Abstract
Purpose
Previous studies have reported conflicting results on the association between Helicobacter pylori infection and osteoporosis. A few studies have discussed the influence of H. pylori eradication therapy on bone mineral density.
Methods
We assessed the prevalence of osteoporosis among the H. pylori-infected population in Taiwan and the influence of early and late H. pylori eradication therapy on bone mineral density.
Results
Using data from Taiwan's National Health Insurance Research Database, we identified 5,447 patients who received H. pylori eradication therapy from 2000 to 2010 and 21,788 controls, frequency-matched according to age, sex, and year of receiving H. pylori eradication therapy. Those who received H. pylori eradication therapy were divided into two groups based on the time interval between the diagnosis of a peptic ulcer and commencement of eradication therapy. The risk of developing osteoporosis was higher in the early H. pylori treatment cohort (hazard ratio [HR] = 1.52, 95% confidence interval [CI] = 1.23–1.89) and late H. pylori treatment cohort (HR = 1.69, 95% CI = 1.39–2.05), compared with the risk in the control cohort. When followed for less than 5 years, both the early and late cohorts had a higher risk of developing osteoporosis (HR = 1.69, 95% CI = 1.32–2.16 and HR = 1.72, 95% CI = 1.38–2.14). However, when the follow-up period was over 5 years, only the late eradication group exhibited a higher incidence of osteoporosis (HR = 1.62, 95% CI = 1.06–2.47).
Conclusion
The development of osteoporosis is complex and multi-factorial. Via this population-based cohort study and adjustment of possible confounding variables, we found H. pylori infection may be associated with an increased risk of developing osteoporosis in Taiwan. Early eradication could reduce the influence of H. pylori infection on osteoporosis when the follow-up period is greater than 5 years. Further prospective studies are necessary to discover the connection of H. pylori and osteoporosis.
Introduction
Osteoporosis is a common, but difficult to detect, disease among elderly people and is characterized by decreased bone mineral density. The World Health Organization claimed that osteoporotic fractures account for 2.8 million disability-adjusted life years annually in Americans and Europeans. Previous studies have reported age, sex, cigarette smoking, low body mass index, steroid use, and chronic alcohol consumption [1–4] as risk factors for osteoporosis.
Gastrointestinal diseases are also believed to contribute to the development of osteoporosis [5]. Both Tovey et al. and Bisballe et al. observed decreased bone mineral density in post-gastrectomy patients [6, 7]. Previous studies have also described an association between celiac disease and osteoporosis [8]. Inflammatory bowel disease was also suspected of increasing the risk of osteoporosis [9]. Recently, peptic ulcers and atrophic gastritis have been suspected of being risk factors for osteoporosis [10, 11].
Research suggests Helicobacter pylori infection is associated with several gastrointestinal diseases, such as chronic gastritis, peptic ulcers, gastric cancer, and mucosa-associated lymphoid tissue lymphoma [12–16]. Recent reports have described the investigation into the extra-gastric effects of H. pylori, including coronary artery disease, chronic kidney disease, dementia, and anemia [17–19].
Whether H. pylori infection is a risk factor for osteoporosis remains controversial [20–23]. However, only a few studies have discussed the influence of H. pylori eradication therapy on bone mineral density. Since H. pylori infection is the most common chronic bacterial infection of the human upper gastrointestinal tract [24, 25], people who received H. pylori eradication therapy may have different durations of chronic H. pylori infection. We suspected that early H. pylori eradication therapy could minimize the influence of chronic H. pylori infection on bone mineral density. We conducted a population-based retrospective cohort study by using records from the Taiwan National Health Insurance Research Database (NHIRD) to investigate the incidence of osteoporosis among people who received H. pylori eradication therapy and the influence of early and late H. pylori eradication therapy on bone.
Materials and Methods
Data Source
A retrospective cohort study was assembled using data from the Longitudinal Health Insurance Database 2000 (LHID2000) provided by Taiwan’s National Health Research Institute (NHRI), which includes information on outpatient, ambulatory, and hospital inpatient care as well as dental services. The National Health Insurance (NHI) program was launched on March 1, 1995 and covered almost 99% of the 23 million residents of Taiwan [26]. The details of the program and the LHID2000 have been adequately described previously [27, 28]. The diagnostic codes in the current study were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The study was approved by the Institutional Review Board (IRB) of the China Medical University and Hospital (CMUH104-REC2-115).
Sampled Participants
We recruited patients who were aged 20 years or above who had been diagnosed with peptic ulcer disease (ICD-9-CM codes 531, 532, and 533) and subsequently received H. pylori eradication therapy. According to the insurance benefits of the NHI system, H. pylori-related treatments are confirmed with gastroscopy and biopsy. H. pylori eradication treatment with triple or quadruple therapy was defined as involving multiple differential medicinal treatments: a proton pump inhibitor or H2 receptor blocker, clarithromycin or metronidazole, and amoxicillin or tetracycline, with or without bismuth (details of all eligible H. pylori eradication regimens have been reported previously) [29]. These drug combinations were prescribed in the same order, and the duration of therapy was between 7 and 14 days. Patients who received H. pylori eradication therapy within 1 year of diagnosis of peptic ulcer disease were included in the early eradication cohort. Patients who received H. pylori eradication therapy after 1 year of being initially diagnosed were included in the late eradication cohort. The index date for patients was set as the date that they first received H. pylori eradication therapy. The exclusion criteria were missing data regarding date of birth, sex, or history of osteoporosis (ICD-9-CM codes 733.0–733.1) before the index date. Controls were randomly selected from the pool of participants without peptic ulcer disease who did not receive H. pylori eradication therapy. Four controls were frequency-matched to each H. pylori eradication case according to age (every 5 y span), sex, and year of receiving H. pylori eradication therapy.
Outcome and Variables of Interest
The general diagnostic process of the NHI program was based on physical examination and quantitative ultrasound. Diagnosis in partial patients was confirmed using dual energy x-ray absorptiometry following the guidance issued by the Health Promotion Administration, Ministry of Health and Welfare in Taiwan on osteoporosis diagnosis and treatment. All patients were followed until a diagnosis of osteoporosis was made or they were censored for loss to follow-up, withdrawal from the NHI program, or December 31, 2011, whichever occurred first. Coronary artery disease (CAD) (ICD-9-CM codes 410–414), alcohol-related illness (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3), stroke (ICD-9-CM codes 430–438), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 491, 492, 496), asthma (ICD-9-CM code 493), rheumatoid arthritis (ICD-9-CM code 714), and steroid use were considered as covariates.
Statistical Analysis
The chi-square test and Student’s t-test were used to determine differences in categorical and continuous variables between H. pylori eradication (including early and late H. pylori eradication therapy) and control cohorts. The cumulative incidence of osteoporosis among the three cohorts was plotted using the Kaplan–Meier method, and the difference was tested using a log-rank test. The incidence density rate of osteoporosis was calculated for each instance of early H. pylori eradication therapy, late H. pylori eradication therapy, and for the control cohort. The incidence rate ratios of the H. pylori eradication cohorts to that of the control cohort and the 95% confidence interval (CI) were estimated using a Poisson regression model. Multivariable Cox proportional hazard regression analysis was performed to estimate the relative hazard ratios (HRs) and 95% CIs of osteoporosis development for the H. Pylori eradication cohorts adjusted for age, sex, and comorbidities of CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis, and steroid use. All data analyses were performed using the SAS statistical package (Version 9.4 for Windows; SAS institute, Inc., Cary, NC, USA). A 2-tailed P<0.05 indicated statistical significance.
Results
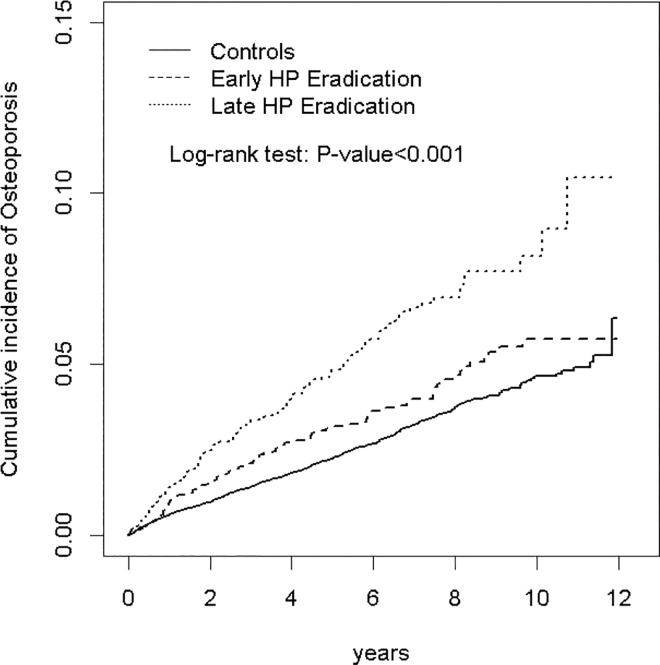
In this study, 5,447 patients who received H. pylori eradication therapy—comprising 2,721 who received early H. pylori eradication therapy and 2,726 who received late H. pylori eradication therapy—and 21,788 controls were investigated (Table 1). Among the three cohorts, most patients were male (62.6% in the early H. pylori eradication cohort, 59.2% in the late H. pylori eradication cohort, and 60.9% in the control cohort, separately). The mean age was 51.1±14.3 years in the H. pylori eradication cohorts and 50.6±14.7 in the control cohort. Both the early and late H. pylori eradication cohorts exhibited a higher prevalence of CAD, alcohol-related illness, COPD, asthma, rheumatoid arthritis, and steroid use compared with the control cohort (P<0.05). The mean follow-up periods were 5.91±3.10 years in the early H. pylori eradication cohort, 5.07±2.77 years in the late H. pylori eradication cohort, and 5.52±2.96 years in the control cohort. After 12 years of follow-up, the cumulative incidence of osteoporosis was higher in the early and late H. pylori eradication cohorts than in the control cohort (P<0.001 and P<0.001, respectively).
Table 1. Comparison of demographics and comorbidity between gastric disease with H pylori eradication and controls.
| Control (N = 21788) | Early HP Eradication (N = 2721) | Late HP Eradication (N = 2726) | Total (N = 5447) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p-value | |
| Age, year | 0.99 | ||||||||
| ≤49 | 10560 | 48.5 | 1518 | 55.8 | 1122 | 41.2 | 260 | 48.5 | |
| 50–65 | 7228 | 33.2 | 834 | 30.7 | 973 | 35.7 | 1807 | 33.2 | |
| ≥65 | 4000 | 18.4 | 369 | 13.6 | 631 | 23.2 | 1000 | 18.4 | |
| Mean (SD) # | 50.6 | 14.7 | 48.5 | 13.9 | 53.6 | 14.3 | 51.1 | 14.3 | 0.03 |
| Sex | 0.99 | ||||||||
| Female | 8528 | 39.1 | 1019 | 37.5 | 1113 | 40.8 | 2132 | 39.1 | |
| Male | 13260 | 60.9 | 1702 | 62.6 | 1613 | 59.2 | 3315 | 60.9 | |
| Comorbidity | |||||||||
| CAD | 2038 | 9.35 | 286 | 10.5 | 647 | 23.7 | 933 | 17.1 | <0.001 |
| Alcohol-related illness | 673 | 3.09 | 132 | 4.85 | 216 | 7.92 | 348 | 6.39 | <0.001 |
| Stroke | 621 | 2.85 | 64 | 2.35 | 116 | 4.26 | 180 | 3.30 | 0.08 |
| COPD | 1449 | 6.65 | 200 | 7.35 | 483 | 17.7 | 683 | 12.5 | <0.001 |
| Asthma | 957 | 4.39 | 127 | 4.67 | 288 | 10.6 | 415 | 7.62 | <0.001 |
| Rheumatoid arthritis | 26 | 0.12 | 6 | 0.22 | 7 | 0.26 | 13 | 0.24 | 0.04 |
| Medication | |||||||||
| Steroid used | 820 | 3.76 | 117 | 4.30 | 269 | 9.87 | 386 | 7.09 | <0.001 |
Chi-square test compared to total gallstone
#Two sample t-test.
The incidence density rates were 6.09, 9.84, and 4.69 per 1000 person-years in the early H. pylori eradication cohort, late H. pylori eradication cohort, and control cohort, respectively (Table 2).
Table 2. Hazard ratios of Osteoporosis between gastric disease with late HP Eradication and control subjects as well as gastric disease with early HP Eradication and control subjects stratified by demographics and comorbidity.
| Control (N = 21788) | Early HP Eradication (N = 2721) | IRR * | Adjusted HR† | Late HP Eradication (N = 2726) | IRR* | Adjusted HR† | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Rate# | Case | Rate# | (95% CI) | (95% CI) | Case | Rate# | (95% CI) | (95% CI) | |
| All | 564 | 4.69 | 98 | 6.09 | 1.30 (1.16, 1.45)*** | 1.52 (1.23, 1.89)*** | 136 | 9.84 | 2.10 (1.91, 2.31)*** | 1.69 (1.39, 2.05)*** |
| Gender | ||||||||||
| Female | 369 | 7.89 | 60 | 10.0 | 1.27 (1.07, 1.50)** | 1.43 (1.09, 1.88)** | 79 | 14.5 | 1.83 (1.57, 2.13)*** | 1.63 (1.27, 2.10)*** |
| Men | 195 | 2.65 | 38 | 3.77 | 1.42 (1.23, 1.64)*** | 1.76 (1.24, 2.49)** | 57 | 6.82 | 2.57 (2.28, 2.90)*** | 1.76 (1.29, 2.40)*** |
| Age | ||||||||||
| ≤49 | 82 | 1.31 | 20 | 2.08 | 1.59 (1.36, 1.86)*** | 1.62 (0.99, 2.65) | 18 | 2.91 | 2.23 (1.89, 2.62)*** | 1.80 (1.06, 3.06)* |
| ≥50 | 482 | 8.38 | 78 | 12.1 | 1.44 (1.24, 1.67)*** | 1.41 (1.11, 1.79)** | 118 | 15.4 | 1.84 (1.63, 2.09)*** | 1.54 (1.25, 1.90)*** |
| Comorbidity‡ | ||||||||||
| No | 366 | 3.68 | 59 | 4.67 | 1.27 (1.12, 1.44)*** | 1.49 (1.13, 1.96)** | 50 | 6.08 | 1.65 (1.44, 1.89)*** | 1.60 (1.19, 2.16)** |
| Yes | 198 | 9.52 | 39 | 11.4 | 1.19 (0.96, 1.49) | 1.57 (1.11, 2.21)* | 86 | 15.4 | 1.61 (1.37, 1.90)*** | 1.70 (1.32, 2.19)*** |
Rate#, incidence rate, per 1000 person-years; IRR*, incidence rate ratio; Adjusted HR†, multiple analysis including age, sex, and co-morbidities of CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis, medication of steroid used; Comorbidity‡: Only to have one of comorbidities (including CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis) classified as the comorbidity group
*p<0.05
**p<0.01
***p<0.001
After adjustment for age, sex, and comorbidities, the risk of developing osteoporosis was higher in the early H. pylori eradication cohort (HR = 1.52, 95% CI = 1.23–1.89) and late H. pylori eradication cohort (HR = 1.69, 95% CI = 1.39–2.05) than in the control cohort. The overall incidence and risk of osteoporosis were compared in the late H. pylori eradication cohort and control cohort according to several variables including age, sex, and comorbidities. The risk of osteoporosis in the late H. pylori eradication cohort was also higher than that of the control cohort. The risk of osteoporosis in patients receiving early H. pylori eradication therapy was also higher than that of the control cohort in all cases, except for those aged ≤49 years.
The risk of osteoporosis was compared in the early H. pylori eradication cohort and the late H. pylori eradication cohort according to several variables including age, sex, and comorbidities. The risk of osteoporosis in patients receiving early H. pylori eradication therapy was lower than that of the late eradication cohort; however, this difference was not statistically significant (Table 3).
Table 3. Hazard ratios of Osteoporosis between all gastric disease patients with early HP Eradication and with late HP Eradication stratified by demographic characteristics and comorbidity.
| Early HP Eradication | Late HP Eradication | |||
|---|---|---|---|---|
| IRR* | Adjusted HR† | IRR* | Adjusted HR† | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| All | 1 (Reference) | 1 (Reference) | 1.61 (1.38, 1.89)*** | 1.11 (0.85, 1.46) |
| Gender | ||||
| Female | 1 (Reference) | 1 (Reference) | 1.44 (1.13, 1.84)** | 1.11 (0.79, 1.57) |
| Men | 1 (Reference) | 1 (Reference) | 1.81 (1.48, 2.22)*** | 1.02 (0.66, 1.58) |
| Age | ||||
| ≤49 | 1 (Reference) | 1 (Reference) | 1.40 (1.09, 1.79)** | 1.15 (0.59, 2.22) |
| ≥50 | 1 (Reference) | 1 (Reference) | 1.28 (1.04, 1.57)* | 1.10 (0.82, 1.48) |
| Comorbidity‡ | ||||
| No | 1 (Reference) | 1 (Reference) | 1.30 (1.07, 1.59)** | 1.05 (0.72, 1.53) |
| Yes | 1 (Reference) | 1 (Reference) | 1.35 (1.04, 1.77)* | 1.09 (0.75, 1.60) |
IRR*, incidence rate ratio; Adjusted HR†, multiple analysis including age, sex, and co-morbidities of CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis; Comorbidity‡: Only to have one of comorbidities (including CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis) classified as the comorbidity group
*p<0.05
**p<0.01
***p<0.001
For patients receiving early H. pylori eradication therapy, a 1.69-fold risk of developing osteoporosis was found within 5 years of follow-up (95% CI = 1.32–2.16) (Table 4). There was no statically significant difference between the control group and early H. pylori eradication group when the follow-up period was over 5 years. However, for patients receiving late H. pylori eradication therapy, higher risks were observed for developing osteoporosis in both follow-up periods (<5 y and >5 y; HR = 1.72, 95% CI = 1.38–2.14 and HR = 1.62, 95% CI = 1.06–2.47, respectively).
Table 4. Trends of Osteoporosis risks by stratified follow-up years.
| Control (N = 21788) | Early HP Eradication (N = 2721) | IRR * | Adjusted HR† | Late HP Eradication (N = 2726) | IRR* | Adjusted HR† | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow time, years | Case | Rate# | Case | Rate# | (95% CI) | (95% CI) | Case | Rate# | (95% CI) | (95% CI) |
| ≤5 years | 405 | 4.62 | 75 | 6.67 | 1.44 (1.29, 1.62)*** | 1.69 (1.32, 2.16)*** | 109 | 10.3 | 2.23 (2.02, 2.46)*** | 1.72 (1.38, 2.14)*** |
| >5 years | 159 | 4.87 | 23 | 4.76 | 0.98 (0.82, 1.16) | 1.14 (0.73, 1.77) | 27 | 8.35 | 1.71 (1.46, 2.01)*** | 1.62 (1.06, 2.47)* |
Rate#, incidence rate, per 1000 person-years; IRR *, incidence rate ratio; Adjusted HR†: multiple analysis including age, sex, and co-morbidities of CAD, alcohol-related illness, stroke, COPD, asthma, rheumatoid arthritis
*p<0.05
***p<0.001
Discussion
The main finding of this population-based retrospective cohort study in Taiwan is that people who received H. pylori eradication therapy, those though to have chronic H. pylori infection, have a higher incidence of osteoporosis. The late eradication group, which had a longer period of chronic H. pylori infection, had a higher incidence of osteoporosis than the early eradication group. When the follow-up period was over 5 years, the incidence of osteoporosis among the early H. pylori eradication cohort returned to the levels observed in the control cohort, while the incidence in the late eradication group remained higher. We believe the higher incidence of osteoporosis within the first 5 years could be the result of chronic H. pylori infection before H. pylori eradication therapy, and people could benefit from early eradication therapy when the follow-up period is greater than 5 years.
The NHIRD covers almost 99% of the population of Taiwan and is a representative data source that includes age, sex, and comorbidity information. It enabled this study—the first of its type nationwide—to be conducted with population-based data and a near-decade follow-up period to date. Although our results do not directly indicate the causal relationship and etiological treatise for this association, based on literature review, we believe that there are several possible explanations.
Chronic H. pylori infection may be the reason for the increased incidence of osteoporosis observed after eradication therapy. First, chronic H. pylori infection may alter calcium absorption. H. pylori can either increase gastric acid production, which predisposes one to duodenal ulceration, or decrease gastric acid production, thus leading to pangastritis or gastric ulceration [15, 30]. The hypochlorhydric stomach impairs calcium homeostasis and alters bone mass [31, 32]. The chronic gastritis from H. pylori results in atrophic gastritis, which increases the likelihood of osteoporosis [10, 33]. Second, a recent population-based cohort study in Taiwan suggested that chronic H. pylori infection increased the subsequent risk of end-stage renal disease (ESRD) [17]. Vitamin D, which is crucial in the gastrointestinal absorption of calcium, is commonly insufficient in patients with chronic kidney disease and ESRD [34, 35]. Furthermore, Ayesh et al. found that people with H. pylori infection had lower levels of vitamin B12 [36]. In addition, low plasma levels of vitamin B12 may be associated with low bone mineral density [37].
H. pylori infection may cause chronic systemic inflammation that negatively affects bone mineral density. H. pylori is the most common chronic bacterial infection of the human upper gastrointestinal tract [25, 38], and causes an increase in the serum level of inflammatory cytokines [39, 40]. An elevated serum level of inflammatory cytokines is considered to be associated with an increased risk of osteoporosis [41]. This is consistent with the increased incidence of osteoporosis among the H. pylori eradication groups in our study. Early eradication of H. pylori may prevent chronic inflammation and the subsequent prolonged elevation of serum levels of inflammatory cytokines that lead to osteoporosis, as described by Moss et al. in 1994 [42].
We believed that the increased incidence of osteoporosis among eradication groups could be the result of chronic H. pylori infection before eradication therapy, rather than the effect of eradication therapy. If it were the effect of eradication therapy, the early eradication group might exhibit a higher incidence of osteoporosis, especially in the early years after therapy. In contrary, although non-significant, we observed a lower incidence of osteoporosis among the early H. pylori eradication group compared with the late H. pylori eradication group (Table 3). The early eradication group had a lower incidence of osteoporosis than the late eradication group among first five years after eradication therapy (Table 4). These were against the hypothesis that the eradication therapy would increase incidence of osteoporosis. As shown in Table 4 and Fig 1, the incidence of osteoporosis among the early eradication group returned to the same level as that of the control group over the course of long-term follow-up, whereas the late eradication group’s incidence rate remained high. These could be the evidence that eradication therapy may eliminate chronic inflammation from H. pylori infection before treatment, which decreased the H. pylori related influence on bone density.
Fig 1. Cumulative incidence of Osteoporosis for patients with gastric disease receiving early HP Eradication and Late HP Eradication compared to those without gastric disease.
H. pylori infection is associated with several gastrointestinal diseases, and the medication used in gastrointestinal diseases may be another cause of osteoporosis. Malabsorption of dietary calcium is a cause of osteoporosis, and gastric acid is necessary in calcium absorption [43]. The use of proton pump inhibitors and H2 receptor blockers in H. pylori eradication therapy can block the secretion of gastric acid that decrease calcium salt absorption, leading to osteoporosis. A meta-analysis by Ngamruengphong et al. demonstrated that using a proton pump inhibitor increased the risk of hip and vertebral fractures [44]. In the current study, we found a higher incidence of osteoporosis in the H. pylori eradication groups. This mechanism treatise could correlate with gastrointestinal calcium malabsorption from H. pylori eradication therapy. However, it still requires further approaches to clarify the causal relationship and detailed mechanism.
This study has several limitations. First, the NHIRD does not disclose patients’ socioeconomic status, family history, personal health behaviors (e.g., smoking habits or alcohol consumption), laboratory data, or biomarkers. Osteoporosis could be affected by these confounding factors, and may have influenced our results. Second, the NHIRD database recorded only patients who received therapy for H. pylori infection and osteoporosis. With symptoms that are difficult to detect, the prevalence of both H. pylori infection and osteoporosis could be underestimated. Third, the severity and duration of H. pylori infection could not be assessed in this study. Fourth, we cannot identify the method used to diagnose osteoporosis and the intensity of osteoporosis in the NHIRD database. Finally, the exact mechanism between H. pylori eradication therapy and osteoporosis could not be identified via a retrospective cohort study using the NHIRD.
Conclusion
In this study, we found a higher incidence of osteoporosis among people with H. pylori infection. Early eradication of H. pylori had a relatively lower incidence of osteoporosis when compared with the late eradication group. When the follow-up period was over 5 years, there was no difference between the control group and early H. pylori eradication group. This provides evidence that encourages physicians to manage H. pylori infections early in patients with a high risk of osteoporosis. Additional studies are necessary to clarify the relationship between H. pylori infection and eradication, and osteoporosis, after adjustment for confounding factors, and to identify the mechanism of the relationship between H. pylori and osteoporosis.
Acknowledgments
We thank the Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan for providing the service of statistical analysis, which is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Bio-signature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039–006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NHIRD
National Health Insurance Research Database
- LHID 2000
Longitudinal Health Insurance Database 2000
Data Availability
The data on the study population that were obtained from the National Health Insurance Research Database (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/).
Funding Statement
The Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Bio-signature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. Miss Cheng-Li Lin received her salary from The Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dolev E. [Cigarette smoking and osteoporosis]. Harefuah 1997;132:511–3. [PubMed] [Google Scholar]
- 2.Suzuki T. Risk factors for osteoporosis in Asia. J Bone Miner Metab. 2001;19:133–41 [DOI] [PubMed] [Google Scholar]
- 3.Kouda K, Iki M, Fujita Y, Tamaki J, Yura A, Kadowaki E, et al. Alcohol intake and bone status in elderly Japanese men: baseline data from the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone 2011;49:275–80. 10.1016/j.bone.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 4.Fujita T. Global assessment of risk factors for osteoporosis. J Bone Miner Metab. 2001;19:131–2 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795–841. [DOI] [PubMed] [Google Scholar]
- 6.Bisballe S, Eriksen EF, Melsen F, Mosekilde L, Sorensen OH, Hessov I. Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodelling, vitamin D metabolites, and bone histomorphometry. Gut 1991;32:1303–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tovey FI, Hall ML, Ell PJ, Hobsley M. Postgastrectomy osteoporosis. Br J Surg. 1991;78:1335–7 [DOI] [PubMed] [Google Scholar]
- 8.Mangione RA. Celiac disease and osteoporosis. Am J Health Syst Pharm. 2008;65:1601; author reply 01–2. 10.2146/ajhp080097 [DOI] [PubMed] [Google Scholar]
- 9.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599–604. 10.1016/j.amjmed.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HW, Kim YH, Han K, Nam GE, Kim GS, Han BD, et al. Atrophic gastritis: a related factor for osteoporosis in elderly women. PloS one 2014;9:e101852 10.1371/journal.pone.0101852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawicki A, Regula A, Godwod K, Debinski A. Peptic ulcer disease and calcium intake as risk factors of osteoporosis in women. Osteoporos Int 2003;14:983–6. [DOI] [PubMed] [Google Scholar]
- 12.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer. Int J Cancer. 2006;118:3030–44. [DOI] [PubMed] [Google Scholar]
- 13.Gillen D, el-Omar EM, Wirz AA, Ardill JE, McColl KE. The acid response to gastrin distinguishes duodenal ulcer patients from Helicobacter pylori-infected healthy subjects. Gastroenterology 1998;114:50–7 [DOI] [PubMed] [Google Scholar]
- 14.Tiwari S, Ghoshal U, Ghoshal UC, Dhingra S, Pandey R, Singh M, et al. Helicobacter pylori-induced apoptosis in pathogenesis of gastric carcinoma. Indian J Gastroenterol. 2005;24:193–6 [PubMed] [Google Scholar]
- 15.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. [DOI] [PubMed] [Google Scholar]
- 16.DeGerome JH. Helicobacter pylori and mucosa-associated lymphoid tissue lymphoma. JAMA 1996;276:1034–5 [DOI] [PubMed] [Google Scholar]
- 17.Lin SY, Lin CL, Liu JH, Yang YF, Huang CC, Kao CH. Association between Helicobacter pylori infection and the subsequent risk of end-stage renal disease: a nationwide population-based cohort study. Int J Clin Pract. 2015;69:604–10. 10.1111/ijcp.12602 [DOI] [PubMed] [Google Scholar]
- 18.Figura N, Palazzuoli A, Vaira D, Campagna M, Moretti E, Iacoponi F, et al. Cross-sectional study: CagA-positive Helicobacter pylori infection, acute coronary artery disease and systemic levels of B-type natriuretic peptide. J Clin Pathol. 2014;67:251–7. 10.1136/jclinpath-2013-201743 [DOI] [PubMed] [Google Scholar]
- 19.Chang YP, Chiu GF, Kuo FC, Lai CL, Yang YH, Hu HM, et al. Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study. Gastroenterol Res Pract. 2013;2013:175729 10.1155/2013/175729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantarhormozi MR, Assadi M, Vahdat K, Asadipooya K, Ostovar A, Raissi K, et al. Chlamydia pneumoniae and Helicobacter pylori IgG seropositivities are not predictors of osteoporosis-associated bone loss: a prospective cohort study. J Bone Miner Metab. 2016;34:422–8. 10.1007/s00774-015-0688-9 [DOI] [PubMed] [Google Scholar]
- 21.Asaoka D, Nagahara A, Shimada Y, Matsumoto K, Ueyama H, Matsumoto K, et al. Risk factors for osteoporosis in Japan: is it associated with Helicobacter pylori? Ther Clin Risk Manag. 2015;11:381–91. 10.2147/TCRM.S80647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fotouk-Kiai M, Hoseini SR, Meftah N, Ghadimi R, Bijani A, Noreddini H, et al. Relationship between Helicobacter pylori infection (HP) and bone mineral density (BMD) in elderly people. Caspian J Intern Med. 2015;6:62–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Kakehasi AM, Mendes CM, Coelho LG, Castro LP, Barbosa AJ. The presence of Helicobacter pylori in postmenopausal women is not a factor to the decrease of bone mineral density. Arq Gastroenterol. 2007;44:266–70. [DOI] [PubMed] [Google Scholar]
- 24.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33–9. [PubMed] [Google Scholar]
- 25.Calvet X, Ramirez Lazaro MJ, Lehours P, Megraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013;18 Suppl 1:5–11. 10.1111/hel.12071 [DOI] [PubMed] [Google Scholar]
- 26.Database NHIR. Taiwan. http://nhird.nhri.org.tw/en/index.html (cited in 2015) 2015.
- 27.Wang CC, Chang CT, Lin CL, Lin IC, Kao CH. Hepatitis C Virus Infection Associated With an Increased Risk of Deep Vein Thrombosis: A Population-Based Cohort Study. Medicine 2015;94:e1585 10.1097/MD.0000000000001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu CW, Lin CS, Chen SJ, Lin SH, Lin CL, Kao CH. Risk of type 2 diabetes mellitus in patients with acute critical illness: a population-based cohort study. Intensive Care Med. 2016;42:38–45. 10.1007/s00134-015-4044-2 [DOI] [PubMed] [Google Scholar]
- 29.Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009;137:1641–8 e1-2. 10.1053/j.gastro.2009.07.060 [DOI] [PubMed] [Google Scholar]
- 30.Majumdar D, Bebb J, Atherton J. Helicobacter pylori infection and peptic ulcers. Medicine 2011;39:154–61. [Google Scholar]
- 31.Keller J, Schinke T. The role of the gastrointestinal tract in calcium homeostasis and bone remodeling. Osteoporos Int. 2013;24:2737–48. 10.1007/s00198-013-2335-4 [DOI] [PubMed] [Google Scholar]
- 32.Schinke T, Schilling AF, Baranowsky A, Seitz S, Marshall RP, Linn T, et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med. 2009;15:674–81. 10.1038/nm.1963 [DOI] [PubMed] [Google Scholar]
- 33.Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet 1995;345:1525–8. [DOI] [PubMed] [Google Scholar]
- 34.Pavlovic D, Orlic L. [Vitamin D insufficiency in patients with chronic renal disease]. Lijec Vjesn. 2007;129:426–7. [PubMed] [Google Scholar]
- 35.Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodialysis international. Hemodial Int. 2007;11:315–21. [DOI] [PubMed] [Google Scholar]
- 36.Ayesh MH, Jadalah K, Al Awadi E, Alawneh K, Khassawneh B. Association between vitamin B12 level and anti-parietal cells and anti-intrinsic factor antibodies among adult Jordanian patients with Helicobacter pylori infection. Braz J Infect Dis. 2013;17:629–32. 10.1016/j.bjid.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker KL, Hannan MT, Qiao N, Jacques PF, Selhub J, Cupples LA, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res. 2005;20:152–8. [DOI] [PubMed] [Google Scholar]
- 38.Nabwera HM, Logan RP. Epidemiology of Helicobacter pylori: transmission, translocation and extragastric reservoirs. J Physiol Pharmacol. 1999;50:711–22. [PubMed] [Google Scholar]
- 39.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–9. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree JE, Farmery SM. Helicobacter pylori and gastric mucosal cytokines: evidence that CagA-positive strains are more virulent. Lab Invest. 1995;73:742–5. [PubMed] [Google Scholar]
- 41.Maugeri D, Russo MS, Franzé C, Motta V, Motta M, Destro G, et al. Correlations between C-reactive protein, interleukin-6, tumor necrosis factor-alpha and body mass index during senile osteoporosis. Arch Gerontol Geriatr. 1998;27:159–63. [DOI] [PubMed] [Google Scholar]
- 42.Moss SF, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut 1994;35:1567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70–3. [DOI] [PubMed] [Google Scholar]
- 44.Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–18; quiz 19. 10.1038/ajg.2011.113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on the study population that were obtained from the National Health Insurance Research Database (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/).