Abstract
Aim
Reactive oxygen species (ROS) are elevated in the retina, and mitochondria are damaged, resulting in capillary cell apoptosis and the development of retinopathy. Dyslipidemia is also considered as one of the major factors in its development, and our aim is to investigate the compounding effect of hyperlipidemia in retinopathy.
Methods
Retinal ROS, mitochondrial damage and vascular pathology were investigated in Zucker diabetic fatty rats (ZDF, type 2 diabetes model), during the age that spans from hyperlipidemia/pre-hyperglycemia (6 weeks), to severe hyperglycemia/moderate hyperlipidemia (~12 weeks), and ultimately to severe hyperglycemia/hyperlipidemia (20–40 weeks). For comparison, retina from streptozotocin-induced Wistar rats (type 1 diabetic for 10–40 weeks) was analyzed.
Results
Compared to age-matched lean rats, despite increased retinal cytosolic ROS in 6 weeks old ZDF rats, mitochondrial dysfunction and DNA damage were not detected, and in 12 weeks old ZDF rats, retinal mitochondria were dysfunctional, but mtDNA damage and vascular pathology (cell apoptosis and degenerative capillaries) were not detectable. Retina from 20 weeks old ZDF rats (hyperglycemic for 14 weeks or less) had significant mitochondrial dysfunction, mtDNA damage and vascular pathology, and similar abnormalities were observed in 40 weeks old ZDF rats. Although retinal mitochondrial dysfunction was observed in Wistar rats diabetic for 20 weeks, mtDNA damage and vascular pathology were not detectable till the duration of diabetes was further extended.
Conclusions
Hyperlipidemia, in a hyperglycemic milieu, potentiates mitochondrial damage and augments the development of retinopathy. Control of dyslipidemia in pre-diabetic patients may prevent/delay the development and the progression of this devastating disease.
Keywords: Diabetic retinopathy, Hyperlipidemia, Mitochondria
1. Introduction
Diabetes has become now the global epidemic of 21st century, and by 2031, 600 million people are projected to suffer from this disease. Among those, 90–95% cases will have type 2 diabetes, and obesity is considered as one of the major contributors to these staggering numbers. Type 2 diabetes comes with an additional burden of moderate to severe hyperlipidemia, and the characteristic features of diabetic dyslipidemia are high plasma triglycerides and low density lipoprotein cholesterol (LDL), and reduced levels of high density lipoprotein cholesterol. These patients are at substantially increased risk of developing macrovascular and microvascular complications including retinopathy and nephropathy [1, 2]. Retinopathy, a slow progressing disease, affects vast majority of patients with 20–25 years of diabetes. The pathogenesis of this blinding disease is complex and many biochemical, metabolic and molecular mechanisms are implicated in its development [3, 4]. Our previous work has shown that during initial stages of the disease, NADPH oxidase-2 (Nox2) is activated producing reactive oxygen species (ROS), and these ROS damage the mitochondria to initiate the apoptotic machinery, a phenomenon, which precedes the development of histopathology characteristic of diabetic retinopathy. Damaged mitochondrial DNA (mtDNA) compromises the electron transport system and continues to fuel into a futile cycle of free radicals [4–10]. Although hyperglycemia remains as one of the major instigators of the development of diabetic retinopathy, other systemic factors, including hyperlipidemia and hypertension are also closely associated with its development [11–13]. Early Treatment of Diabetic Retinopathy Study (ETDRS) has shown a relationship between retinal hard exudates and total and LDL cholesterol [14]. In addition, clinical trials using long-term administration of lipid-lowering therapy, fenofibrate, has documented a significant relative reduction of progression of diabetic retinopathy in patients with background retinopathy [15]. In a rat model of type 2 diabetes, dietary supplementation with docosahexaenoic acid prevents the development of retinopathy [16]. In vitro models have also shown close correlation between lipoxygenase pathway and vascular permeability, and between modified LDL and pericyte loss in diabetic retinopathy [17–19]. Recent study from our laboratory using retinal endothelial cells has further documented the role of lipotoxicity, and the results have shown that in a glucotoxic environment, addition of lipotoxicity exacerbates ROS production and mtDNA damage [10]. The exact mechanism by which hyperlipidemia impacts the development of retinopathy in type 2 diabetic patients, however, remains elusive.
The aim of this study is to investigate how hyperlipidemia contributes to the development of diabetic retinopathy. Mitochondrial damage and vascular pathology were investigated in the retina from Zucker diabetic fatty (ZDF) rats during the age that spans from pre-diabetic, but hyperlipidemic (6 weeks), to overt hyperglycemic and hyperlipidemic (40 weeks). For comparison, the retina from streptozotocin-induced Wistar rats (type 1 diabetes) was also analyzed at duration of diabetes when retinal mitochondria are intact and the histopathology characteristic of diabetic retinopathy is not detectable (10 weeks of diabetes), to the duration when mitochondrial damage and histopathology can be detected in the retinal vasculature (40 weeks of diabetes).
2. Methods
2.1. Animal models
Male, ZDF rats (Charles River Laboratories, MA, USA) at 6 weeks (normal glycemia with mild hyperlipidemia), 12 weeks (hyperglycemia and moderate hyperlipidemia), and 20–40 weeks of age (hyperglycemia and overt hyperlipidemia) were used as type 2 diabetes animal model [20]. For comparison with type 1 diabetic animal model, diabetes was induced in 7–8 weeks old male Wistar rats (Harlan Laboratories, Indianapolis, IN, USA) by a single injection of streptozotocin (55mg/kg BW), and the rats were maintained diabetic for 10–40 weeks. Age-matched Zucker lean rats and normal Wistar rats served as their respective controls. The entire animal colony was weighed two times/week, non-fasting blood glucose was monitored once every week, and glycated hemoglobin (GHb) was quantified every 8–9 weeks. While ZDF rats started to present hyperglycemia around 7 weeks of age, Wistar rats were hyperglycemic within 3 days of streptozotocin administration. Animals were sacrificed by CO2 asphyxiation, and blood (2–3 ml) was collected by heart puncture. One eye was fixed in formalin for histopathology, and the retina isolated from the other eye was frozen immediately in liquid nitrogen, as reported previously by us [6, 9, 21]. Treatment of the animals conformed to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research and NIH guidelines, and was approved by the Wayne State University’s Animal Care and Use Committee.
2.1.1. Serum lipid profile
Serum triglycerides and cholesterol/triglyceride-rich very low-density lipoprotein (VLDL) were quantified using commercial assay kits (#ab65336 and ab65390 respectively from Abcam Inc., Cambridge, MA, USA) following the manufacturer’s instructions.
2.2. Retinal damage
2.2.1
Mitochondrial dysfunction and damage were investigated by evaluating their membrane permeability, activity of the electron transport system and mtDNA damage. Mitochondria were isolated from the retina using mitochondria isolation kit and following the manufacture’s instructions (Pierce, Rockford, IL); these preparations are devoid of nuclear contamination [21]. Permeability of the mitochondrial membrane was quantified by measuring its swelling; briefly, mitochondrial protein (5–10 μg) was equilibrated for 30 seconds at 25°C in an a 60μl assay volume containing 3 mM HEPES buffer (pH 7.4) supplemented with 215 mM mannitol, 71 mM sucrose and 5 mM succinate. To induce the transition, calcium chloride (400 μM) was added, and decrease in the absorbance at 540nm was followed for 5 minutes. The extent of swelling was calculated as a percentage of swelling with respect to the maximum swelling achieved by exposure to calcium chloride [5].
The activity of coenzyme Q cytochrome-c reductase (complex III) was assayed in a 60μl total assay volume containing 0.3–0.5μg mitochondrial protein, 40 μM reduced decylubiquinone and 2 mM KCN. The reaction was initiated by 50 μM cytochrome c, and its reduction was measured at 550nm [5].
Damage to mtDNA was assessed by extended-length PCR; the long (13.4kb= Fwd AAAATCCCGCAAACAATGACCACCCC and Rev GGCAATTAAGAGTGGGATGGAGCCAA), and the short (210bp= Fwd CCTCCCATTCATTATCGCCGCCCTTGC and Rev GTCTGGGTCTCCTAGTAGGTCTGGGAA) regions of mtDNA were amplified, and the decrease in the ratio of long to short amplicons indicated damage to the mtDNA [6, 9, 10, 21].
2.2.2
Reactive oxygen species were quantified in the retinal homogenate (5–10μg protein) using 2′,7′-dichlorofluorescein diacetate (DCHFDA; Sigma- Aldrich, St Louis, MO, USA) by measuring fluorescence at 485nm and 530nm as excitation and emission wavelengths respectively [9, 10, 22].
2.2.3
Nox2 activity was measured in 10μg retinal protein using lucigenin as an electron acceptor and NADPH as substrate, in the presence or absence of 0.2 mM apocynin. Since a small molecular weight GTP-binding protein Ras-related C3 botulinum toxin substrate 1 (Rac1) helps in the membrane stability of Nox2 holoenzyme, Rac1 activation was determined by a G-LISA based colorimetric assay (Cytoskeleton, Denver, CO, USA) [9, 10, 22].
2.2.4
Co-localization of Nox2 and Rac1 was determined in 10μm retinal cryosections using antibodies against Rac1 and Nox2 (Santa Cruz Biotechnology, CA, USA and Abcam Inc., Cambridge, MA, USA, respectively, both at a dilution of 1:500). The secondary antibodies included Texas red-conjugated for Nox2 (Molecular Probes, Eugene, OR, USA) and fluorescein isothiocyanate (FITC)-conjugated for Rac1 (Vector Laboratories, Burlingame, CA, USA). After mounting the slides in 4″,6-diamidino-2-phenylindole, dihydrochloride (DAPI)- containing medium (Vectashield-DAPI; Vector Laboratories), they were imaged under a ZEISS ApoTome fluorescence microscope at 40X magnification (Carl Zeiss, Chicago, IL, USA) [22, 23].
2.2.5. Retinal capillary cell apoptosis and histopathology
The retina from formalin-fixed eyes was rinsed overnight with running water, and was incubated at 37°C with 3% crude trypsin (Invitrogen-Gibco, Grand Island, NY, USA) containing 200 mM sodium fluoride for 45 to 70 minutes. After gently brushing away the neuro-retinal tissue, trypsin-digested microvasculature mounted on a glass slide was stained with terminal deoxyribonucleotide transferase-mediated dUTP nick-end labelling (TUNEL) stain (In Situ Cell Death kit; Roche Molecular Biochemicals, Indianapolis, IN, USA). The microvessels were then rinsed, and stained with periodic acid Schiff-hematoxylin to count the degenerative capillaries [21, 24].
2.2.6
Retinal function was determined by evaluating the multi-focal electroretinogram (ERG) responses in dark adapted animals using Ocuscience HMsERG system [24]. The amplitude of ‘b’ wave and implicit time were measured using a silver embedded thread eye electrode, placed at the corneal surface through a thin layer of 1% methylcellulose, and the dark-adapted intensity-response series was recorded using a series of Ganzfeld flashes with 100–25,000 mcd.s/m^2 intensities.
2.3. Statistical analysis
Statistical analysis was carried out by Sigma Stat software. Data are expressed as means ± SD. The Shapiro-Wilk test was used to test for normal distribution of the data, and for variables with normal distribution, student’s t test was used for comparing two groups and one way analysis of variance followed by Bonferroni’s was applied for multiple groups. For data that did not present normal distribution, Mann-Whitney U or Kruskal-wallis followed by Dunn’s test was performed. A p value <0.05 compared to age-matched lean rat (for type 2 diabetes animal model), or normal rat (type 1 diabetes animal model), was considered as statistically significant.
3. Results
3.1. Animal model of type 2 diabetes
As shown in table I, although 6–20 weeks old ZDF rats had consistently higher body weights compared to age-matched lean rats (p<0.05), 40 weeks old ZDF rats and lean rats had similar body weights. Around 7 weeks of age, ZDF rats began to present some hyperglycemia. While 6 weeks old ZDF rats had normal blood glucose with 60% increase in triglycerides and cholesterol compared to age-matched lean rats, 12 weeks old ZDF rats were severely hyperglycemic and hyperlipidemic with GHb, serum triglyceride and VLDL levels elevated by ~2 fold. Although 20–40 weeks old ZDF rats had 2 fold higher GHb values, serum triglycerides and VLDL levels were elevated by over 3 fold compared to age-matched lean rats.
Table I.
Hyperglycemia and hyperlipidemia in animal models
| Group | BW (g) | Glucose (mg/dl) | GHb (%) | Triglycerides (mg/dl) | VLDL (mg/dl) | Cholesterol (mg/dl) | ||
|---|---|---|---|---|---|---|---|---|
| Zucker rats (Type 2 diabetes) | ||||||||
| Age | ||||||||
| 6 weeks | Lean | 118±6 | 79±6 | ND | 280±17 | 96±21 | 111±3 | |
| ZDF | 157±7* | 86±11 | ND | 460±77* | 250±67* | 181±9* | ||
| 12 weeks | Lean | 261±49 | 71±7 | 6.7±1.0 | 329±45 | 82±18 | 136±14 | |
| ZDF | 372±63* | 228±47* | 12.7±2.8* | 755±119* | 309±23* | 230±8* | ||
| 20 weeks | Lean | 328±20 | 69±3 | 6.9±0.9 | 359±54 | 178±38 | 153±6 | |
| ZDF | 430±45* | 200±96* | 10.9±3.3* | 1402±42* | 420±29* | 254±7* | ||
| 40 weeks | Lean | 377±106 | 72±20 | 6.4±2.2 | 463±97 | 144±64 | 156±16 | |
| ZDF | 420±42 | 289±78* | 12.0±1.8* | 1666±178* | 444±27* | 272±18* | ||
| Duration of diabetes | Wistar rats (Type 1 diabetes) | |||||||
| 10 weeks | Normal | 363±33 | 89±24 | 5.8±0.4 | 233±38 | 105±19 | ND | |
| Diabetes | 292±16 | 351±89* | 11.3±1.3* | 212±32 | 149±41 | ND | ||
| 20 weeks | Normal | 455±23 | 79±10 | 6.0±1.3 | 194±19 | 110±16 | ND | |
| Diabetes | 385±11 | 388±60* | 12.7±0.3* | 310±46* | 191±23* | ND | ||
| 40 weeks | Normal | 522±67 | 76±3 | 5.4±0.6 | 306±50 | 137±17 | ND | |
| Diabetes | 362±31* | 349±70* | 11.1±1.2* | 410±78 | 213±31* | ND | ||
Values are represented as mean ± SD from 5–8 rats in each group, with each measurement made in duplicate.
p<0.05 compared to age-matched lean rat (for type 2 diabetes model), or normal rat (type 1 animal model).
BW= body weight; GHb= glycated hemoglobin; VLDL= very low density lipoproteins; ND=not determined.
3.1.1
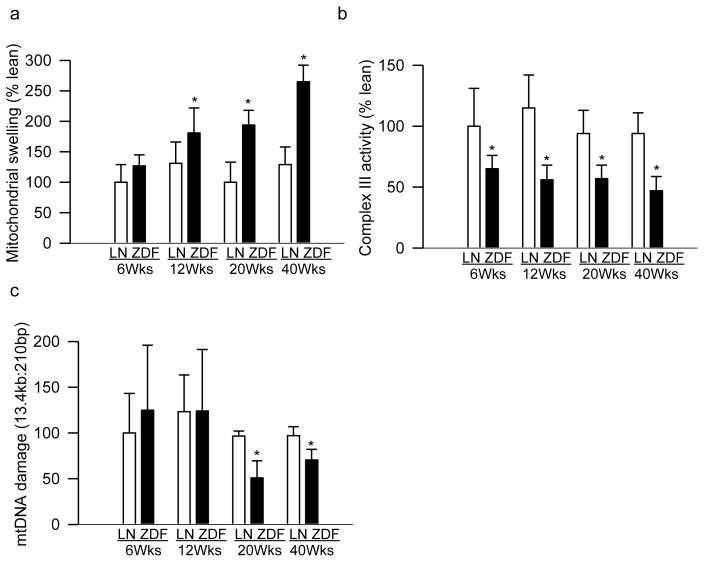
Mitochondrial dysfunction is implicated in the development of diabetic retinopathy including in the accelerated apoptosis of capillary cells [4, 25, 26]. To understand possible effects of hyperlipidemia on mitochondrial dysfunction, mitochondrial membrane permeability was evaluated. As early as 6 weeks of age, when ZDF rats were normoglycemic with slightly elevated triglycerides, retinal mitochondrial membrane permeability was increased by ~20% compared to age-matched lean rats, but the values failed to achieve any statistical significance. However, compared to age-matched lean rats, 12 weeks old ZDF rats (hyperglycemia for 6 weeks or less) had 30% increase in mitochondrial membrane permeability (p=0.042), and 20 weeks old ZDF rats (hyperglycemia for 14 weeks or less) had ~2 fold increase in mitochondrial membrane permeability (Figure 1a).
Figure 1.
Temporal relationships between hyperglycemia-hyperlipidemia and retinal mitochondrial damage. (a) Mitochondrial swelling was determined by quantifying the collapse of mitochondrial membrane potential and decrease in absorbance at 540nm, induced by calcium chloride, was recorded. (b) Complex III activity was determined by measuring reduction of cytochrome c at 550nm. (c) Damage to mtDNA was assessed by extended-length PCR and the ratio of 13.4kb to 210bp amplicons was calculated; decrease in long and short amplicons ratio is the indicative of mtDNA damage. Each measurement was performed in duplicate in 5–8 animals/group, and the values are represented as mean ± SD. Values obtained from 6 weeks (Wks) old lean rats were considered as 100%. *p< 0.05 compared to age-matched lean rat (LN); ZDF=ZDF rats.
The integrity of complex III of the respiratory chain is critical for mitochondrial homeostasis and its activity is decreased in the retina in diabetes [5, 27]; mitochondrial dysfunction was further confirmed by quantifying complex III activity. Complex III activity was decreased by 35% in the retinal mitochondria from 6 weeks old ZDF rats compared to age-matched lean rats. However, further worsening of hyperlipidemia in a hyperglycemic milieu did not exacerbate damage to the complex III, and the enzyme activity remained 40–50% lower in 20–40 weeks old ZDF rats (Figure 1b).
Retinal mtDNA is damaged in diabetes, and damaged mtDNA, by compromising electron transport system, fuels into a futile cycle of free radicals [4]; the effect of hyperlipidemia-hyperglycemia was investigated on retinal mtDNA damage. Although mtDNA damage was not observed in 6–12 weeks old ZDF rats, it was 35–50% higher in 20–40 weeks old ZDF rats compared to their age-matched lean rats (Figure 1c).
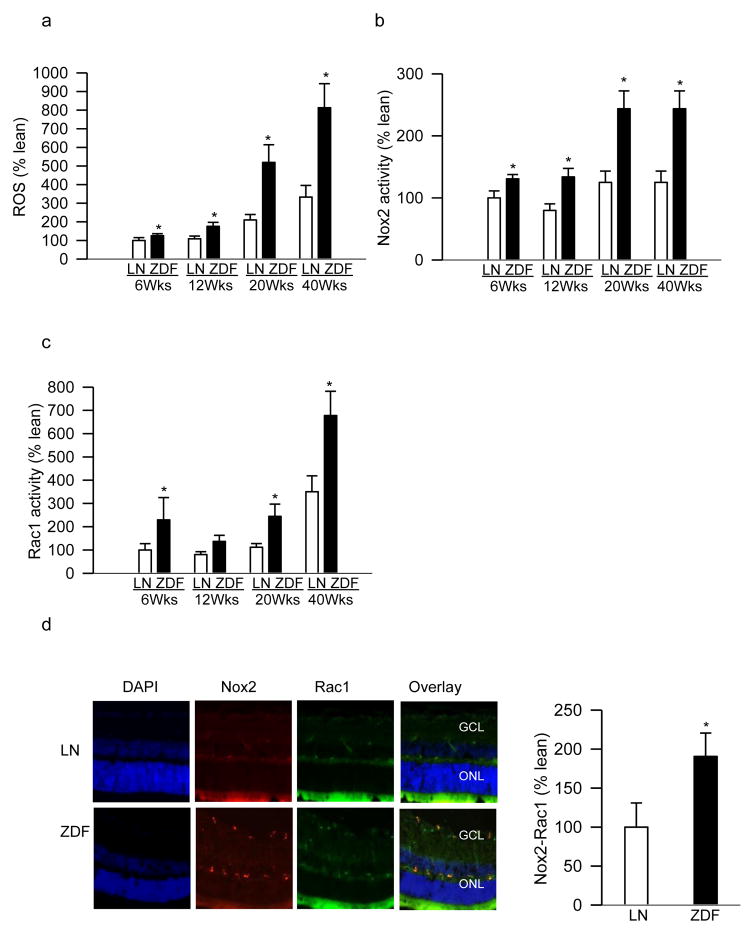
Increased diabetes-induced ROS in the retina are considered to precede mitochondrial damage [9, 10]; to investigate the mechanism of mitochondrial damage, total ROS levels were quantified. Compared to their age-matched lean rats, 6–12 weeks old ZDF rats had 60–80% increase in retinal ROS levels, and this increase in ROS was 3 fold in 20–40 weeks old ZDF rats (Figure 2a).
Figure 2.
Rac1-Nox2-ROS signaling in the retina of ZDF and lean rats. (a) Total ROS levels were quantified fluorometrically in 5–10μg retinal protein by DCHFDA. (b) Apocynin-sensitive Nox2 activity was measured in 5–10μg retinal protein using lucigenin as electron acceptor and NADPH as a substrate. (c) Rac1 activation was quantified (20–30μg protein) by a G-LISA colorimetric assay. (d). Nox2 and Rac1 were immuno-stained in retinal cryosections from 20 weeks (Wks) old rats (ZDF and lean) using Texas Red (red) and FITC (green)- conjugated secondary antibodies for Nox2 and Rac1 respectively. Sections were mounted with DAPI (blue) containing Vectashield mounting medium; the accompanying histogram represents co-staining of Nox2-Rac1 in the vasculature. Results are expressed as mean ± SD from 5–7 rats in each group, with each measurement made in duplicate. Values obtained from 6 weeks old lean rats are considered as 100%. *p<0.05 compared with age-matched lean rats (LN).
Initial increase in retinal ROS in diabetes is shown to involve cytosolic Nox2 activation [9, 10]; the effect of lipids on retinal Nox2 activity was investigated. Figure 2b shows that compared to their age-matched lean rats, Nox2 activity was increased by 30–50% in 6–12 weeks old ZDF rats, and by over 3 fold in 20–40 weeks old ZDF rats. In the same animals, 6 weeks old ZDF rats presented ~2 fold increase in retinal Rac1 activity, and Rac1 remained elevated in 20–40 weeks old ZDF rats compared to their age-matched lean rats (Figure 2c). The role of Rac1 in Nox2 activation was further confirmed by immunofluorescence microscopy. ZDF rats had significantly increased expressions of Rac1 and Nox2 compared to lean rats, and co-localization of these two proteins was ~90% higher in the retinal microvessels from ZDF rats, suggesting their involvement in vascular pathology (Figure 2d).
3.1.2. Vascular pathology
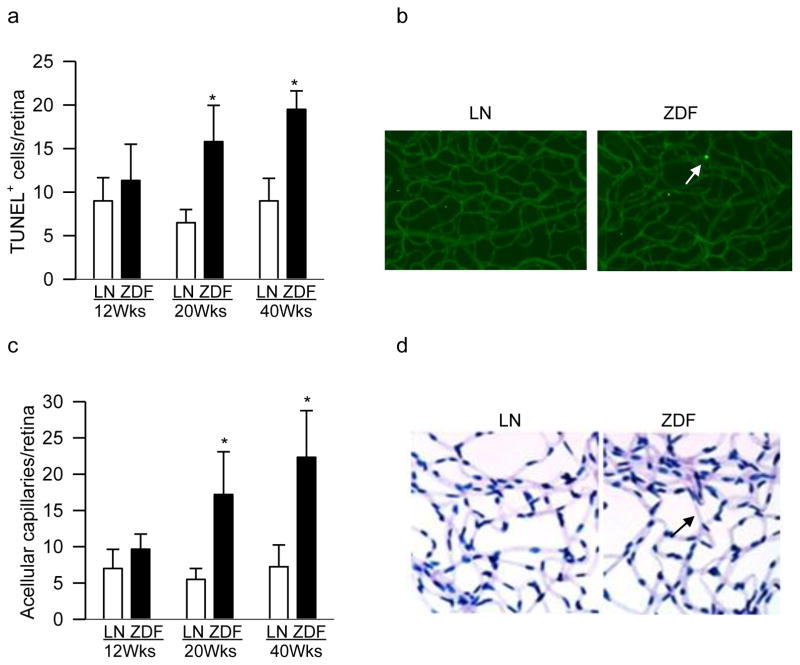
Despite severe hyperglycemia in 12 weeks old ZDF rats, retinal vasculature did not present any increase in TUNEL positive capillary cells (apoptotic), but in 20 weeks and 40 weeks old ZDF rats >2 fold increase in apoptotic capillary cells was detected as compared to the values obtained from their age-matched normal rats (Figure 3a). Figure 3b shows representative TUNEL staining in the retinal vasculature. Similarly, 12 weeks old ZDF and lean rats had only 4–6 degenerative retinal capillaries, and the numbers became 3–4 fold higher in 20–40 weeks old ZDF rats compared to age-matched lean rats (Figures 3c&d).
Figure 3.
Capillary cell apoptosis and histopathology in retinal microvasculature: (a) Trypsin-digested retinal microvessels from 12–40 weeks (Wks) old ZDF rats were TUNEL-stained, and TUNEL-positive cells were counted. (b) Representative TUNEL-stained retinal microvasculature from 40 weeks old ZDF and lean (LN) rats. A TUNEL positive cell is indicated with an arrow. (c) After TUNEL staining, the retinal microvasculature was stained with periodic acid Schiff-hematoxylin for acellular capillaries. (d) Representative periodic acid Schiff-hematoxylin-stained microvasculature from 40 weeks old ZDF and lean rats; the arrow indicates an acellular capillary. Values are represented as mean ± SD from 5–7 rats/group; *p< 0.05 compared with age-matched lean rat.
3.1.3. Retinal function
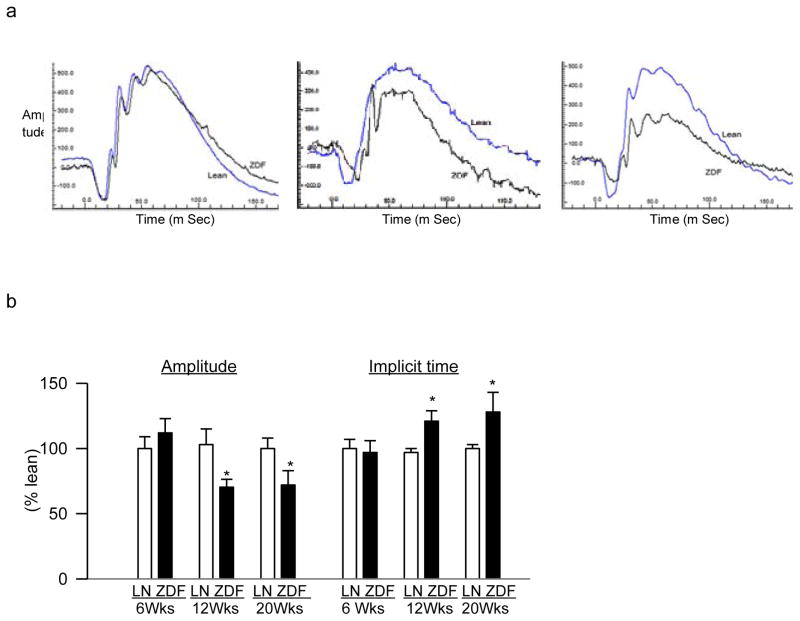
The hallmark histopathology associated with diabetic retinopathy is seen in the retinal microvasculature, but changes in the multifocal ERG are now also considered as predictive of its onset and progression [28]. Six weeks old ZDF and lean rats had similar amplitudes of ‘b’ wave (at 10,000 mcd.s/m^2) and implicit times, suggesting that the initial mild hyperlipidemia did not produce any detrimental effect on retinal function. However, 12 weeks old ZDF rats showed significant decrease in ‘b’ wave amplitude and prolonged implicit time, and with sustained severe hyperglycemia-hyperlipidemia (20 to 40 weeks old ZDF rats), amplitude of ‘b’ wave and the implicit time remained lower and higher respectively, compared to age-matched lean rats (Figure 4). The values obtained from 40 weeks old ZDF rats were similar to those obtained from 20 weeks old ZDF rats.
Figure 4.
Effect of hyperglycemia-hyperlipidemia on retinal dysfunction. ERG was performed in dark-adapted 6–40 weeks (Wks) old ZDF rats (ZDF) and lean rats (LN) using Ocuscience HMsERG system. A dark-adapted intensity-response series was recorded using a series of Ganzfeld flashes with intensities ranging from 100–25,000 mcd.s/m^2. Representative plots at 10,000 mcd.s/m^2 from 6, 12 and 20 weeks old ZDF (black) and lean (blue) are shown. (b) Amplitude of ‘b’ wave and implicit time, obtained from 6 weeks old lean rats are considered as 100%. The results are representative of 5 or more rats in each age group. *p< 0.05 vs age-matched lean rats.
3.2. Animal model of type 1 diabetes
Compared to age-matched normal rats, body weights of diabetic rats were 20–30% lower and the GHb values were ~2 fold higher. Triglyceride and VLDL levels were comparable in rats diabetic for 10 weeks and age-matched normal rats, but serum triglycerides were elevated by >30% and VLDL levels by about 80% in rats diabetic for 20–40 weeks. However, the values in diabetic Wistar rats were more than 2 fold lower than those observed in 20–40 weeks old ZDF rats (Table I).
3.2.1
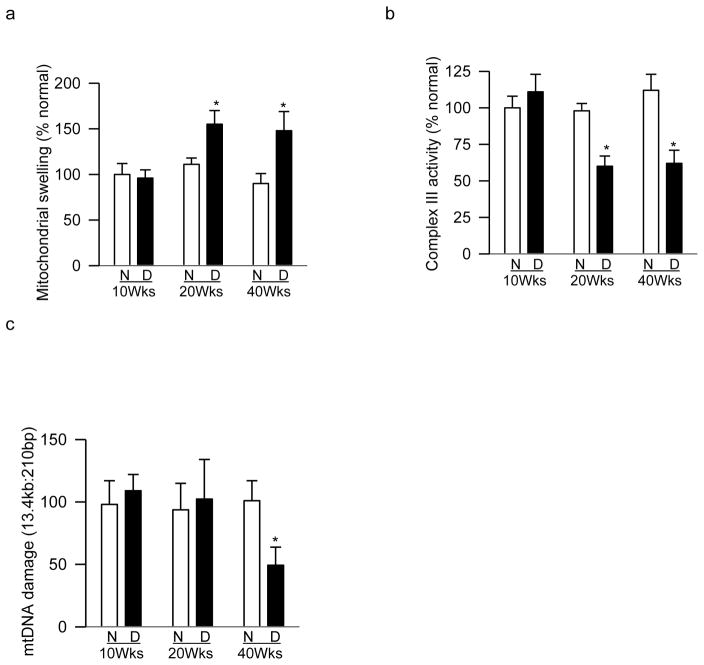
Ten weeks of diabetes had no effect on retinal mitochondrial membrane permeability, but rats diabetic for 20 weeks showed 35–60% increase in membrane permeability compared to values obtained from their age-matched normal rats (Figure 5a). Consistent with this, 10 weeks of diabetes did not alter complex III activity, but 40–50% decrease in complex III activity was observed in rats diabetic for 20 weeks (Figure 5b). In agreement with our previous results [29], although 20 weeks of diabetes produced no significant retinal mtDNA damage, significant increase in mtDNA damage was observed in rats diabetic for 40 weeks (Figures 5c).
Figure 5.
Duration of type 1 diabetes and retinal mitochondrial damage. (a) Mitochondrial swelling determined spectrophotometrically using calcium loading. (b) Complex III activity was determined by measuring reduction of cytochrome c at 550nm. (c) mtDNA damage was assessed by quantifying ratio of 13.4kb to 210bp amplicons. Each measurement was performed in duplicate in the retina from 6–8 animals/group, and represented as mean ± SD. Values obtained from normal rats are considered as 100%. N=normal and D= diabetes; *p< 0.05 compared to age-matched normal control rats.
3.2.2. Vascular pathology
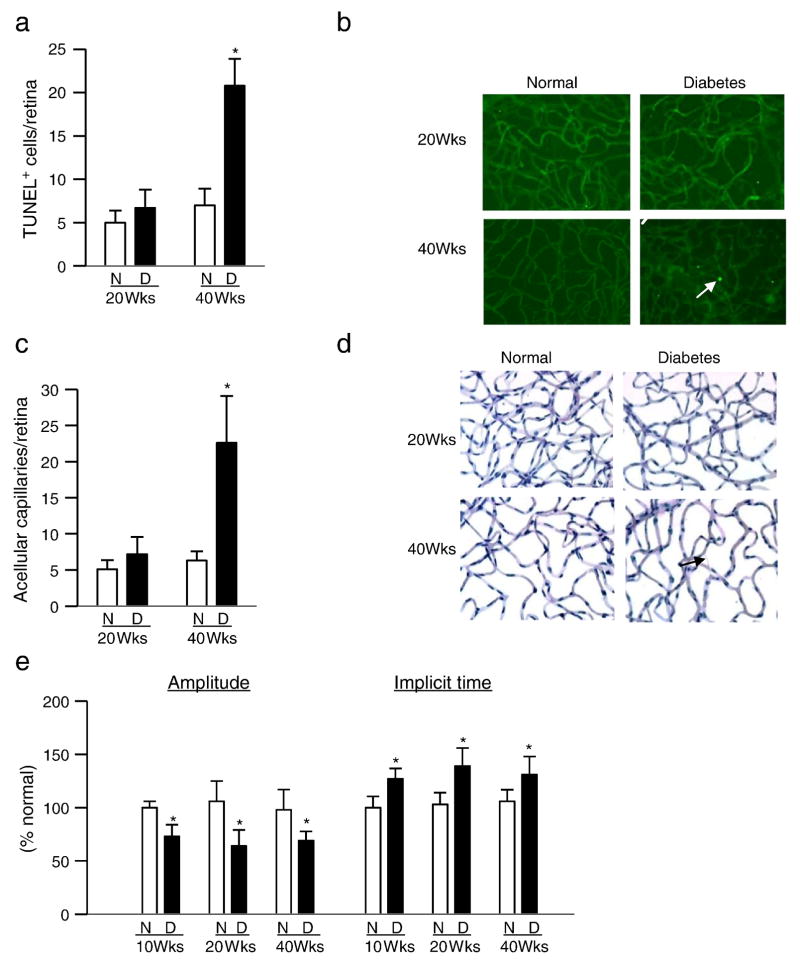
Compared to their age-matched normal rats, while Wistar rats diabetic for 40 weeks had over 4 fold increase in TUNEL-positive cells and degenerative capillaries in their retinal vasculature, as expected, no increase in either apoptotic cells or degenerative capillaries was observed in rats diabetic for up to 20 weeks (Figure 6a–d).
Figure 6.
Retinal capillary cell apoptosis, histopathology and ERG in type 1 diabetic rat model. (a) TUNEL staining was performed in trypsin-digested retinal microvasculature from rats diabetic for 20–40 weeks. (b) Representative TUNEL-stained microvessels from rats diabetic for 20 and 40 weeks and age-matched normal rats; the arrow indicates TUNEL-positive cell. (c) Acellular capillaries were counted in retinal microvasculature stained with periodic acid Schiff-hematoxylin stain, and (d) representative periodic acid Schiff-hematoxylin-stained microvessels from rats diabetic for 20 and 40 weeks; an acellular capillary is marked with an arrow. (e) For ERG, a dark-adapted intensity-response series was recorded using Ganzfeld flashes, and ‘b’ wave amplitude and implicit time were plotted. Values obtained from normal rats are considered as 100%, and represented as mean ± SD from 6–8 rats/group; N=normal and D= Diabetes; p< 0.05 compared to age-matched normal control rats.
3.2.3. Retinal function
The amplitude of ‘b’ wave was significantly decreased and the implicit time was prolonged in rats diabetic for 10 weeks compared to age-matched normal control rats, and the values obtained from rats diabetic for 20–40 weeks were not significantly different from those obtained from the rats diabetic for 10 weeks (Figure 6e). Similar alterations in retinal function were observed in rats diabetic for 4–6 weeks (data not shown).
Discussion
Diabetic retinopathy is a slow progressing disease, which takes over decades in humans to develop [3, 4]. Type 1 diabetic rat model has shown that retinal capillary cell apoptosis, which precedes the development of histopathology characteristic of diabetic retinopathy, is not observed till 5–6 months of streptozotocin-induced diabetes [30, 31]. Although high circulating glucose is the key instigator, hyperlipidemia is also considered to play an important role in the development of diabetic retinopathy [10–15, 17, 18]. Triglycerides and LDL cholesterol are shown to have a direct correlation with the incidence and severity of diabetic retinopathy, and long-term supplementation with lipid-lowering therapy, fenofibrate, reduces the need for laser treatment in patients with proliferative diabetic retinopathy [12, 14, 32]. Here, we present data demonstrating the augmentation of the development of diabetic retinopathy in a hyperlipidemic environment, and our results suggest that the combination of hyperlipidemia and hyperglycemia, via accelerating mitochondrial damage, potentiates activation of the apoptotic machinery. Using ZDF rat as a model of type 2 diabetes, during the age of these rats that spans from pre-diabetes with hyperlipidemia to overt hyperglycemia and hyperlipidemia, our results show that despite significant decrease in the retinal function (b wave amplitude and the implicit time) during the initial stages of hyperglycemia accompanied with moderate hyperlipidemia (12 weeks of age, when these rats are hyperglycemic for 6 weeks or less), retinal histopathology is not detectable, but by 20 weeks of age, an age when ZDF rats are hyperlipidemic for their entire life, but are hyperglycemic for 14 weeks or less, number of apoptotic capillary cells and degenerative capillaries is significantly increased compared to age-matched lean rats. However, in type 1 diabetic animals, in spite of severe hyperglycemia, which is accompanied with mild hyperlipidemia, vascular histopathology and accelerated capillary cell apoptosis are not observed even after 20 weeks of diabetes. Taken together, these results show a significant interplay between hyperglycemia and hyperlipidemia, and suggest that in type 2 diabetes, hyperlipidemia could be potentiating the deleterious effect of the hyperglycemia.
Landmark trials with type1 and type 2 diabetic patients have clearly documented a reduction in the incidence of retinopathy in patients with tight blood glucose control [33, 34], and clinical studies have suggested a role of hyperlipidemia in its development [12, 32]. Altered lipid metabolism is observed in both type 1 and type 2 diabetes, but the severity is higher in type 2 patients with generally higher levels of serum triglycerides, low-density lipoprotein and VLDL [2]. The data presented here show that, compared to age-matched lean rats and type 1 diabetic rats, ZDF rats have 2–4 fold higher triglyceride and VLDL, and in the same animals, increase in the number degenerative capillaries is observed when these rats are hyperglycemic for 14 weeks or less. Consistent with previous reports [30], 20 weeks of severe hyperglycemia in type 1 animal model has no detrimental effects on retinal capillaries. But, when the duration of diabetes is extended, retinal histopathology is comparable between these two animal models, suggesting that the initial hyperlipidemia could be playing an important role in potentiating the development of diabetic retinopathy. In support, others have shown significant increase in acellular capillaries in the retinal vasculature from 8 months old ZDF rats [35]. Although compared to ZDF rats, type 1 diabetic rats had 50% lower VLDL levels, these VLDL values were still significantly higher than generally observed in diabetic patients, suggesting the effect of hyperlipidemia on diabetic retinopathy could be much milder in the patients than observed in our animal model. However, our previous study using isolated retinal endothelial cells has clearly demonstrated that mitochondrial damage experienced by these cells in lipotoxic environment alone is not significant, but addition of lipotoxicity in a glucotoxic environment exacerbates ROS production, and also accelerates their damaging effects on mitochondrial homeostasis [10], suggesting that hyperlipidemia itself may not be sufficient to produce retinopathy. The rats used in the present study for type 1 and type 2 diabetic animal models come from two different genetic backgrounds, and different genetic backgrounds have presented differences in susceptibility/resistance to retinal vascular damage [36]. Thus, the effect of genetic background in the differences observed in ZDF rats and streptozotocin-induced diabetic Wistar rats, however, cannot be ruled out.
In pathogenesis of diabetic retinopathy, retinal mitochondrial dysfunction and DNA damage precede the development of diabetic retinopathy. Mitochondrial membrane potential is increased and cytochrome c leaks out into the cytosol, which activates the apoptotic machinery. In addition, the electron transport system is damaged, and superoxide levels are elevated [5–7, 9]. Here, we show that in a type 2 diabetic animal model, despite absence of hyperglycemia in 6 weeks old ZDF rats, the respiratory chain in the retina is significantly damaged, and their mitochondrial membrane permeability is modestly, but not significantly, elevated.
Twelve weeks old ZDF rats, an age when these rats have hyperlipidemia for their entire life, but are hyperglycemic for 6 weeks or less, although do not present any retinal vasculature histopathology, the mitochondria are damaged with ~50% alterations in membrane permeability and complex III activity. In contrast, in type 1 model, 10 weeks of similar hyperglycemia (11–12% GHb), which is instead, accompanied with a mild dyslipidemia, fails to produce any damage to the retinal mitochondria. These results suggest that the electron transport chain system could be more sensitive to the lipid environment, and continued exposure to high lipids in a hyperglycemic milieu could be contributing to accelerated mitochondrial damage. In support, lipids are shown to play a crucial role in the structure and function of mitochondrial membrane polarization [37], complex III, which is integrated in the inner mitochondrial membrane, requires phospholipids for its function [38], and lipid peroxidation products effectively inhibit the electron-transport complexes [39].
Mitochondrial damage is closely associated with apoptosis, and accelerated apoptosis of retinal capillary cells, which precedes the development of diabetic retinopathy, is mediated, in part, by mitochondrial damage [4, 25, 26, 40, 41]. Dysfunctional mitochondria, by inducing permeability of the mitochondrial outer membrane and allowing pro-apoptotic Bax to translocate into the mitochondria, initiate the apoptotic machinery [42]. Here, we show that within 14 weeks of hyperglycemia, which is accompanied by severe dyslipidemia (20 weeks old ZDF rats), retinal capillary cell apoptosis is increased significantly, but in contrast, even after 20 weeks of severe hyperglycemia, which is complemented with mild hyperlipidemia (type 1 diabetes), capillary cell apoptosis is not increased. These results further strengthen the role of hyperlipidemia in accelerating capillary cell apoptosis. In accordance, fatty acids are shown to increase membrane permeability and Bax translocation [43], induce mitochondrial dysfunction and consequent apoptosis [44], and accelerate glucose-induced mitochondrial damage and apoptosis [10]. Due to technical difficulties in isolating mitochondria from retinal vasculature, mitochondria prepared from the whole retina were used, and contribution of other retinal cells, including highly active photoreceptors, in such measurements cannot be ruled out. However, we have measured apoptosis and histopathology in trypsin-digested retinal microvasculature, which is devoid of non-vascular cells, and supports the compounding role of lipids in vascular pathology seen in diabetic environment.
Lipid-sensitive Nox2 is activated in the retina during the early stages of diabetes, increasing the levels of cytosolic ROS [9, 10, 17, 18], and increased cytosolic ROS serve as a trigger to damage retinal mitochondria [9, 10, 45]. Our study using in vitro model has shown that the addition of lipotoxic insult in a glucotoxic environment exacerbates cytosolic ROS production and accelerates mitochondrial damage [10]. Here, our data demonstrate that retinal ROS levels are increased during the initial mild hyperlipidemia in ZDF rats during the age when these rats had normal blood glucose levels. The results also show that Rac1 is activated before hyperglycemia begins to appear, further confirming the role of Rac1-Nox2 signaling in increased ROS. In support, Rac1-Nox2 signaling is shown to regulate lipid-dependent ROS generation [9, 10, 45], and release of arachidonic acid by activation of phospholipase A2 in hyperglycemia, is implicated in the generation of Nox-mediated ROS [18]. Our data also suggest that the Rac1-Nox2 signaling axis continues to generate ROS, and consistent with the results from the in vitro model in which addition of high glucose in a sustained lipotoxic environment exacerbates ROS levels [10], a temporal relationship is also observed between prolonged hyperlipidemia and increase in retinal Nox2-ROS. Despite 2.5 fold higher Rac1 activity in 40 weeks old ZDF rats compared to 6 weeks old rats, due to concomitant increase in Rac1 activity in age-matched lean rats, the difference between ZDF and their age-matched lean rats at 6 weeks and 40 weeks of age are similar, suggesting that chronic hyperlipidemia by itself also activates Rac1.
Although ‘ETDRS’ and ‘Action to Control Cardiovascular Risk in Diabetes’ studies have shown associations between hyperlipidemia and diabetic retinopathy [12, 14], despite some cross-sectional relationship between total cholesterol and the prevalence of hard exudates, patients enrolled in Wisconsin Epidemiologic Study of Diabetic Retinopathy study have failed to demonstrate any relationship between lipid levels and the severity of diabetic retinopathy [46, 47]; the reasons for such discrepancies are not clear. Our study has employed ZDF rats from the age when these animals are mildly hyperlipidimic without any hyperglycemia, to the age when they are both severely hyperglycemic and hyperlipidemic, and the results clearly demonstrate the importance of hyperlipidemia in accelerating mitochondrial damage and the development of diabetic retinopathy. The possibility that there could be some inherent differences between rodents and humans in responding to the hyperlipidemic-hyperglycemic environment, cannot be ruled out. However, in support, our recent in vitro study using isolated retinal endothelial cells has shown accelerated mitochondrial damage and cell apoptosis in a gluco-lipotoxic milieu [10].
ZDF rats have a defect in their leptin receptors, which is not seen in type 2 diabetic patients [48], and this raises the possibility that this defect could also be contributing to the retinal abnormalities observed in the present study. Previous reports associating leptin defects with diabetic retinopathy, however, have been inconclusive; while leptin is shown to stimulate ischemia-induced retinal neovasucularization, possibly via upregulating vascular endothelial growth factor [49], others have failed to observe a strong association between leptin levels and micro- or macro- vascular complications in type 2 diabetic patients [50]. Furthermore, the two animal models used in our study have different backgrounds; responses from one animal model may not necessarily extrapolate to another animal model, and to what is observed in diabetic patients [51]. Thus, the influence of genetic background on the differences observed in ZDF and Wistar rat models cannot be ruled out. However, our previous study using retinal endothelial cells in culture has shown a similar cross-talk between lipotoxicity and glucotoxicity [10], and supports that the leptin abnormalities in ZDF rat model may not be playing a contributory role in the retinal abnormalities observed in the present study, further validating clinical implications of our results for type 2 diabetic patients.
In summary, this study compares the results from type 1 and type 2 diabetic animal models demonstrating that the addition of hyperlipidemia, in a hyperglycemic environment (commonly observed in type 2 diabetic patients), accelerates mitochondrial damage, resulting in accelerated apoptosis of capillary cells, and ultimately, in the development of diabetic retinopathy. We recognize that our study has some limitations, including genetic differences in Wistar rats and ZDF rats used as models of type 1 and type 2 diabetes respectively, and genetic background may influence the outcome of retinal damage observed in diabetic conditions. Streptozotocin-induced diabetic rats (type 1 diabetes model) had much higher VLDL values than generally observed in type 1 diabetic patients, suggesting that humans and rodents could have some differences in responding to the gluco-lipotoxicity insult. Furthermore, in contrast to type 2 diabetic patients, the ZDF rats have defective leptin receptors. However, how this defect could have affected the retinal abnormalities presented in our study is unclear because the influence of leptin on diabetic complications remains inconclusive, and our recent in vitro results using retinal endothelial cells in culture are in agreement with the results obtained from ZDF rats. Thus, despite these limitations, we clearly demonstrate that combination of hyperlipidemia and hyperglycemia, via activating cytosolic Rac1-Nox2 signaling, potentiates and exacerbates ROS production, and due to accelerated mitochondrial damage, augmenting the development of retinopathy. These results with clinical corollaries suggest that, without undermining the importance of control of hyperglycemia, the management of hyperlipidemia in pre-diabetic patients, those with even mild hyperlipidemia, could prove to be a good strategy to slow down the development/progression of diabetic retinopathy.
Acknowledgments
We thank Mangayarkarasi ThandampallayamAjjeya of the Wayne State University for her help with the maintenance of rodent colony. This study was supported in part by grants from the National Institutes of Health (EY014370, EY017313 and EY022230 to RAK; DK74921 and EY022230 to AK), Juvenile Diabetes Research Foundation (5-2012-313 to RAK; and 5-2012-257 to AK), the Department of Veterans Affairs (1BX000469 to AK), and an unrestricted grant to the Ophthalmology Department from Research to Prevent Blindness. AK is the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs (13S-RCS-006).
Abbreviations
- DAPI
4″,6-Diamidino-2-phenylindole, dihydrochloride
- DCHFDA
2′,7′-dichlorofluorescein diacetate
- ERG
Electroretinogram
- ETDRS
Early Treatment of Diabetic Retinopathy Study
- FITC
Fluorescein isothiocyanate
- GHb
Glycated hemoglobin
- mtDNA
Mitochondrial DNA
- LN
Lean
- ND
Not determined
- Nox2
NADPH oxidase 2
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ROS
Reactive oxygen species
- TUNEL
Terminal deoxyribonucleotide transferase-mediated dUTP nick-end label
- VLDL
Very low-density lipoprotein
- Wks
Weeks
- ZDF
Zucker diabetic fatty
Footnotes
Duality of interest: The authors declare that no competing financial interests exist that might create a conflict of interest associated with this manuscript.
Author contribution: RAK was responsible for the experimental plan, literature search, manuscript writing and editing, AK for experimental plan, literature search and editing of the manuscript, and MM and BK for planning and conducting the experiments. RAK has final approval from each author to publish the manuscript, and takes full responsibility for the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 2.Tremblay J, Hamet P. Biomarkers of vascular complications in type 2 diabetes. Metabolism. 2015;65:S28–S32. doi: 10.1016/j.metabol.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Frank RN. Diabetic Retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 4.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Inves Ophtahlmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 6.Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antiox Redox Signal. 2010;13:797–805. doi: 10.1089/ars.2009.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos JM, Tewari S, Goldberg AFX, Kowluru RA. Mitochondria biogenesis and the development of diabetic retinopathy. Free Rad Biol Med. 2011;51:1849–1860. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewari S, Santos JM, Kowluru RA. Damaged mitochondrial DNA replication system and the development of diabetic retinopathy. Antiox Redox Signal. 2012;17:492–504. doi: 10.1089/ars.2011.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowluru RA, Kowluru A, Veluthakal R, et al. TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia. 2014;57:1047–1056. doi: 10.1007/s00125-014-3194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Inves Ophtahlmol Vis Sci. 2015;56:2985–2992. doi: 10.1167/iovs.15-16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA. 2008;299:1185–1187. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- 12.Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443–2451. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busik JV, Esselman WJ, Reid GE. Examining the role of lipid mediators in diabetic retinopathy. Clin Lipid. 2012;7:661–675. doi: 10.2217/clp.12.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 15.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikhonenko M, Lydic TA, Opreanu M, et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PloS one. 2013;8:e55177. doi: 10.1371/journal.pone.0055177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Othman A, Ahmad S, Megyerdi S, et al. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PloS one. 2013;8:e57254. doi: 10.1371/journal.pone.0057254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim AS, Elshafey S, Sellak H, et al. A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. J Lipid Res. 2015;56:599–611. doi: 10.1194/jlr.M056069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu D, Wu M, Zhang J, et al. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55:3128–3140. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hempe J, Elvert R, Schmidts HL, Kramer W, Herling AW. Appropriateness of the Zucker Diabetic Fatty rat as a model for diabetic microvascular late complications. Laboratory animals. 2012;46:32–39. doi: 10.1258/la.2011.010165. [DOI] [PubMed] [Google Scholar]
- 21.Kowluru RA, Mohammad G, dos Santos JM, Zhong Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60:3023–3033. doi: 10.2337/db11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veluthakal R, Kumar B, Mohammad G, Kowluru A, Kowluru RA. Tiam1-Rac1 Axis Promotes Activation of p38 MAP Kinase in the Development of Diabetic Retinopathy: Evidence for a Requisite Role for Protein Palmitoylation. Cell Physiol Biochem. 2015;36:208–220. doi: 10.1159/000374065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammad G, Kowluru RA. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012;227:1052–1061. doi: 10.1002/jcp.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowluru RA, Zhong Q, Santos JM, Thandampallayam M, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab (Lond) 2014;11:8. doi: 10.1186/1743-7075-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrin G, Linares CI, Muntane J. Mitochondrial drug targets in cell death and cancer. Curr Pharm Des. 2011;17:2002–2016. doi: 10.2174/138161211796904803. [DOI] [PubMed] [Google Scholar]
- 26.Kale J, Liu Q, Leber B, Andrews DW. Shedding light on apoptosis at subcellular membranes. Cell. 2012;151:1179–1184. doi: 10.1016/j.cell.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Mustata GT, Rosca M, Biemel KM, et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats: improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes. 2005;54:517–526. doi: 10.2337/diabetes.54.2.517. [DOI] [PubMed] [Google Scholar]
- 28.Bearse MA, Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–448. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos JM, Tewari S, Kowluru RA. A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radic Biol Med. 2012;53:1729–1737. doi: 10.1016/j.freeradbiomed.2012.08.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: Comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- 31.Kowluru RA, Chan PS. Metabolic memory in diabetes - from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2010;81:297–302. doi: 10.1016/j.brainresbull.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 34.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 35.Wohlfart P, Lin J, Dietrich N, et al. Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta Diabetol. 2014;51:553–558. doi: 10.1007/s00592-013-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern TS, Miller CM, Tang J, Du Y, Ball SL, Berti-Matera L. Comparison of three strains of diabetic rats with respect to the rate at which retinopathy and tactile allodynia develop. Mol Vis. 2010;16:1629–1639. [PMC free article] [PubMed] [Google Scholar]
- 37.Kogot-Levin A, Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2014;100:88–94. doi: 10.1016/j.biochi.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 39.Musatov A, Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res. 2012;46:1313–1326. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- 40.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 41.Kowluru RA. Mitochondria damage in the pathogenesis of diabetic retinopathy and in the metabolic memory associated with its continued progression. Curr Med Chem. 2013;20:3226–3233. doi: 10.2174/09298673113209990029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosentino K, Garcia-Saez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75. doi: 10.1016/j.chemphyslip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Mignard V, Lalier L, Paris F, Vallette FM. Bioactive lipids and the control of Bax pro-apoptotic activity. Cell Death Dis. 2014;5:e1266. doi: 10.1038/cddis.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol metab. 2010;299:E1096–1105. doi: 10.1152/ajpendo.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol. 2010;80:874–883. doi: 10.1016/j.bcp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–1265. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 47.Klein BE, Myers CE, Howard KP, Klein R. Serum Lipids and Proliferative Diabetic Retinopathy and Macular Edema in Persons With Long-term Type 1 Diabetes Mellitus: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Ophthalmol. 2015;133:503–510. doi: 10.1001/jamaophthalmol.2014.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin W, Patti ME. Genetic determinants and molecular pathways in the pathogenesis of Type 2 diabetes. Clin Sci (Lond) 2009;116:99–111. doi: 10.1042/CS20080090. [DOI] [PubMed] [Google Scholar]
- 49.Suganami E, Takagi H, Ohashi H, et al. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes. 2004;53:2443–2448. doi: 10.2337/diabetes.53.9.2443. [DOI] [PubMed] [Google Scholar]
- 50.Sari R, Balci MK, Apaydin C. The relationship between plasma leptin levels and chronic complication in patients with type 2 diabetes mellitus. Metab Syndr Relat Disord. 2010;8:499–503. doi: 10.1089/met.2009.0127. [DOI] [PubMed] [Google Scholar]
- 51.Paul W, Queen LR, Page CP, Ferro A. Increased platelet aggregation in vivo in the Zucker Diabetic Fatty rat: differences from the streptozotocin diabetic rat. Br J Pharmacol. 2007;150:105–111. doi: 10.1038/sj.bjp.0706957. [DOI] [PMC free article] [PubMed] [Google Scholar]