Abstract
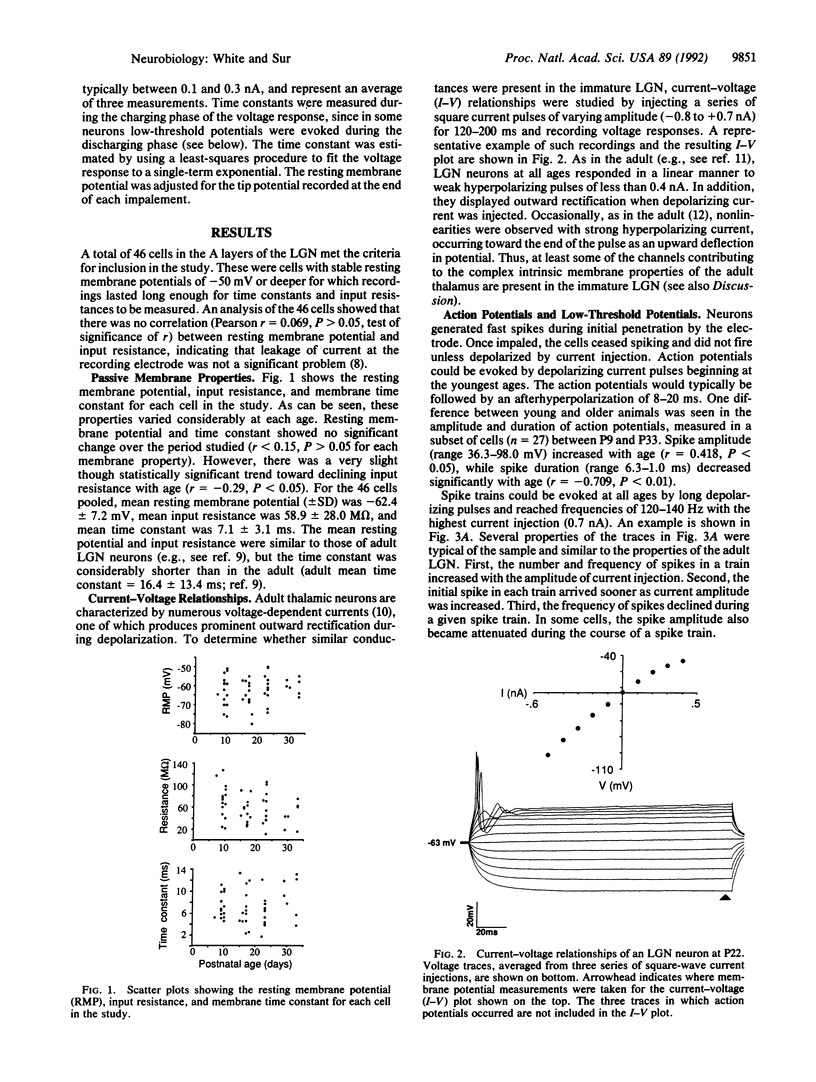
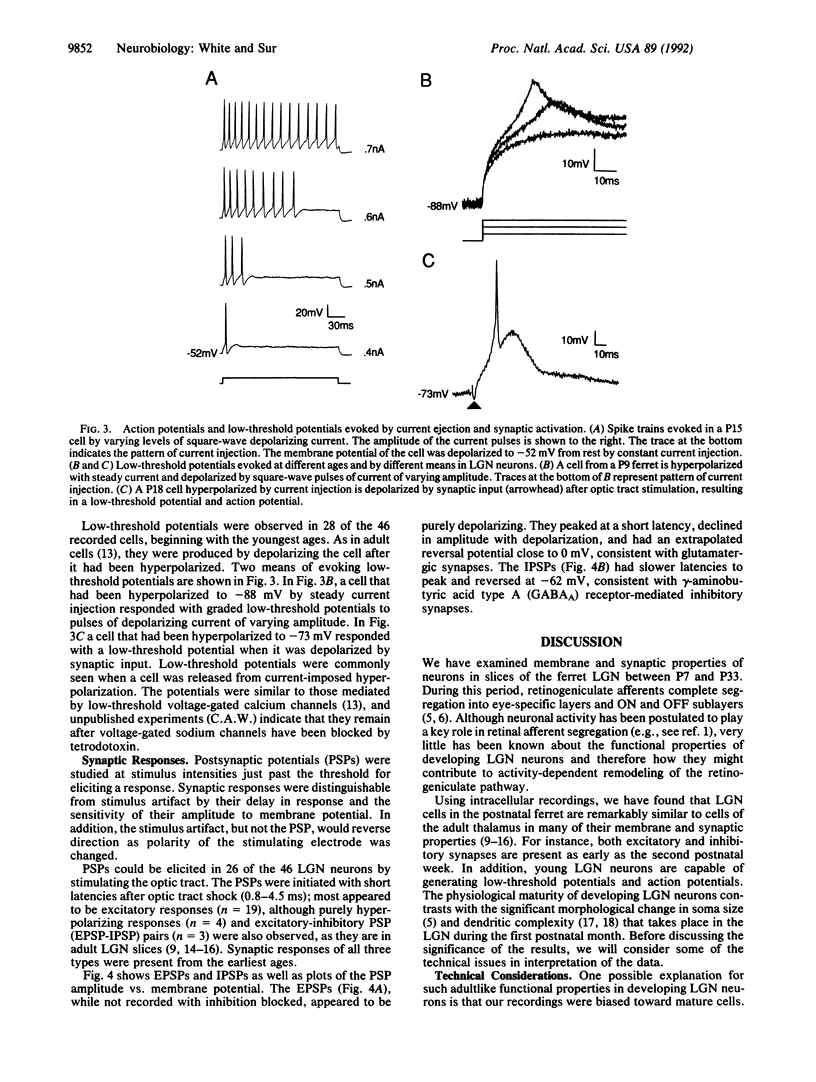
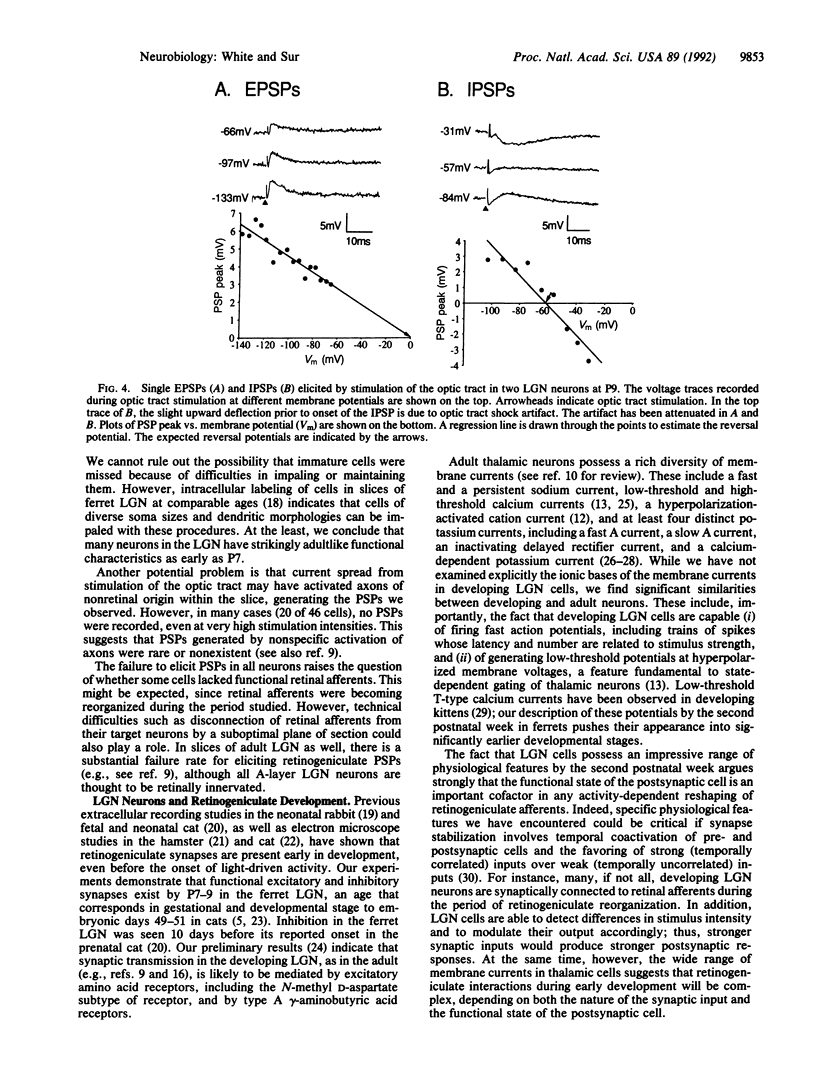
During the first postnatal month in the ferret (Mustela putorius furo), the projections from the retina to the lateral geniculate nucleus (LGN) become segregated into eye-specific layers and ON and OFF sublayers, a process that is thought to depend in part on neuronal activity. Remarkably, virtually nothing is known about the physiological features of LGN neurons during this period. We have recorded intracellularly from 46 A-layer neurons in slices of the ferret LGN between the ages of postnatal days 7 and 33. The passive membrane properties and current-voltage relationships of the developing neurons were similar in many, though not all, respects to those of adult LGN neurons. Action potentials in younger animals were smaller in amplitude and longer in duration than in older animals, but cells at all ages were capable of producing spike trains whose latency and spike number varied with stimulus intensity. In addition, cells at all ages responded with low-threshold potentials upon release from hyperpolarization. Slightly more than half of the LGN neurons responded to optic tract stimulation with excitatory postsynaptic potentials (EPSPs), inhibitory postsynaptic potentials (IPSPs), or EPSP-IPSP pairs, beginning with the youngest ages. Thus, as early as the second postnatal week, and much before the onset of pattern vision, LGN neurons have many of the membrane and synaptic properties of adult thalamic neurons. These data are consistent with LGN cells playing a significant role in activity-dependent reshaping of the retinogeniculate pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., Shatz C. J. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci. 1992 May;12(5):1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., So K. F., Lieberman A. R. Normal postnatal development of retinogeniculate axons and terminals and identification of inappropriately-located transient synapses: electron microscope studies of horseradish peroxidase-labelled retinal axons in the hamster. Neuroscience. 1984 Nov;13(3):743–759. doi: 10.1016/0306-4522(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987 Mar;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. Membrane properties of morphologically identified X and Y cells in the lateral geniculate nucleus of the cat in vitro. J Physiol. 1987 Sep;390:243–256. doi: 10.1113/jphysiol.1987.sp016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M., Kwon Y. H., Sur M. Retinogeniculate EPSPs recorded intracellularly in the ferret lateral geniculate nucleus in vitro: role of NMDA receptors. Vis Neurosci. 1992 Jun;8(6):545–555. doi: 10.1017/s0952523800005642. [DOI] [PubMed] [Google Scholar]
- Galli L., Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988 Oct 7;242(4875):90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Hahm J. O., Langdon R. B., Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991 Jun 13;351(6327):568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A., Pape H. C. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J Neurophysiol. 1989 Jun;61(6):1270–1283. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Coulter D. A., Prince D. A. A fast transient potassium current in thalamic relay neurons: kinetics of activation and inactivation. J Neurophysiol. 1991 Oct;66(4):1304–1315. doi: 10.1152/jn.1991.66.4.1304. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Prince D. A. Slow inactivation of a TEA-sensitive K current in acutely isolated rat thalamic relay neurons. J Neurophysiol. 1991 Oct;66(4):1316–1328. doi: 10.1152/jn.1991.66.4.1316. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Guillery R. W., Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981 Dec 1;203(2):189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Llinás R., Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982 Jun 3;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991 Oct;66(4):1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Pirchio M., Lightowler S., Crunelli V. Postnatal development of the T calcium current in cat thalamocortical cells. Neuroscience. 1990;38(1):39–45. doi: 10.1016/0306-4522(90)90372-b. [DOI] [PubMed] [Google Scholar]
- Rapisardi S. C., Chow K. L., Mathers L. H. Ontogenesis of receptive field characteristics in the dorsal lateral geniculate nucleus of the rabbit. Exp Brain Res. 1975 Mar 27;22(3):295–305. doi: 10.1007/BF00234771. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Lu S. M., Guido W., Adams P. R., Sherman S. M. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Kirkwood P. A. Prenatal development of functional connections in the cat's retinogeniculate pathway. J Neurosci. 1984 May;4(5):1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988 Oct 7;242(4875):87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Sutton J. K., Brunso-Bechtold J. K. A Golgi study of dendritic development in the dorsal lateral geniculate nucleus of normal ferrets. J Comp Neurol. 1991 Jul 1;309(1):71–85. doi: 10.1002/cne.903090106. [DOI] [PubMed] [Google Scholar]



