and the stochastic variables of brain function such as those obtained from fMRI imaging. In addition, we cannot from this small study determine the clinical significance of increased or decreased brain activation. However, the present fMRI findings add to our previous report3 implicating that brain function is reversibly affected by a lower dietary PA/OA ratio, which, in turn, consistently is associated with lower inflammation. Future studies might explore effects on cognitive performance, such as episodic memory, and additional biomarkers for the effects of dietary FA composition, such as circulating BDNF concentration.
4.1 Conclusion
This crossover study in young women revealed reversible effects on brain functioning and cytokine production of a high PA diet and a low PA, high OA diet over a brief period. Diet may be an intervention that can enhance or impair brain performance, possibly via a mechanistic pathway that involves inflammation, brain insulin signaling, and perhaps neurotrophic effects (e.g. brain levels of BDNF).
Supplementary Material
Acknowledgments
This work was supported by NIDDK R01DK082803 and DoE SC 0001753. The authors thank the research nursing staff of the University of Vermont CRC for their hard work and support of this study and our volunteers for their dedication to clinical research. The authors declare no competing conflicts of interest. JAD, CLK, and MEP designed the study. JAD, CLK, MEP, JN, KIC, DBE, JM, and EKT conducted the research. JAD, CLK, JYB, MEP, KIC, and JM analyzed the data and performed the statistical analyses. JN, JAD, and CLK reviewed MRI data. JAD, CLK, and MEP wrote the paper. JAD and CLK each have equal and primary responsibility for final content. All authors read and approved the final manuscript.
List of abbreviations
- FA
fatty acid
- fMRI
functional magnetic resonance imaging
- HOA
low PA, high OA diet
- HPA
high PA diet
- IL
interleukin
- LPS
lipopolysaccharide
- MUFA
monounsaturated fatty acids
- NLRP3
Nucleotide Oligomerization Domain (NOD)-Like Receptor Protein
- OA
oleic acid
- PA
palmitic acid
- SFA
saturated fatty acids
- TLR4
toll-like receptor-4
- TNFα
tumor necrosis factor-α
Footnotes
This study has been registered at http://www.clinicaltrials.gov/ as University of Vermont Protocol Record R01DK082803.
Clinicaltrials.gov registration number: University of Vermont Protocol Record R01DK082803.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age. Epidemiology (Cambridge, Mass) 2013;24:490–9. doi: 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson AJ, Bayer JL, Baker LD, Cholerton B, VanFossen B, Trittschuh E, Rissman RA, Donohue MC, Moghadam SH, Plymate SR, Craft S. Differential Effects of Meal Challenges on Cognition, Metabolism, and Biomarkers for Apolipoprotein E varepsilon4 Carriers and Adults with Mild Cognitive Impairment. J Alzheimers Dis. 2015;48:205–18. doi: 10.3233/JAD-150273. [DOI] [PubMed] [Google Scholar]
- 3.Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr. 2013;97:689–97. doi: 10.3945/ajcn.112.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kien CL, Bunn JY, Poynter ME, Stevens R, Bain J, Ikayeva O, Fukagawa NK, Champagne CM, Crain KI, Koves TR, Muoio DM. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes. 2013;62:1054–63. doi: 10.2337/db12-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kien CL, Bunn JY, Fukagawa NK, Anathy V, Matthews DE, Crain KI, Ebenstein DB, Tarleton EK, Pratley RE, Poynter ME. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. The Journal of nutritional biochemistry. 2015;26:1599–606. doi: 10.1016/j.jnutbio.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr. 2014;99:436–45. doi: 10.3945/ajcn.113.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartorius T, Ketterer C, Kullmann S, Balzer M, Rotermund C, Binder S, Hallschmid M, Machann J, Schick F, Somoza V, Preissl H, Fritsche A, Haring HU, Hennige AM. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes. 2012;61:1669–79. doi: 10.2337/db11-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartorius T, Lutz SZ, Hoene M, Waak J, Peter A, Weigert C, Rammensee HG, Kahle PJ, Haring HU, Hennige AM. Toll-like receptors 2 and 4 impair insulin-mediated brain activity by interleukin-6 and osteopontin and alter sleep architecture. Faseb j. 2012;26:1799–809. doi: 10.1096/fj.11-191023. [DOI] [PubMed] [Google Scholar]
- 9.Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kien CL, Matthews DE, Poynter ME, Bunn JY, Fukagawa NK, Crain KI, Ebenstein DB, Tarleton EK, Stevens RD, Koves TR, Muoio DM. Increased palmitate intake: higher acylcarnitine concentrations without impaired progression of beta-oxidation. Journal of lipid research. 2015;56:1795–807. doi: 10.1194/jlr.M060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumas JA, Kutz AM, McDonald BC, Naylor MR, Pfaff AC, Saykin AJ, Newhouse PA. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiology of Aging. 2013;34:1145–7. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;6625:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 15.Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, Floel A. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. 2013;81:1746–52. doi: 10.1212/01.wnl.0000435561.00234.ee. [DOI] [PubMed] [Google Scholar]
- 16.Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino acids. 2013;45:419–30. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 17.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7:e30571. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baddeley AD. Working Memory. Oxford: Clarendon Press; 1986. [Google Scholar]
- 19.Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18:247–56. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 20.Marklund P, Larsson A, Elgh E, Linder J, Riklund KA, Forsgren L, Nyberg L. Temporal dynamics of basal ganglia under-recruitment in Parkinson’s disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–46. doi: 10.1093/brain/awn309. [DOI] [PubMed] [Google Scholar]
- 21.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 22.Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 23.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 24.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–86. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 25.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–20. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 27.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Archives of medical science: AMS. 2015;11:1164–78. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas CB, Kalinine E, Zimmer ER, Hansel G, Brochier AW, Oses JP, Portela LV, Muller AP. Brain Insulin Administration Triggers Distinct Cognitive and Neurotrophic Responses in Young and Aged Rats. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9494-6. [DOI] [PubMed] [Google Scholar]
- 29.Takach O, Gill TB, Silverman MA. Modulation of insulin signaling rescues BDNF transport defects independent of tau in amyloid-beta oligomer-treated hippocampal neurons. Neurobiol Aging. 2015;36:1378–82. doi: 10.1016/j.neurobiolaging.2014.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.