Abstract
Neonatal CD4+ T cells have traditionally been viewed as deficient in their capacity to produce Th-1 cytokines in response to polyclonal or antigen specific stimuli. Thus, defining unique aspects of CD4+ T cell activation and development into Th-1 effector cells in neonates is essential to the successful development of novel vaccines and immunotherapies to protect infants from intracellular pathogens. Using highly purified naïve CD4+ T cells derived from cord and adult peripheral blood, we compared the impact of anti-CD3 stimulation plus co-stimulation through TLR-2 performed in the absence of APC, on CD4+ T cell cytokine production, proliferation, and expression of activation markers. In both age groups, TLR-2 co-stimulation elicited activation of naïve CD4+ T cells, characterized by robust production of IL-2 as well as key Th-1 type cytokines IFN-γ and TNF-α. TLR-2 co-stimulation also dramatically reduced naïve T cell production of the immunosuppressive cytokine IL-10. We observed that neonatal naïve CD4+ T cells are uniquely sensitive to TLR-2 mediated co-stimulation, which enabled them to produce equivalent amounts of IFN-γ and more IL-2 when compared to adult responses. Thus, neonatal CD4+ T cells have a distinctive propensity to utilize TLR-2 mediated co-stimulation for development into pro-inflammatory Th-1 effectors, and interventions that target CD4+ T cell TLR-2 mediated responses may be exploited to enhance neonatal adaptive immunity.
Introduction
Following birth, the neonatal immune system must rapidly evolve to successfully recognize pathogens and mount effective, protective immune responses while simultaneously developing tolerance to benign environmental antigens and commensal organisms. Although the majority of newborns successfully navigate these immunologic adaptations, this period of transition and early infancy are notable for an increased risk of invasive infections from a broad variety of pathogens.
Research comparing the effector capacity of human neonatal and adult CD4+ T cells suggests that newborn T cells are deficient in production of the prototypical Th-1 cytokine IFN-γ in response to polyclonal stimulation and/or mitogen, and display an inherent propensity to generate the regulatory cytokine IL-10 (1–5). Thus, the neonatal adaptive immune system is considered biased towards anti-inflammatory or Th-2 adaptive responses, and this bias is thought to predispose newborns to infection. However, some investigators have found that when provided with optimal co-stimulation, neonatal naïve CD4+ T cells produce equivalent amounts of IFN-γ as compared to adult naïve cells (6, 7). Therefore, the capacity of neonatal CD4+ T cells to function as pro-inflammatory effectors may not be inherently defective. Rather, external influences on the polarization of T cell subsets, such as the intensity and composition of co-stimulatory signals (8) and the surrounding cytokine mileu (9) may determine the fate of naïve neonatal CD4+ T cells (10).
Understanding how the neonatal adaptive immune response is optimally activated is critical to identification of successful interventions to enhance neonatal antimicrobial immunity, and to the advancement of techniques utilizing cord-derived T cells for adoptive immunotherapy. The traditional model of naïve CD4+ T cell activation requires TCR mediated antigen-recognition, plus a secondary co-stimulatory signal provided by an APC. However, CD4+ T cells can be activated independently from APC-provided co-stimulatory signals. Specifically, ex vivo studies have demonstrated that recognition of pathogen associated molecular patterns (PAMPS) by TLR expressed by CD4+ T cells, in conjunction with TCR signaling provided by anti-CD3 antibody, can lead to CD4+ T cell activation in the absence of APC. Such direct TLR-mediated co-stimulation of CD4+ T cells in the absence of APC, has been reported most consistently with TLR-2 ligands, and among adults is primarily observed in cells with a memory (CD45R0+) phenotype (11–19). The ability of the primarily naïve (20) CD4+ T cell compartment of neonates to utilize TLR to directly augment cellular immune responses in the absence of APC is unclear, and studies regarding neonatal T cell TLR expression and function are limited (12, 21, 22).
Prior work has demonstrated that neonatal monocytes and dendritic cells are deficient in their activation response to select TLR ligands (23–28). Given these findings and the predominately naïve phenotype of neonatal T cells, we hypothesized that pro-inflammatory responses of neonatal CD4+ T cells to TLR-2 co-stimulation would be deficient when compared to adult responses. Using cord blood mononuclear cells (CBMC) as a readily accessible source of human neonatal blood, we found that TLR-2 co-stimulation of neonatal naïve CD4+ T cells resulted in a robust Th-1 type cytokine response, suggesting that interventions that target CD4+ T cell TLR-2 mediated responses may enhance neonatal adaptive immunity.
Materials and Methods
Study Subjects
Human subjects protocols and consent forms were approved by the Oregon Health & Science University (OHSU) Institutional Review Board. PBMC were obtained from healthy adult donors aged 18–65 years by apheresis following written informed consent. Umbilical cord blood was obtained from healthy, singleton, term infants ≥36 weeks gestational age born at OHSU. As cord blood is considered medical waste, and no identifying information was collected from the newborns or their mothers, we obtained cord blood under approved institutional guidelines that do not require informed consent. Cord blood was collected into CPT Vacutainer (BD) tubes, and CBMC isolated per manufacturer’s instructions within 24 h of delivery. PBMC and CBMC were washed twice in sterile 1X PBS and cryopreserved in freezing medium until use.
Cell isolation
Cryopreserved CBMC and PBMC were thawed in the presence of DNAse, assessed for viability using trypan blue exclusion, and rested overnight in serum-free complete medium (XVNS-15; Lonza) plus low dose IL-2 (30 U/ml; Prometheus) in 6 well ULA tissue culture plates (Fisher Scientific). Following overnight rest, CBMC/PBMC were subjected to CD4+ enrichment using negative isolation columns (Miltenyi), and resulting CD4+ T cells labeled with anti-CD4-PECy7 (BD), anti-CD45RA-allophycocyanin (Biolegend), and anti-CD14/CD19/CD123/CD56 (Biolegend)/CD1c -PE (Miltenyi), and sorted to obtain CD4+CD45RA+(CD14/CD19/CD123/CD1c/CD56)negative T cells using a FACS InFlux sorter (BD). To assess the impact of contaminating CD4+CD45RA+RO+ and CD4+CD45RO+RA− T cells on the effector cytokine response among adult donors, PBMC from a subset of adult donors underwent CD4+ enrichment using negative isolation columns followed by parallel FACS purification for CD4+/CD45RA+/CD14, CD19, CD123, CD1c, CD56negative T cells and CD4+/CD45RA+/CD45RO− (BV 421; BD)/CD14, CD19, CD123, CD1c, CD56negative T cells (Supplemental Figure 1 A/B).
Flow cytometric analysis
To perform phenotypic analysis of FACS purified CD4+CD45RA+ T cell subsets (Table I), isolated cells were labeled with anti-CD4-PECy7, anti-CD45RA-allophycocyanin anti-CD3-APC H7 (BD), anti-CD45RO-BV650 (BD), anti-CCR7-FITC (BD), anti-CD27-BV421 (Biolegend), and anti-CD95-PerCP-Cy5.5 (BD). To assess for TLR-2, CD45RA and CD45RO expression on resting and freshly thawed CBMC and PBMC, specimens were stained with anti-CD3-phycoerythrin (BD), anti-CD4-PECy7, anti-CD45 RA APC-H7 (BD), anti-CD45R0 BV 421 (Biolegend), and anti-TLR-2-Alexa 647 (Biolegend). To assess for intracellular cytokine production, cells were harvested from tissue culture plates after 18h (IL-2) or 72h (IFN-γ) of stimulation (see T cell stimulation assays) and stained with LIVE/DEAD Aqua (Invitrogen) and anti-CD4-PECy7, followed by fixation and permeabilization in Cytofix/Cytoperm buffer (BD) and stained with anti-IL-2-BV421 (Biolegend) or anti-IFN-γ-FITC (BD). To assess for Ki-67 expression, stimulated CD4+CD45RA+ T cells were harvested from tissue culture plates following 96 h of stimulation and stained with LIVE/DEAD Aqua and anti-CD4-PECy7, followed by fixation and permeabilization in FoxP3 staining buffer (BD) and stained with anti-Ki-67-BV421 (Biolegend). To assess for T cell expression of CD25, CD69, and CD154, anti-CD154-PECy5 (Biolegend) was included during 18 h stimulation assay to optimize detection (29); CD4+ T cells were then harvested from tissue culture plates and stained with LIVE/DEAD Aqua, anti-CD4-PECy7, anti-CD25-APC H7 (BD), and anti-CD69-FITC (Biolegend). For all experiments, appropriate isotype controls and fluorescent-minus-one controls for each fluorochrome were included to assess for non-specific staining and to determine gating strategy, respectively. Data was acquired using a Fortessa flow cytometer (BD) and data analyzed with FlowJo software (Tree Star, Inc).
Table I.
Phenotypic analysis of T cell populations obtained by two-step purification process1
| Neonate | Adult | |
|---|---|---|
| CD3+CD4+ | 99.6% ± 0.3% | 99.6% ± 0.2% |
| CD3+CD4+CD45RA+ | 98.7% ± 1.3% | 98.5 ± 1% |
| CD3+CD4+CD45RA+CD45RO− | 94.6% ± 1.8% | 96% ± 3% |
| CD3+CD4+CD45RA+CD45RO+ | 2.4% ± 0.5% | 2.4% ± 2.3% |
| CD3+CD4+CD45RA−CD45RO+ | 1.2% ± 0.3% | 1.5% ± 0.8% |
| CD3+CD4+CD45RA+CD45RO−CCR7+CD27+CD95+ | 0.4% ± 0.5% | 0.2% ± 0.2% |
Shown are mean percentages ± SEM from 9 cord and 9 adult donors
T cell stimulation assays
For cytokine secretion assays, FACS purified neonatal and adult CD4+CD45RA+ T cells were cultured in 96-well flat bottom plates (105 cells/well) in serum free medium (XVNS-15) and recombinant IL-2 (30 U/ml) with and without: plate-bound αCD3 (10 μg/ml; clone Hit3a; BD), soluble αCD28 (0.5 – 8 μg/ml; clone 28.2; BD), the synthetic TLR-1/2 ligand Pam3Cys4 (P3C; 0.5 – 10 μg/ml; Invivogen), or ultra-purified LPS (1 μg/ml; Invivogen) as a TLR-4 ligand. Autologous, unfractionated CBMC and PBMC (105 cells/well) treated with PHA (5 μg/ml; Sigma) were also included in each experiment. All conditions were performed in triplicate. Cell-free culture supernatants were collected at 18 h (IL-2, TNF-α, IL-8, and IL-17a) and 72 h (IFN-γ, IL-10, IL-8, and IL-17a) for ELISA. For measurement of IL-2 or IFN-γ by intracellular flow cytometry, stimulations were performed as described using P3C at 10 μg/ml (for IL-2) or 1 μg/ml (for IFN-γ) in 24-well flat bottom plates (106 cells/well). Cells were harvested from tissue culture plates following 18 h (IL-2) or 72 h (IFN-γ) of stimulation, with Brefeldin A (5 μg/ml; Sigma Aldrich) added during the last 12 h of incubation. Autologous, unfractionated CBMC and PBMC stimulated with Staph enterotoxin B (SEB; 5 μg/ml; Sigma Aldrich) were included as a positive control for cytokine production. For measurement of T cell expression of CD25, CD69, CD154, and Ki-67, and TLR-2 RT-PCR, stimulations were performed as described for 18 h in 24-well flat bottom plates (106 cells/well).
Cytokine quantification by ELISA
Cytokine quantifications in cell free culture supernatants were performed using commercial kits according to the manufacturer’s instructions: IL-2 (eBioscience), IFN-γ, IL-10, TNF-α, IL-8, IL-17a (Biolegend).
RT-PCR
TLR-2 expression was analyzed by RT-PCR on FACS purified cord and adult CD4+CD4RA+ T cells. Total cytoplasmic RNA was extracted using RNeasy minikit (Qiagen), including treatment with RNase-free DNase (Qiagen). A total of 0.2 μg of total RNA was used for each RT reaction, using SuperscriptIII/RNaseOUT (Invitrogen) per manufacturer’s instructions. Resulting cDNA underwent a 40-cycle PCR amplification using TaqMan Universal 2X gene expression master mix and the ready-made primer and probe sets for TLR-2 (catalog number Hs01014511_m1; Applied Biosystems) in a Step-one Plus system (Applied Biosystems). TLR-2 mRNA expression was normalized to 18s mRNA and calculated using the comparative cycle threshold method.
Statistical analysis
Data was analyzed as indicated in figure legends. Statistical Analysis System (SAS Institute) was used for all analysis.
Results
T cell isolation protocol results in highly purified populations of neonatal and adult naïve CD4+ T cells
To understand the capacity of naïve CD4+ T cells from neonatal and adult donors to directly utilize TLR-2 as a co-stimulatory receptor, it is essential to eliminate confounding effects from APC (16, 30), and CD4+ T cells expressing memory markers such as CD45RO, in T cell purifications. Among a subset of adult donors (n=4), the use of a FACS sorting protocol that excluded CD45RO+ cells had no impact on IL-2 or IFN-γ production in our T cell stimulation assays compared to naïve CD4 + T cells obtained without explicit exclusion of CD45RO+ cells (Supplemental Figure 1A/B). Subsequently, naïve CD4+ T cells were purified from CBMC and PBMC by CD4+ enrichment using negative isolation columns followed by sorting for CD4+CD45RA+(CD14/CD19/CD123/CD1c/CD56)negative T cells. T cell preparations from neonatal and adult donors were highly purified for CD3+CD4+ T cells and expressed a predominately naïve phenotype (Table I and Supplemental Figure 1C). Frequency of cells with a memory stem T cell phenotype (CD3+CD4+CD45RA+CD45RO−CCR7+CD27+CD95+) in naïve CD4+ T cell preparations was low in both age groups (31, 32). Terminally differentiated effector memory (“TEMRA”) cells expressing a CD45RA+CCR7− phenotype, best characterized in conditions of repetitive antigen exposure with low antigen load as is encountered in CMV+ adults (33, 34), were rare in naïve CD4+ T cell preparations from adult donors (1.4 ± 0.01%). Lack of IFN-γ production by naïve T cells in response to PHA confirmed that T cell preparations were not contaminated with APC (Supplemental Figure 1D) (35–37).
TLR-2 surface expression is equivalent between neonatal and adult CD4+ T cells
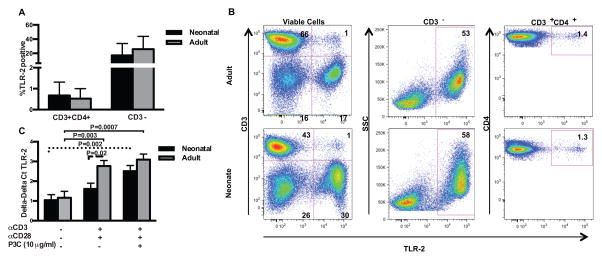
We investigated TLR-2 surface expression on CD3+CD4+ T cells and CD3-mononuclear cells from unfractionated CBMC compared to unfractionated adult PBMC. TLR-2 surface expression was low but detectable on both neonatal and adult resting CD3+CD4+ T cells, with no significant differences noted (Figure 1A/B; p > 0.05). TLR-2 surface expression did not differ in the non-T cell (CD3−) population between neonatal and adult donors (Figure 1A/B; p > 0.05). We also compared expression of CD45RA and CD45RO isoforms on CD3+CD4+TLR-2+ versus the total CD3+CD4+ T cell populations from neonatal and adult donors. In adult PBMC (n=5), the median percentage of CD3+CD4+ T cells that were CD45RA+CD45R0− was 43% (range 27–51%); among CD3+CD4+TLR-2+ T cells, the median percentage of CD45RA+CD45R0− cells was 26% (range 1.8–30%). In neonatal CBMC (n=4), the median percentage of CD3+CD4+ T cells that were CD45RA+CD45R0− was 82% (range 74–87%); among CD3+CD4+TLR2+ T cells, the median percentage of CD45RA+CD45R0− cells was 74% (range 57–82%). Thus expression of CD45RA and CD45R0 by CD3+CD4+TLR-2+ T cells tends to mirror that of the total CD3+CD4+ T cell population (see Supplemental Figure 2). We wanted to determine if highly purified naïve CD4+ T cells from neonatal and adult donors increase TLR-2 mRNA expression with activation, as previously shown in adult CD4+ T cells (Figure 1C) (11, 12). Here, increased TLR-2 expression by neonatal naïve CD4+ T cells required the presence of the TLR-2 ligand P3C, in addition to αCD3 plus αCD28. In adult donors, αCD3 plus αCD28 alone was sufficient to significantly increase TLR-2 mRNA expression.
Figure 1. TLR-2 mRNA and TLR-2 surface expression in adult and neonatal CD4+ T cells.
To assess for TLR-2 expression on resting CBMC and PBMC, freshly thawed, un-fractionated CBMC and PBMC specimens were stained with anti-CD3-PE, anti-CD4-PECy7, and anti-TLR-2-Alexa 647. Shown are percentage of CD3+CD4+ and CD3-cells with TLR-2 surface expression in 12 neonatal and 12 adult samples (A; shown are mean values ± SD). Data were analyzed using two-way ANOVA followed by Tukey-Kramer adjustment for multiple comparisons. There were no statistically significant differences in surface expression of TLR-2 between adult and neonatal donors (p > 0.05). Representative dot plots from an adult and neonatal donor are shown (B). Changes in TLR-2 mRNA expression were evaluated by RT-PCR in purified adult and neonatal naïve CD4+ T cells stimulated for 18 h as indicated (n=6 both age groups; C). Repeated measures ANOVA with technical replicates as a within subject factor was used to compare mean responses under different stimulation conditions, and to compare neonatal and adult responses. Tukey-Kramer adjustment was performed to adjust for multiple comparisons. Adjusted p values < 0.05 were considered significant and are indicated in figure. Dashed lines indicate comparisons within neonatal group, solid lines indicate comparisons within adult group, and long dashed line indicates comparisons between neonatal and adult donors.
TLR-2 is a potent co-stimulator of IL-2 production by neonatal and adult CD4+ T cells
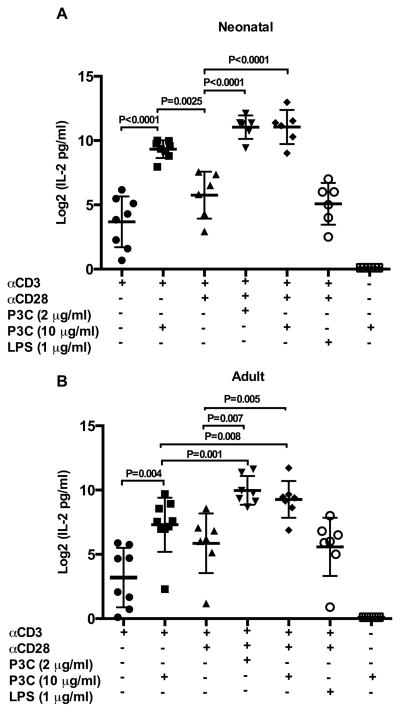
We sought to explore the potential for TLR-2 to function as a direct co-stimulatory receptor and activate naïve CD4+ T cells from neonatal and adult donors. Dose response experiments comparing IL-2 production following stimulation with αCD3 plus either the TLR-2 ligand P3C or αCD28, demonstrated that P3C elicited more IL-2 production than αCD28 at all concentrations tested in both neonatal and adult donors (Supplemental Figure 3 A/B). As shown in Figure 2A/B, the addition of P3C to αCD3 alone or αCD3 plus αCD28 significantly increased IL-2 production by neonatal and adult naïve CD4+ T cells. Notably, the addition of high concentration (10 μg/ml) P3C to αCD3 was a more potent inducer of IL-2 production than αCD3 plus αCD28 in neonatal donors only (p = 0.0025). Stimulation with P3C alone did not result in IL-2 production above resting levels, confirming that TCR stimulation through αCD3 is required for TLR-2 mediated T cell cytokine production. The addition of the TLR-4 ligand LPS to αCD3 plus αCD28 did not increase IL-2 production above αCD3 plus αCD28 alone in either age group (p > 0.5 both age groups). Thus, co-stimulation mediated specifically by TLR-2 triggers the initiation of T cell activation and effector function as evidenced by potent induction of IL-2 production by human naïve CD4+ T cells. Moreover, TLR-2 co-stimulation provides more robust induction of IL-2 production by TCR-stimulated naïve CD4+ T cells than αCD28.
Figure 2. IL-2 production following direct TLR-2 co-stimulation of neonatal and adult naïve CD4+T cells.
Purified neonatal (A) and adult (B) naïve CD4+CD45RA+ T cells were stimulated as indicated in triplicate. Culture supernatants were harvested at 18 h and cytokine production measured by ELISA. As IL-2 was present in culture medium, resting (medium alone) values were subtracted prior to analysis (data not shown). Cytokine data was log transformed with base 2 for analysis due to skewed distribution of data. Shown are log 2 transformed means of technical replicates for each donor ± SD. Repeated measures ANOVA with technical replicates as a within subject factor was used to compare mean cytokine responses under different stimulation conditions. Tukey-Kramer adjustment was performed to adjust for multiple comparisons. Adjusted p values < 0.05 were considered significant and are indicated in figure.
TLR-2 co-stimulation induces naïve CD4+ T cells to differentiate into Th-1 effectors characterized by robust IFN-γ production in both neonatal and adult donors
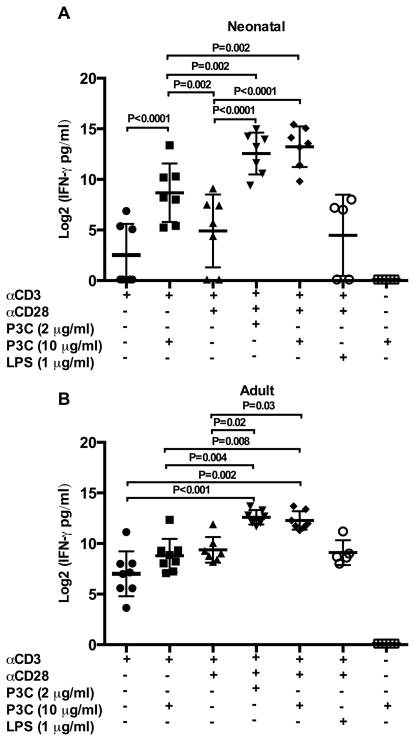
We wanted to determine if naïve CD4+ T cells activated by direct TLR-2 co-stimulation would differentiate into effector T cells productive of the prototypical Th-1 cytokines IFN-γ and TNF-α. Thus, we measured production of these cytokines by highly purified naïve CD4+ T cells from both neonatal and adult donors in response to direct TLR-2 co-stimulation. Dose response experiments comparing IFN-γ production following stimulation with αCD3 plus either P3C or αCD28, demonstrated that low concentration (0.5 μg/ml) P3C elicited substantially more IFN-γ production than αCD28 at all concentrations tested (0.5 – 8 μg/ml) in both neonatal and adult donors (Supplemental Figure 3 C/D). As shown in Figure 3A/B, the addition of P3C to αCD3 plus αCD28 resulted in significant increases in IFN-γ production compared to αCD3 plus αCD28 alone in both age groups. Stimulation with P3C alone did not result in detectable IFN-γ production, and the addition of LPS to αCD3 plus αCD28 did not increase IFN-γ production above αCD3 plus αCD28 alone in either age group (p > 0.5 both age groups). We noted that high concentration (10 μg/ml) P3C significantly augmented IFN-γ production by neonatal CD4+ T cells stimulated by αCD3 alone (p < 0.0001), whereas this effect was not observed in adult CD4+ T cells (p > 0.05). High concentration TLR-2 mediated co-stimulation also resulted in significantly more IFN-γ production than αCD3 plus αCD28 in neonatal but not adult donors (p = 0.002 and p = 0.9, respectively).
Figure 3. IFN-γ production following direct TLR-2 co-stimulation of neonatal and adult naïve CD4+T cells.
Purified neonatal (A) and adult (B) naïve CD4+CD45RA+ T cells were stimulated as indicated in triplicate. Culture supernatants were harvested at 72 h and cytokine production measured by ELISA. Cytokine data was log transformed with base 2 for analysis due to skewed distribution of data. Shown are log 2 transformed means of technical replicates for each donor ± SD. Statistical analysis was performed exactly as described in Figure 2. Adjusted p values < 0.05 were considered significant and are indicated in figure.
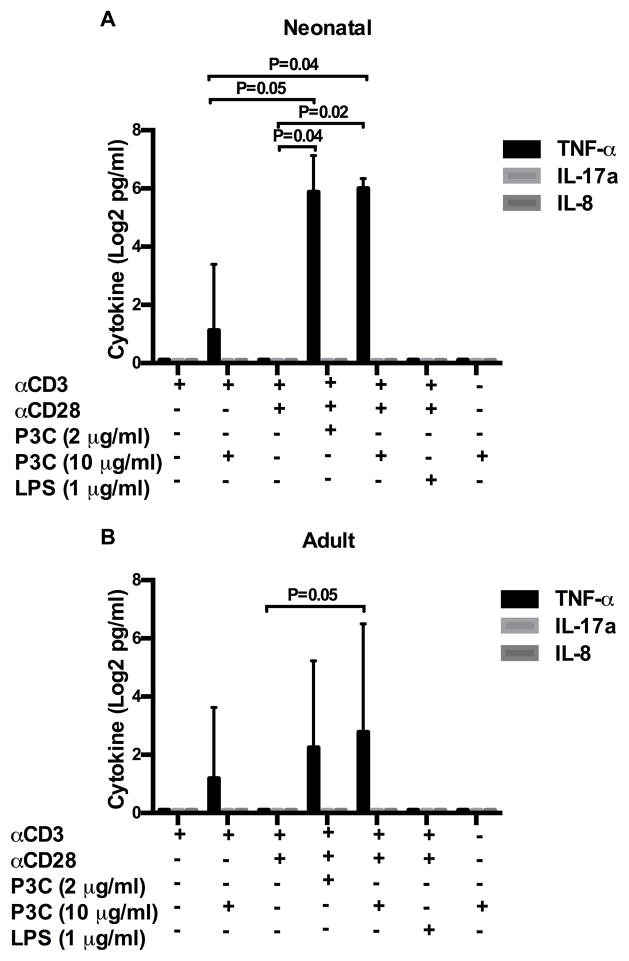
Regarding TLR-2 induced TNF-α production, addition of P3C to αCD3 plus αCD28 resulted in significantly more TNF-α than αCD3 plus αCD28 alone in both neonatal and adult donors (Figure 4A/B). However, compared to IFN-γ, peak TNF-α responses were minimal in both age groups and increased variability was noted among donors. Stimulation with P3C alone did not result in TNF-α production, and the addition of LPS to αCD3 plus αCD28 did not increase TNF-α production above αCD3 plus αCD28 alone in either age group (p > 0.5 both age groups).
Figure 4. TNF-α, IL-17a, and IL-8 production following direct TLR-2 co-stimulation of neonatal and adult naïve CD4+T cells.
Purified neonatal (A) and adult (B) naïve CD4+CD45RA+ T cells were stimulated as indicated in triplicate. Culture supernatants were harvested at 18 h (TNF-α, IL-8 and 72 h (IL-17a) and cytokine production measured by ELISA. Cytokine data was log transformed with base 2 for analysis due to the skewed distribution of data. Shown are log 2 transformed mean group responses ± SD from 6 neonatal and 6 adult donors. Statistical analysis was performed exactly as described in Figure 2. Adjusted p values < 0.05 were considered significant and are indicated in figure.
The prototypical Th17 cytokine, IL-17a, was not produced under any of the tested conditions by either neonatal or adult donors (Figure 4A/B). IL-8, a pro-inflammatory chemokine recently reported to be preferentially produced by neonatal T cells (38), was also not produced by TLR-2 co-stimulated naïve CD4+ T cells (Figure 4A/B). We did observe, however, that stimulation of unfractionated CBMC and PBMC with PHA resulted in robust IL-8 production (mean responses at 18h of 12367 pg/ml ± 4146 and 9704 pg/ml ± 3274, respectively).
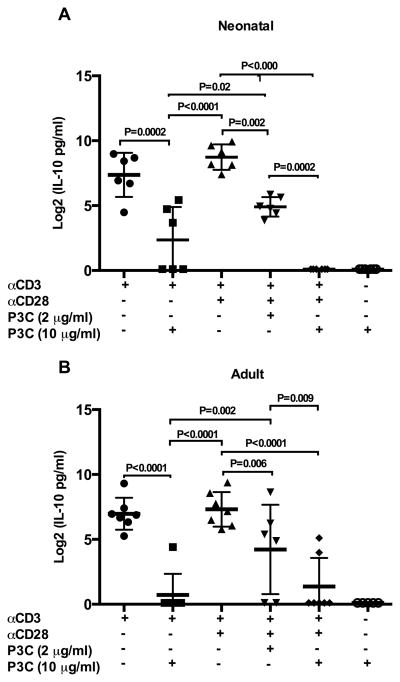
We also measured the prototypical Th-2 cytokine IL-10 in culture supernatants from neonatal and adult naïve CD4+ T cells exposed to direct TLR-2 co-stimulation. Notably, for both neonatal and adult naïve T cells, addition of P3C to αCD3 alone significantly reduced IL-10 production (Figure 5A/B; p = 0.002 and p < 0.001, respectively). Statistically significant reductions in IL-10 production were also observed in both age groups when low or high concentration P3C was added to the combination of αCD3 plus αCD28 (Supplemental Figure 3 E/F). Stimulation with P3C alone did not result in altered IL-10 production compared to resting levels. The addition of LPS to αCD3 plus αCD28 did not alter IL-10 production from that observed following stimulation with αCD3 plus αCD28 alone in either age group (data not shown).
Figure 5. IL-10 production following direct TLR-2 co-stimulation of neonatal and adult naïve CD4+T cells.
Purified neonatal (A) and adult (B) naïve CD4+CD45RA+ T cells were stimulated as indicated in triplicate. Culture supernatants were harvested at 72 h and cytokine production measured by ELISA. Cytokine data was log transformed with base 2 for analysis due to the skewed distribution of data. Shown are log 2 transformed means of technical replicates for each donor ± SD. Statistical analysis was performed exactly as described in Figure 2. Adjusted p values < 0.05 were considered significant and are indicated in figure.
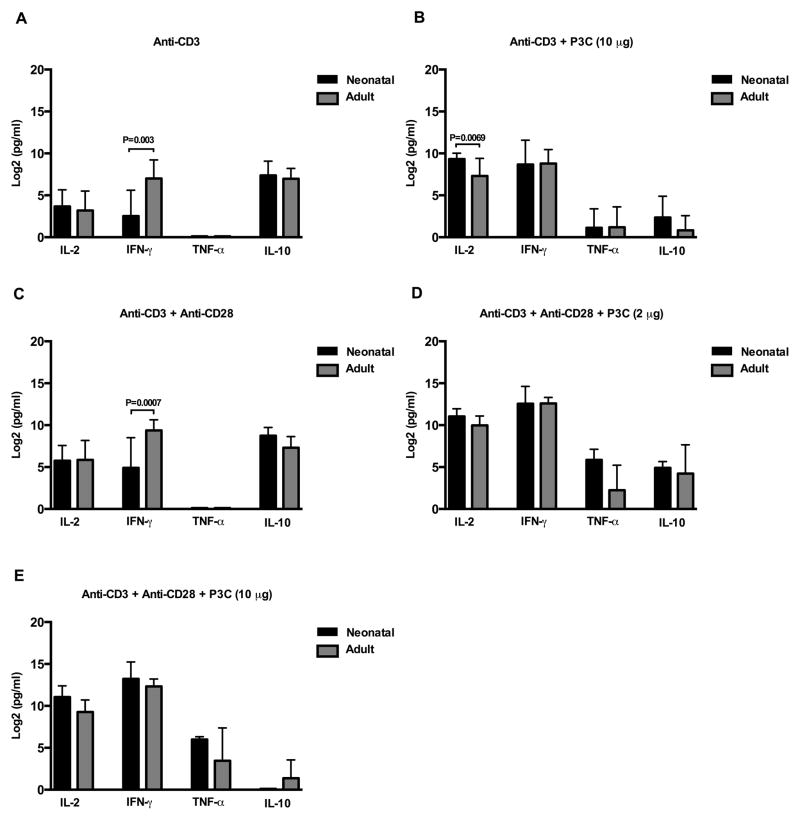
TLR-2 co-stimulation endows neonatal CD4+ T cells with equivalent capacity to produce IFN-γ and enhanced IL-2 production compared to adult responses
As CD4+ T cells from newborns are often characterized as deficient in pro-inflammatory cytokine production, we sought to directly compare neonatal and adult naïve CD4+ T cell production of IL-2, IFN-γ, TNF-α, and IL-10 under each of our previously tested stimulatory conditions (Figure 6A–E). As has been previously recognized (5, 39), naïve cord CD4+ T cells make significantly less IFN-γ in response to αCD3 alone or αCD3 plus αCD28 when compared to naïve adult CD4+ T cells (p = 0.0003 and p = 0.0007, respectively), but comparable quantities of IL-2 (p > 0.05 both conditions). However, following TLR-2 co-stimulation, production of IFN-γ by neonatal CD4+ T cells was equivalent to that of adult (p > 0.05 all conditions). In addition, neonatal CD4+ T cells produced significantly more IL-2 than their adult counterparts when stimulated with αCD3 plus high concentrations of P3C (p = 0.0069).
Figure 6. Comparison between adult and neonatal TLR-2 induced cytokine responses.
Purified neonatal and adult naïve CD4+CD45RA+ T cells were stimulated as indicated in triplicate. Culture supernatants were harvested at 18 h (IL-2 and TNF-α) and 72 h (IFN-γ and IL-10), and cytokine production measuring using commercial ELISAs. As IL-2 was present in culture medium, resting (medium alone) values were subtracted from measured IL-2 values prior to analysis. Cytokine data was log transformed with base 2 for analysis due to the skewed distribution of data. Shown are log 2 transformed mean group responses in pg/ml ± SD from N=6–8 neonatal and N=6–8 adult donors. Repeated measures ANOVA with technical replicates as a within subject factor was used to compare neonatal and adult responses. Tukey-Kramer adjustment was performed to adjust for multiple comparisons. Adjusted p values < 0.05 were considered significant and are indicated in figure.
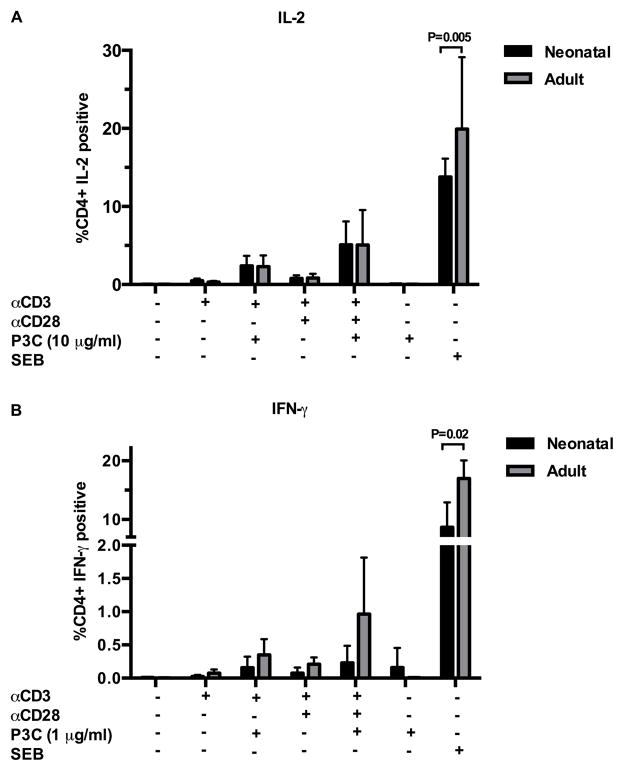
We next wanted to determine if the percentages of naïve CD4+ T cells that generated IL-2 or IFN-γ in response to TLR-2 mediated co-stimulation differed between adult and neonatal donors. Using intracellular flow cytometry, we found that in both age groups a minority of naïve CD4+ T cells generate IL-2 or IFN-γ in response to TLR-2 co-stimulation, and that the percentage of cytokine producing naïve CD4+ T cells does not differ by age (Figure 7A, B and Supplemental Figure 4). This is in striking contrast to the percentages of CD4+ T cells in unfractionated CBMC and adult PBMC that produced IL-2 or IFN-γ in response to SEB, where the frequency of cytokine producing cells was significantly greater in adult donors (p=0.005 and p=0.02, respectively; Figure 7A, B). Production of TNF-α and IL-10 did not differ between age groups under any of the tested conditions.
Figure 7. Percentage of CD4+CD45RA+ T cells productive of cytokine following direct TLR-2 co-stimulation.
Purified CD4+CD45RA+ T cells (see T cell stimulation assays) were stimulated as indicated in 24 well tissue culture plates for 18 – 72 h, with Brefeldin A (5 μg/ml) added during the last 12 h of incubation. Cells were harvested and stained with LIVE/DEAD Aqua and anti-CD4-PECy7, followed by fixation and permeabilization in Cytofix/Cytoperm buffer (BD) and stained with anti-IL-2-BV421 or anti-IFN-γ FITC. Expression of IL-2 (A) or IFN-γ (B) by viable CD4+ T cells was assessed by flow cytometry. Autologous, unfractionated CBMC and PBMC stimulated with Staph enterotoxin B (SEB; 5 μg/ml) were included as positive controls. Shown are mean percentages of viable CD4+ T cells with IL-2 expression ± SD (A; n=8 neonate; n=8 adult) and mean percentages of viable CD4+ T cells with IFN-γ expression ± SD (B; n=5 neonate; n=5 adult). Data were analyzed using two-way ANOVA followed by Tukey-Kramer adjustment for multiple comparisons. Adjusted p values < 0.05 were considered significant and are indicated in figure. Representative flow plots from neonatal donors are shown in Supplemental Figure 4.
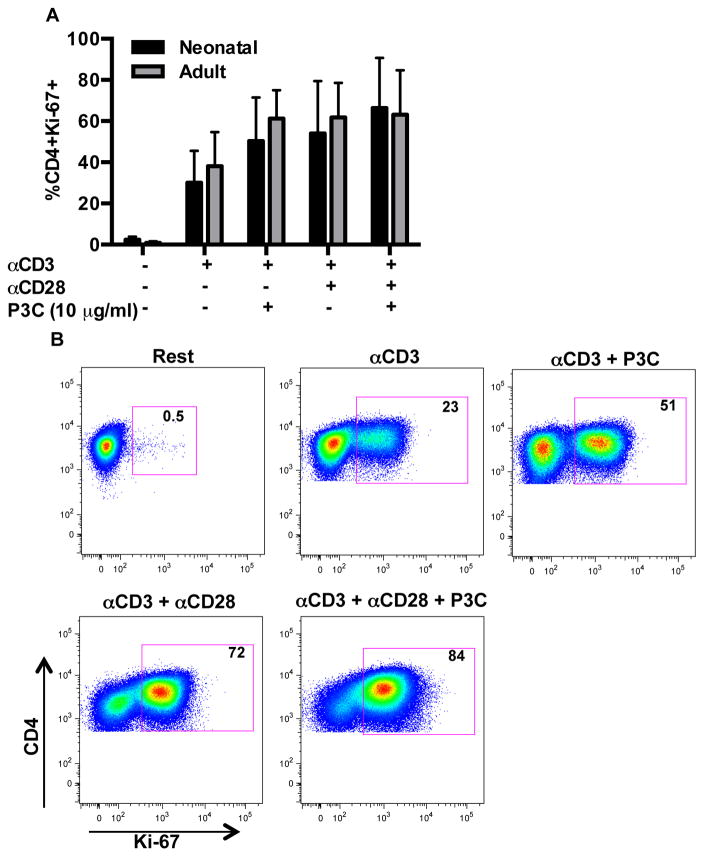
Neonatal naïve CD4+ T cells do not display a proliferation defect in response to TLR-2 mediated co-stimulation
We examined the impact of TLR-2 co-stimulation on proliferation of naïve neonatal and adult CD4+ T cells utilizing Ki-67 expression as a marker of cell cycle entry (Figure 8A/B) (40). Ki-67 expression significantly increased following stimulation of naïve CD4+ T cells from both neonatal and adult donors over the course of 96 hours with αCD3 plus αCD28 (p = 0.003 and p < 0.001 compared to resting condition, respectively). Minimal increases in Ki-67 expression were observed with addition of P3C to αCD3 with or without αCD28 in both age groups (p > 0.05 all comparisons). Notably, the proliferative response of neonatal and adult naïve CD4+ T cells did not differ significantly under any of the tested conditions (p > 0.05 all comparisons).
Figure 8. TLR-2 induced CD4+ T cell proliferation in neonatal and adult donors.
To assess for T cell proliferation, purified CD4+CD45RA+ T cells were stimulated as indicated in 24 well tissue culture plates for 96 h and subsequently stained with LIVE/DEAD Aqua and CD4-PECy7, followed by fixation and permeabilization in FoxP3 staining buffer and staining with anti-Ki-67-BV421. (A) Shown are mean percentages of viable CD4+ T cells with Ki-67 expression ± SD (n=7 cord; n=7 adult). Data were analyzed using two-way ANOVA followed by Tukey-Kramer adjustment for multiple comparisons. Adjusted p values < 0.05 were considered significant. Minimal increases in Ki-67 expression were observed with addition of P3C to αCD3 ± αCD28 in both age groups (p > 0.05 all comparisons). The proliferative response of neonatal and adult naïve CD4+ T cells did not differ significantly under any of the tested conditions.
(B) A representative flow plot from a neonatal donor is shown.
Neonatal naïve CD4+ T cells are not deficient in up-regulation of activation markers CD25, CD69, and CD154 following TLR-2 co-stimulation
TCR stimulation drives up-regulation of T cell activation markers such as CD25, CD69 and CD154, and TLR-2 co-stimulation of human adult CD4+ T cells has been shown to significantly increase expression of CD25 (11). In both age groups, we observed up-regulation of CD25, CD69, and CD154 when highly purified, resting, naïve CD4+ T cells were exposed to αCD3 with or without αCD28 (Table II). Although a trend towards increased expression of these markers was observed with the addition of P3C to αCD3 with or without αCD28, statistical comparisons were not significant (p > 0.05 all comparisons).
Table II.
Expression of activation markers by naïve CD4+ T cells from neonatal and adult donors following TLR-2 co-stimulation1
| Rest | αCD3 | αCD3 + P3C (10 μg/ml) | αCD3 + αCD28 | αCD3+ αCD28 + P3C (10 μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Neonate | Adult | Neonate | Adult | Neonate | Adult | Neonate | Adult | Neonate | Adult | |
| CD25 | 2.6 ± 1 | 2.4 ± 1 | 29 ± 7 | 23 ± 8 | 53 ± 8* | 39* ± 9 | 31* ± 7 | 28 ± 9 | 55 ± 7* | 40 ± 10* |
| CD69 | 0.2 ± 0.1 | 0.3 ± 0.1 | 40 ± 8* | 59 ± 5* | 57 ± 8* | 62 ± 5* | 40 ± 9* | 61 ± 6* | 56 ± 9* | 64 ± 4* |
| CD154 | 1.1 ± 1 | 0.2 ± 0.1 | 24 ± 8 | 29 ± 4 | 49 ± 14* | 48 ± 9* | 24 ± 8 | 28 ± 5 | 50 ± 14* | 47 ± 10* |
Expression of individual activation markers (CD25, CD69, and CD541) on highly purified naïve CD4+ T cells from neonatal and adult donors stimulated for 18 h under the indicated conditions (shown are mean percentage ± SEM from 8 experiments using 8 adult and 8 cord donors). There were no significant differences in expression of any activation markers under the tested conditions between neonatal and adult donors.
Indicates p-value < 0.01 for within age-group comparison of stimulated to resting condition using two-way ANOVA followed by Tukey-Kramer adjustment for multiple comparisons.
Discussion
The majority of work detailing contributions of TLR to immunity has focused on the response of APC, such as monocytes, dendritic cells, and B cells, to PAMPs. This includes a body of research detailing aberrant TLR-induced cytokine responses in whole blood, mononuclear cell populations, and fractionated monocytes and dendritic cells from cord, neonatal, and infant peripheral blood (23–27, 41–44). Subsequent in vitro models have demonstrated that in conjunction with anti-CD3 signaling, some TLR can function as direct (in the absence of APC) co-stimulatory receptors on memory CD4+ T cells (11, 12, 16, 19, 30). The impact of TLR co-stimulation on naïve CD4+ T cell activation and effector phenotype, however, has remained unclear. This area of immunobiology is particularly relevant to understanding unique features of neonatal T cell activation and development as recognition of PAMPs by TLR culminates in activation of NF-κB, and initiates cytokine and chemokine production to initiate, support, and shape subsequent immune responses (45). As reduced production of Th-1 supporting cytokines such as IL-12p70 by APC has been observed in cord and infant blood, the identification of an APC-independent mechanism to promote pro-inflammatory T cell immunity could represent a novel immune-based therapy against intracellular pathogens for this vulnerable population (16, 42, 43). Thus, in our current work we explored the impact of TLR-2 co-stimulation on naïve CD4+ T cells from neonates and adults. We chose to specifically study CD4+ T cell use of TLR-2 as this TLR has been most consistently demonstrated to serve as a co-stimulatory molecule on human CD4+ T cells (12, 30, 46). We focused on CD4+ T cells with a naïve phenotype as these are the predominate CD4+ T cells found in newborn infants, and are most relevant for exploring how T cell TLR co-stimulation could shape primary immune responses to pathogens.
Our study demonstrates that naïve CD4+ T cells from both neonatal and adult blood generate a robust, Th-1 type cytokine response characterized by increased IL-2 and IFN-γ production, and reduced IL-10 secretion, in response to TLR-2 co-stimulation with the prototypical, synthetic TLR-1/2 ligand P3C. Moreover, we have shown that for naïve CD4+ T cells from neonates, P3C alone provides a potent co-stimulatory signal for pro-inflammatory cytokine production, and corrects deficient IFN-γ production from neonatal CD4+ T cells that is observed following polyclonal activation with αCD3 with or without αCD28. These findings confirm that neonatal naïve CD4+ T cells are not intrinsically deficient compared to adult naïve CD4+ T cells in their capacity to generate pro-inflammatory cytokines; rather neonatal CD4+ T cells require co-stimulation through an alternative co-receptor (TLR-2) to reach and/or exceed adult level cytokine production. Our findings suggest that vaccination strategies utilizing TLR-2 based adjuvants could directly drive T cells towards a Th-1 type immune response in populations such as newborns that are otherwise predisposed towards Th-2 and Th-17 responses (44).
Although the mechanisms permissive to altered cytokine production by TLR signaling in APC such as macrophages and dendritic cells have been elucidated (47), this is an under-studied area of T cell biology. As shown in Figure 2, among both neonatal and adult donors, TLR-2 mediated co-stimulation in the absence of CD28 signaling significantly increased IL-2 secretion compared to stimulation with plate-bound αCD3 alone. Through its activation of PI-3 kinase (PI3K) signaling and the Akt kinase, CD28 has a pivotal role in eliciting T cell IL-2 production following TCR engagement (48). Our findings are notable in that direct TLR-2 co-stimulation can replace CD28-mediated signaling to elicit robust IL-2 production, and that this effect is more potent for naïve CD4+ T cells derived from neonatal donors (Figure 6). Earlier publications have shown that activation of murine CD4+ T cells via TLR-9, and activation of murine CD8+ T cells via TLR-2, has been linked to the PI3K pathway (49, 50). Thus, we hypothesize that signaling through the PI3K pathway is critical to TLR-2 mediated production of IL-2 by human naïve CD4+ T cells, and this is an on-going area of research by our group. We also observed that direct TLR-2 co-stimulation with P3C results in IFN-γ secretion that is most pronounced at low concentrations of the TLR-2 agonist, and exceeds that observed following co-stimulation with αCD28 (Supplemental Figure 3). TLR-2 signaling was recently shown to enhance Tbet expression in a MyD88- dependent fashion and results in improved IFN-γ production by chronically stimulated human Th-1 cells. However, the precise cellular mechanisms governing IFN-γ production following TLR-2 mediated co-stimulation of CD4+ T cells have not yet been described (51).
A recent in vivo study demonstrated the protective benefits of priming naïve CD4+ T cells in conjunction with direct TLR-2 co-stimulation in a murine challenge model with Mycobacterium tuberculosis (Mtb) infection (52). Here, provision of Mtb-specific TCR transgenic CD4+ T cells to TLR-2−/− animals subsequently immunized with corresponding antigen plus P3C resulted in increased frequency of antigen-specific IFN-γ T cells compared to immunization with antigen alone. Moreover, priming transgenic CD4+ T cells with antigen plus P3C prior to transfer provided enhanced protection against disease progression compared to priming with antigen alone (52). Although this study demonstrates that TLR-2 mediated co-stimulation of CD4+ T cells impacts both the nature and effectiveness of a resulting immune response in vivo, the precise mechanisms responsible for this protective effect remain unclear. Specifically, whether all naïve CD4+ T cells express and utilize TLR-2, or if this is a feature of a unique subset of cells, remains unknown. In fact, our own data and that of others (11, 52) suggest that a fraction of resting CD4+ T cells express cell surface TLR-2, and that a minority of naïve CD4+ T cells generate cytokine in response to TLR-2 co-stimulation. Thus, TLR-2 co-stimulation of naïve CD4+ T cells may act to intensify cytokine production by a subset of T cells.
The role of TLR in autoimmune disease is an active area of study, and various TLR have been associated with the pathogenesis of disorders relevant to young children such as insulin dependent diabetes, allergy, and inflammatory bowel disease (53). Moreover, reversal of regulatory T cell suppressive function has been reported following TLR-2 co-stimulation (18, 54), and if this occurs in vivo, could promote proinflammatory conditions associated with autoimmunity. It is notable that the precise role of TLR in the development of these disorders remains controversial, however, as both induction of tolerance and exacerbation of autoreactivity have been described. Given the widespread expression of TLR on immune and non-immune cells throughout the body, the mechanism(s) that determines the dominant effect of TLR stimulation in vivo are likely extremely complex. Moreover, the context or microenvironment in which TLR stimulation occurs (ie, lymph node, gut mucosa, pancreas) likely shapes the resulting dominant immune phenotype. The in vitro model utilized in our current study demonstrated that Th-1 is the dominant phenotype elicited by direct TLR-2 stimulation of naïve CD4+ T cells. However, in vivo models have revealed a more complex picture. Using murine, naïve CD4+ T cells in vitro, Reba et. al. found that direct TLR-2 co-stimulation did not result in production of IL-17 (52). However, in their in vivo model, co-administration of the TLR-2 agonist P3C with antigen did significantly increase the number of IL-17 producing antigen-specific CD4+ T cells. Thus, conditions present in vivo (activated APC, additional co-stimulatory signals, and endogenous cytokines such as TGF-β and IL-6) are clearly required to reveal the full consequences of TLR-2 modulation of CD4+ T cell phenotype and function.
Prior studies have demonstrated significant increases in T cell proliferation and expression of activation markers such as CD25 following TLR-2 mediated co-stimulation of unfractionated or memory, adult CD4+ T cells (11, 12, 16, 30). In our model, TLR-2 co-stimulation did not significantly increases any of these activation parameters beyond what was observed with αCD3 with or without αCD28. These differences are likely secondary to our use of highly purified naïve T cells. Our data do confirm prior reports that neonatal T cells do not demonstrate a proliferation defect to polyclonal stimuli compared to adult responses (2, 3, 55), and provide new data demonstrating that neonatal CD4+ T cells are not deficient in their capacity to up-regulate activation markers CD25, CD69, or CD154.
Co-stimulation is essential for naïve T cell priming; therefore, utilization of TLR-2 for co-stimulation of naïve CD4+ T cells could serve to augment immune responses to pathogens in situations where APC co-stimulatory function is compromised, or when cytokines to direct Th-1 cell development are not readily produced. Therefore, we utilized a model to specifically address the intrinsic capacity of naïve CD4+ T cells to utilize TLR-2 as a co-stimulatory receptor, and to avoid confounding effects of APC present in cell culture. This approach limits extension of our findings to in vivo immune responses where TCR stimulation is provided by APC presenting peptide-loaded MCH-II molecules, and immunomodulatory extrinsic factors such as adenosine are present (41). Our use of naïve CD4+ T cells from neonatal blood allowed us to study T cell TLR-2 responses at an age when susceptibility to a variety of pathogens is at an extreme. Future studies by our group will determine how T cell responses to TLR co-stimulation change with normal development throughout infancy.
In summary, we have demonstrated that human naïve CD4+ T cells demonstrate a robust pro-inflammatory, Th-1 type cytokine profile in response to direct TLR-2 co-stimulation, characterized by increased IL-2 and IFN-γ and diminished IL-10 production. Moreover, we have demonstrated that neonatal naïve CD4+ T cells are fully capable of utilizing TLR-2 as a co-stimulatory receptor to elicit IL-2 and IFN-γ production, and that TLR-2 mediated stimulation enables these cells to exhibit features of potent effectors. We believe our findings have implications for the use of TLR-2 ligands as adjuvants for vaccines targeting cell-mediated immune responses in newborns and infants, and could be exploited in protocols to generate antigen specific T cells from cord blood for adoptive immunotherapy.
Supplementary Material
Acknowledgments
We thank the Labor & Delivery staff at OHSU for their collection of cord blood for this study. We thank Dr. Ruth Napier and Dr. Luke Uebelhoer for their critical reviews of this manuscript.
This work was supported by the National Institutes of Health/National Institute of Allergy and Immunology through grants AI104229 and AI083739.
Abbreviations used in this article
- P3C
Pam3Cys4
- PAMPs
pathogen-associated molecular patterns
- CBMC
cord blood mononuclear cells
References
- 1.Miyawaki T, Seki H, Taga K, Sato H, Taniguchi N. Dissociated production of interleukin-2 and immune (gamma) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clin Exp Immunol. 1985;59:505–511. [PMC free article] [PubMed] [Google Scholar]
- 2.Wakasugi N, Virelizier JL. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985;134:167–171. [PubMed] [Google Scholar]
- 3.Bryson YJ, Winter HS, Gard SE, Fischer TJ, Stiehm ER. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980;55:191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- 4.Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. Intracellular cytokine profile of cord blood T-, and NK- cells and monocytes. Haematologica. 2000;85:675–679. [PubMed] [Google Scholar]
- 5.Wilson CB, Westall J, Johnston L, Lewis DB, Dower SK, Alpert AR. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986;77:860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainsford E, Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116:702–709. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- 7.Chipeta J, Komada Y, Zhang XL, Azuma E, Yamamoto H, Sakurai M. Neonatal (cord blood) T cells can competently raise type 1 and 2 immune responses upon polyclonal activation. Cell Immunol. 2000;205:110–119. doi: 10.1006/cimm.2000.1718. [DOI] [PubMed] [Google Scholar]
- 8.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 9.O’Garra A, Murphy K. Role of cytokines in development of Th1 and Th2 cells. Chem Immunol. 1996;63:1–13. doi: 10.1159/000319475. [DOI] [PubMed] [Google Scholar]
- 10.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 11.Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, Pecora ND, Ziady AG, Shank S, Harding CV, Boom WH, Rojas RE. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect Immun. 2011;79:663–673. doi: 10.1128/IAI.00806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood. 2010;116:3494–3504. doi: 10.1182/blood-2010-02-268169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini JC, Speiser D, Romero P. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 15.Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- 16.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 17.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 18.Nyirenda MH, Sanvito L, Darlington PJ, O’Brien K, Zhang GX, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 19.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarron M, Reen DJ. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J Immunol. 2009;182:55–62. doi: 10.4049/jimmunol.182.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Komai-Koma M, Gilchrist DS, Xu D. Direct recognition of LPS by human but not murine CD8+ T cells via TLR4 complex. Eur J Immunol. 2009;39:1564–1572. doi: 10.1002/eji.200838866. [DOI] [PubMed] [Google Scholar]
- 23.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 25.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Shey MS, Nemes E, Whatney W, de Kock M, Africa H, Barnard C, van Rooyen M, Stone L, Riou C, Kollmann T, Hawn TR, Scriba TJ, Hanekom WA. Maturation of innate responses to mycobacteria over the first nine months of life. J Immunol. 2014;192:4833–4843. doi: 10.4049/jimmunol.1400062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maecher HT. Multiparameter Flow Cytometry Monitoring of T cell Responses. In: Prasad VR, Kalpana GV, editors. HIV Protocols: Methods in Molecular Biology. 2. Humana Press; 2009. pp. 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancioni CL, Thomas JJ, Rojas RE. Activation requirements and responses to TLR ligands in human CD4+ T cells: comparison of two T cell isolation techniques. J Immunol Methods. 2009;344:15–25. doi: 10.1016/j.jim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, Roederer M. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries JE, Caviles AP, Jr, Bont WS, Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979;122:1099–1107. [PubMed] [Google Scholar]
- 36.Kern JA, Daniele RP, Nowell PC. Accessory cells provide more than one signal for lectin mitogen-stimulated proliferation of human lymphocytes. J Leukoc Biol. 1985;38:495–507. doi: 10.1002/jlb.38.4.495. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstreich DL, Farrar JJ, Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976;116:131–139. [PubMed] [Google Scholar]
- 38.Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 39.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 40.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES, 3rd, Kollmann TR. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 46.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176:3885–3889. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- 47.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 49.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol. 2009;182:1860–1867. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 51.Chodisetti SB, Gowthaman U, Rai PK, Vidyarthi A, Khan N, Agrewala JN. Triggering through Toll-like receptor 2 limits chronically stimulated T-helper type 1 cells from undergoing exhaustion. J Infect Dis. 2015;211:486–496. doi: 10.1093/infdis/jiu472. [DOI] [PubMed] [Google Scholar]
- 52.Reba SM, Li Q, Onwuzulike S, Ding X, Karim AF, Hernandez Y, Fulton SA, Harding CV, Lancioni CL, Nagy N, Rodriguez ME, Wearsch PA, Rojas RE. TLR2 engagement on CD4(+) T cells enhances effector functions and protective responses to Mycobacterium tuberculosis. Eur J Immunol. 2014;44:1410–1421. doi: 10.1002/eji.201344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulden E, Wen L. Toll-Like Receptor Activation in Immunity vs. Tolerance in Autoimmune Diabetes. Front Immunol. 2014;5:119. doi: 10.3389/fimmu.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyirenda MH, Morandi E, Vinkemeier U, Constantin-Teodosiu D, Drinkwater S, Mee M, King L, Podda G, Zhang GX, Ghaemmaghami A, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J Immunol. 2015;194:5761–5774. doi: 10.4049/jimmunol.1400472. [DOI] [PubMed] [Google Scholar]
- 55.Trivedi HN, HayGlass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.