Abstract
Background
Free radial forearm flap (FRFF) reconstruction is a valuable technique in head and neck surgery, that allows closure of large defects while striving to maintain functionality. Anticoagulative drugs are often administered to improve flap survival, although evidence regarding effectiveness is lacking.
Objective of review
To investigate the effectiveness of postoperative anticoagulants to improve survival of the FRFF in head and neck reconstruction.
Type of review
Systematic review and multicenter, individual patient data meta-analysis.
Search strategy
MEDLINE, EMBASE, Web of Science and CINAHL were searched for synonyms of ‘anticoagulants’ and ‘free flap reconstruction’.
Evaluation method
Studies were critically appraised for directness of evidence and risk of bias. Authors of the highest quality publications were invited to submit their original data for meta-analysis.
Results
Five studies were of adequate quality and data from four studies (80%) were available for meta-analysis, describing 759 FRFF procedures. Anticoagulants used were: aspirin (12%), low-molecular weight dextran (18.3%), unfractioned heparin (28.1%), low-molecular weight heparin (49%) and prostaglandin-E1 (2.1%). Thirty-one percent did not receive anticoagulants. Flap failure occurred in 40 of 759 patients (5.3%) On univariate analysis, use of unfractioned heparin was associated with a higher rate of flap failure. However, these regimens were often administered to patients who had revision surgery of the anastomosis. In multivariate logistic regression analysis, anticoagulant use was not associated with improved flap survival or flap-related complications.
Conclusions
The studied anticoagulative drugs did not improve FRFF survival or lower the rate of flap-related complications. In addition some anticoagulants may cause systemic complications.
Keywords: Free radial forearm flap, free flap reconstruction, anticoagulants, head and neck cancer, meta-analysis
Background
In head and neck surgery microsurgical free flap reconstructions following ablative surgical procedures are often used. Free flap reconstruction has several advantages over primary closure. Wound surface is minimized, to reduce pain and risk of infection1. Second, tissue volume is maintained, leading to better functional outcomes in terms of speech and swallowing2, 3. And thirdly, delicate structures such as vessels or nerves are covered to offer protection, for instance if postoperative radiotherapy is performed. Flaps can be raised from various sites on the body and the success rate is generally high (90–99%)4– 6. In head and neck reconstruction, the free radial forearm flap (FRFF) is the most commonly used fasciocutaneous flap.
Flap loss, however, is considered a serious complication, often caused by arterial or venous thrombus formation in the anastomosis7. To improve flap patency and prevent thrombus formation, anticoagulative drugs can be administered in the postoperative period. Nowadays, administering anticoagulative drugs postoperatively is still popular: 96% of surgeons use some form of anticoagulative treatment after microvascular surgery, in particular aspirin, low-molecular weight dextran and subcutaneous heparin7,10.
The efficacy of anticoagulative drugs to prevent flap loss has been the focus of many studies5, 6, 11–13. However, the effect of anticoagulants on flap survival, and the FRFF in particular, is unclear. We performed a systematic review and multicenter (meta-) analysis of the current literature to investigate the effect of anticoagulants on survival of the FRFF used for reconstruction in the head and neck region.
Methods
Search and selection
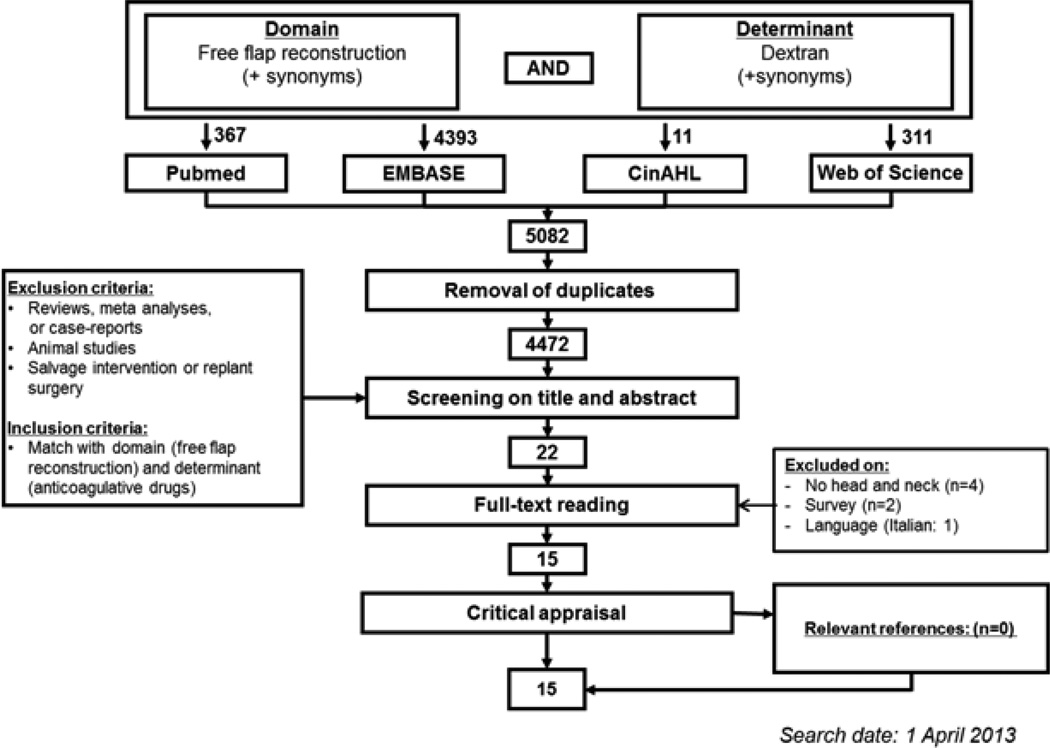
A literature search was performed using PubMed, EMBASE, the Cochrane database and Web of Science for a combination of the domain (free tissue transfer) and the determinant (anticoagulants) (Appendix A). Duplicates were removed using RefWorks. Title and abstract screening was performed using predefined in- and exclusion criteria (Figure 1).
Figure 1. Flowchart.
Appraisal of the evidence
The full text of relevant publications was retrieved. To select only high quality evidence, studies were critically appraised for directness of evidence and risk of bias, using predefined criteria (Table 1). Every study received a satisfactory score (sufficient or insufficient) for each criterion. When information concerning an item was not reported in the study, it was classified as insufficient. Concerning treatment, the following drugs have been described best in current literature and were seen as most relevant: aspirin, dextran-40, prostaglandin-E1, heparin or low molecular-weight heparin. Studies describing these drugs were valued highest in the critical appraisal.
Table 1. Critical appraisal.
Studies that had at least one ‘insufficient’ score in the directness of evidence appraisal were excluded from the risk of bias analysis.
| Study characteristics | Directness of evidence | Risk of bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Treatment | Comparator | Outcome | n | Study design | Randomization | Concealed allocation |
Treatment standardization |
Outcome standardization |
Complete data | |
| Disa 2003 | ● | ● | ● | ● | 100 | RC | ● | ○ | ● | ● | ● |
| Gerressen 2013 | ● | ● | ● | ● | 406 | RC | ○ | ○ | ● | ● | ● |
| Lighthall 2012 | ● | ● | ● | ● | 390 | RC | ○ | ○ | ● | ● | ● |
| Riva 2012 | ● | ● | ● | ● | 1,351 | RC | ○ | ○ | ● | ● | ● |
| Sun 2003 | ● | ● | ● | ● | 55 | RC | ○ | ○ | ● | ● | ● |
| Ashjian 2007 | ○ | ● | ● | ● | |||||||
| Blackburn 2012 | ⊠ | ● | ● | ● | |||||||
| Jayaprasad 2013 | ● | ● | ○ | ● | |||||||
| Kroll 1995 | ○ | ● | ● | ● | |||||||
| Chen 2006 | ○ | ○ | ● | ● | |||||||
| Deutinger 1998 | ○ | ● | ○ | ● | |||||||
| Jones 2007 | ○ | ○ | ● | ● | |||||||
| Khouri 1998 | ○ | ● | ○ | ● | |||||||
| Khouri 2001 | ○ | ○ | ● | ● | |||||||
| Okochi 2012 | ● | ○ | ○ | ● | |||||||
| Directness of evidence | |
| Domain | Included only head and neck reconstruction, >10% FRFFs |
| Determinant | Patients received either aspirin, dextran, LMWH, heparin or prostaglandin-E postoperatively for prevention of flap loss |
| Comparator | Placebo, no anticoagulation or other drug, but not combination of drugs |
| Outcome | Complete flap failure |
| Risk of bias | |
| Randomization | Random allocation of patient to treatment groups |
| Concealed allocation | Computer generated sequence, central allocation (e.g. telephone service or web-based) |
| Treatment standardization | Protocolled, uniform |
| Outcome standardization | Protocolled, uniform assessment of outcome |
| Complete data | Adequate reporting of all included patients (<10% missing data) |
| ● | Satisfactory |
| ○ | Not satisfactory |
| ⊠ | Insufficient information |
| n | Total number of patients |
| RC | Retrospective cohort |
Statistical analysis
Authors who performed a study that compared at least two types of anticoagulants (or anticoagulant versus placebo) and included at least 10% FRFFs were invited to participate in the meta-analysis. Authors were invited to submit the raw data file of their original study. Data on age, sex, smoking, drinking, T- and N-staging (in the case of reconstruction after oncological resections), American Society of Anaesthesiologists (ASA-) score for physical status, type of anticoagulant, flap survival, revision surgery and flap or systemic complications were requested if available, since these factors were suspected for confounding the outcome. When the anticoagulative regimen was missing, patients were excluded from further analysis.
The main outcome was final flap failure, defined as flap failure with or without revision surgery. First, per study, the rate of initial flap failure, successful revision surgery, final flap failure, and complications (flap or systemic complications) according to anticoagulation type were determined. Flap complications included bleeding, wound infection, seroma, fistula formation and (partial) flap failure. Absolute risk (AR), absolute risk reduction (ARR) and number needed to treat (NNT) or harm (NNH) were calculated for the studies that had available data of controls that did not use anticoagulation.
Second, to identify factors associated with flap failure and complications, data were pooled and logistic regression analyses were performed. Variables that were significantly associated with initial flap failure, final flap failure, or complications in the univariable model were added to the corresponding multivariable model. Statistical significance was set at α=0.05. Data were analyzed using IBM SPSS Statistics 20 (SPSS Inc., Chicago, Illinois).
Results
Search and selection
The search yielded 4,472 unique publications. After title/abstract screening, 22 publications were deemed potentially relevant. Two studies did not collect individual patient data9, 14, but described a survey held among surgeons. Four studies described only free flap reconstruction outside the head and neck region (lower leg15–17 and post-mastectomy18). One study was not in English, Dutch or German19. These seven studies were excluded; hence 15 publications remained for critical appraisal20–34 (Table 1).
Critical appraisal
Most studies were retrospective cohort studies, only two were randomized trials. There was a great variety in the type of anticoagulants studied, and the flap patency was high in all studies (>91%). The studied drugs included aspirin, low-molecular-weight dextrans, low molecular-weight heparins, unfractioned heparin, prostaglandin-E1, but also milrinone, which is a vasodilator31, and recombinant human tissue factor pathway inhibitor, which inhibits the tissue factor pathway of coagulation33.
After critical appraisal on directness of evidence and risk of bias, five studies met the criteria for participating in the meta-analysis21–25. Four authors replied positively to participating in the meta-analysis. Finally, raw data from four studies (80%) were received and analysed21–24. The remaining study by Sun et al could not be included in the meta-analysis25. Sun et al described 55 cases of free flap transfer after tumor ablation in the upper aerodigestive tract. Of these patients, 25 received dextran-40 intravenously, while the remaining 30 did not. There were 18 FRFF procedures in each arm. One patient required anastomosis revision after venous congestion in the dextran-treated group. All flaps survived, indicating no beneficial effect of Dextran administration.
Meta-analysis
Baseline patient characteristics of the four studies in the meta-analysis, as well as the entire cohort are shown in Table 2. All patients underwent FRFF reconstruction in the head and neck area. Data on pre-operative ASA classification were often not available and are not shown. Only two studies had data available on T- and N-staging. Compared to the other studies, the patients described by Riva et al. were younger and more often male. Excessive alcohol use was more prevalent in the study of Lighthall et al., compared to the other studies.
Table 2.
Patient characteristics of studies included in the meta-analysis.
| Disa et al. (2003) | Gerressen et al. (2013) | Riva et al. (2012) | Lighthall et al. (2013) | Pooled cohort | ||
|---|---|---|---|---|---|---|
| Free radial forearm flaps (n) | 55 | 254 | 280 | 170 | 759 | |
| Anticoagulation use | 90.9% | 100% | 47.1% | 52.4% | 69% | |
| Age (Mean (SD)) | 59.3 (12.5) | 58.1 (12.1) | 51.7 (10.7) | 63.1 (15.8) | 56.9 (13.3) | |
| Sex | ||||||
| Male | 61.8% | 71.7% | 94.6% | 52.9% | 75.2% | |
| Female | 38.2% | 28.3% | 5.4% | 47.1% | 24.8% | |
| Smoking | ||||||
| Non-smoker/quit > 5 years ago | 61.1% | 36.8% | 57.5% | 12% | 39.4% | |
| Active smoker/quit < 5 years ago | 38.9% | 63.2% | 42.5% | 88.0% | 51.8% | |
| Alcohol use | ||||||
| 3 units or less/day | 77.4% | 56.2% | 65.4% | 21.2% | 53.0% | |
| More than 3 units/day | 22.6% | 43.8% | 34.6% | 78.8% | 47.0% | |
| cT-stage (%) | ||||||
| T1/T2 | 44% | 64.8% | - | - | 62.3% | |
| T3/T4a/b | 56% | 35.2% | - | - | 37.7% | |
| cN-stage (%) | ||||||
| N0 | 65.0% | 56.3% | - | - | 57.2% | |
| N+ | 35.0% | 43.7% | - | - | 42.8% | |
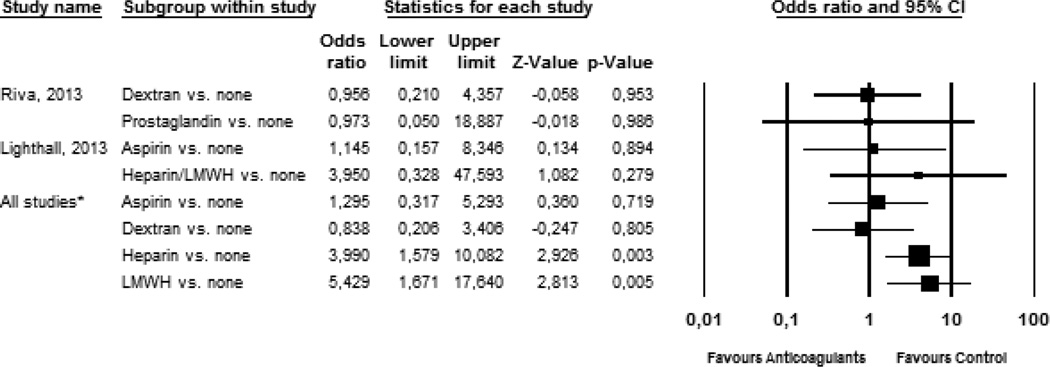
Table 3 describes the failure and flap-related complication rates per study according to type of coagulation, along with risk differences. In two studies, data were available on a sufficient number of patients that did not use anticoagulants (Figure 2). A comparison of Dextran-40 or prostaglandin-E1 use versus no anticoagulant use did not show a significant difference on flap failure in the study of Riva et al27. In the study of Lighthall et al, comparisons could be made between patients using aspirin, (low molecular-weight) heparin and no anticoagulative drugs23. The use of heparin resulted in a higher frequency of flap failure (OR: 3.950, p=.279) as compared to no anticoagulative drugs, but this difference was not statistically significant. However, heparin was administered to patients in whom intraoperative anastomosis revision had to be performed. Flap-related complications were available from the studies of Riva, Lighthall and Disa. Flap complications occurred in 26.7% (135/505) of patients. Wound infection was most often observed (10.1%, n=51), followed by fistula (5.7%) and hematoma (3.8%). In both the studies of Riva and Lighthall, the use of anticoagulative drugs resulted in a higher number of flap-related complications.
Table 3.
Flap failure and complications according to type of coagulation per study.
| % | Initial flap failure | Final flap failure | RD (95% CI) | NNT/NNH | Complications* | |
|---|---|---|---|---|---|---|
| Disa et al. (2003) | ||||||
| Aspirin | 36.3% | 1/20 (5%) | 1/20 (5%) | +5% (−4,6 to +14,6) | 20 | 7/20 (35%) |
| Dextran | 41.8% | 2/23 (8.7%) | 0/23 (0%) | - | 4/23 (17,4%) | |
| Heparin | 9.1% | 0/5 (0%) | 0/5 (0%) | - | 0/5 (0%) | |
| Combination | 3.6% | 0/2 (0%) | 0/2 (0%) | - | 1/2 (50%) | |
| No anticoagulation | 9.1% | 0/5 (0%) | 0/5 (0%) | Reference | 0/5 (0%) | |
| Lighthall et al. (2013) | ||||||
| Aspirin | 41.8% | 13/71 (18.3%) | 2/71 (2.8%) | +0,3% (−4,8 to +5,5) | 33 | 23/71 (32,4%) |
| Heparin | 2.4% | 3/4 (75%) | 1/4 (25%) | +22,5% (−20 to +65,1) | 4.4 | 2/4 (50%) |
| LMWH | 4.1% | 0/7 (0%) | 0/7 (0%) | −2,5% (−5,8 to +0,9) | 40 | 1/7 (14,3%) |
| Combination | 4.1% | 4/7 (57.1%) | 1/7 (14.3%) | +10% (−13,1 to +33,2) | 8.5 | 3/7 (42,9%) |
| No anticoagulation | 48.6% | 4/81 (4.9%) | 2/81 (2.5%) | Reference | 13/81 (16%) | |
| Riva et al. (2012) | ||||||
| Dextran | 41.4% | 12/116 (10.3%) | 3/116 (2.9%) | −0,1% (−4 to +3,8) | 1000 | 26/116 (22,4%) |
| Prostaglandin-E1 | 5.7% | 0/16 (0%) | 0/16 (0%) | −2,7% (−5,3 to −0,1) | 37 | 3/16 (18.8%) |
| No anticoagulation | 52.9% | 10/148 (6.7%) | 4/148 (2.7%) | Reference | 49/148 (33,1%) | |
| Gerressen et al. (2013)† | ||||||
| Heparin | 83.9% | 33/209‡ (15.8%) | 20/212 (9.4%) | - | - | NA |
| LMWH | 16.1% | 8/40 (20%) | 6/41 (14.6%) | - | - | NA |
| Pooled cohort | ||||||
| Aspirin | 12% | 14/91 (15.4%) | 3/91 (3.3%) | +2.4 (−7.3 to 12.2) | 41 | 30/91 (33%) |
| Dextran | 18.3% | 14/139 (10.1%) | 3/139 (2.2%) | +0.7 (−3.5 to 4.9) | 137 | 30/139 (21.6%) |
| Heparin | 29.1% | 36/218 (16.5%) | 21/21 (9.5%) | −0.4 (−3.6 to 2.7) | 246 | 2/9 (22.2%) |
| LMWH | 6.5% | 8/47 (17%) | 6/48 (12.5%) | +6.9 (2.6 to 11.3) | 14 | 1/7 (14.3%) |
| Prostaglandin-E1 | 2.1% | 0/16 (0%) | 0/16 (0%) | +9.9 (0.4 to 19.5) | 10 | 3/16 (18.8%) |
| Combination | 1.2% | 4/9 (44%) | 1/9 (11.1%) | −2.6 (−4.6 to −0.5) | −39 | 4/9 (44.4%) |
| No anticoagulation | 30.8% | 14/234 (6%) | 6/234 (2.6%) | reference | 62/234 (26.5%) |
RD: risk difference, calculated for final flap failure. Positive values indicate more failure in treated, compared to untreated patients. NA: not available. NNT/NNH: number needed to treat/harm. Total: sum of patients that used any kind of anticoagulative drugs.
Only flap-related complications are shown.
No patients were available that did not use anticoagulation, therefore RD and NNT could not be calculated. Also, no data were available on flap complications.
Data on revisional surgery were missing in some patients, therefore the numbers in the ‘initial flap failure’ and ‘final flap failure’ are not always equal.
Figure 2. Results and meta-analysis of final flap failure.
Univariate forest plot of studies that had a sufficient number of controls are shown (Riva and Lighthall23,24), as well as the meta-analysis of all included studies (Disa, Gerressen, Lighthall and Riva21–24). Limits represent 95% confidence interval. Flap failure occurred more often in patients taking heparin or LMWHs. Flap success in other treatment regimens did not differ from untreated patients.
Finally, pooled analyses of all 759 patients were performed. Initial flap failure occurred in 90 (12%) patients. Of these patients, 74 underwent revision surgery, which was successful in 50 cases (67.6%). Sixteen patients with flap failure did not receive revision surgery, or data were not available. Thus, final flap failure was observed in 40 of 758 patients (5.3%, data missing for 1 patient). In the meta-analysis, the use of heparin and LMWH was associated with an increased risk of flap failure in univariate analysis (Figure 2).
Univariate regression analysis identified that several factors were associated with flap failure (Table 4). In univariate analysis, final flap failure occurred more often in patients that used anticoagulants, especially heparin and aspirin, compared to patients not using anticoagulants. The study by Gerressen22 was chosen as the reference standard, as this population was most average in terms of alcohol use, male-female ratio and age, compared to the other studies. Compared to this study, significantly less flap failure occurred in the studies of Riva and Lighthall. Age, smoking and alcohol use were not associated with flap failure. Two multivariate models were constructed. In the first model (Model A), the use of anticoagulant (yes/no) and the study cohort a patient participated in were included. Flap failure occurred significantly less in patients in the cohort of Lighthall23 and Riva24. The use of anticoagulants was not significantly associated with a higher flap patency. A second model (Model B) was constructed to compare the effect of the individual anticoagulants. In this multivariate model, the use of anticoagulation was no longer a determinant for final flap failure, also there was no longer an association between cohort and flap failure when the type of anti-coagulation was taken into account.
Table 4.
Univariate and multivariate analysis of flap failure and complications.
| Flap failure | Flap Complications | |||||
|---|---|---|---|---|---|---|
| Determinant | OR (95%CI) Univariate |
OR (95%CI) Multivariate1 Model A |
OR (95%CI) Multivariate1 Model B |
OR (95%CI) Univariate2 |
OR (95%CI) Multivariate1,2 |
|
| Anticoagulation use (yes vs. no) | 2.6 (1.1–6.4)* | 1.2 (0.4–3.7) | - | 2.2 (1.1–4.1)* | 1.5 (0.7–3.5) | |
| Type of anticoagulation | ||||||
| None | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Aspirin | 2.9 (1.3–6.3)* | - | 1.8 (0.3–10.8) | 0.4 (0.1–1.7) | - | |
| Dextran | 1.8 (0.8–3.8) | - | 0.8 (0.2–3.6) | 0.9 (0.2–3.8) | - | |
| Heparin/LMWH | 3.1 (1.7–5.9) | - | 3.6 (0.3–42.4) | 1.5 (0.3–7.7) | - | |
| Age (per year increase) | 1.0 (1.0–1.0) | - | - | 1.0 (1.0–1.0) | - | |
| Female gender (yes vs. no) | 1.3 (0.7–2.7) | - | - | 1.0 (0.5–1.8) | - | |
| Smoking (yes vs. no) | 1.2 (0.6–2.3) | - | - | 0.4 (0.2–0.9)* | 0.5 (0.2–1.1) | |
| Alcohol use (yes vs. no) | 0.9 (0.5–1.8) | - | - | 0.8 (0.4–1.6) | - | |
| Study | ||||||
| Gerressen et al (2013) | Reference | Reference | Reference | - | - | |
| Disa et al. (2003) | 0.2 (0.0–1.2) | 0.2 (0.0–1.2) | 0.4 (0.0–6.0) | Reference | - | |
| Riva et al. (2012) | 0.2 (0.1–0.5)* | 0.3 (0.1–0.7)* | 0.9 (0.1–12.8) | - | - | |
| Lighthall et al. (2013) | 0.3 (0.1–0.8)* | 0.4 (0.1–1.0)* | 0.7 (0.1–5.6) | 0.8 (0.4–1.6) | - | |
OR= Odds ratio, 95%CI= 95% Confidence interval.
p<0.05.
Variables that were significantly related to the outcome in the univariate model were included.
Only the studies of Riva, Disa and Lighthall reported flap complications. Model A: Anticoagulation use as central determinant. Model B: Type of coagulation as central determinant.
Flap complications were reported by Riva24, Disa21 and Lighthall23. Using univariate logistic regression, use of anticoagulants was associated with higher risk of flap complications, while smoking was associated with lower risk of complications. However, in multivariate analysis these associations were no longer present.
Discussion
Summary of main results
With our comprehensive search and meta-analysis on the effect of anticoagulants on survival of the free radial forearm flap used for reconstruction in head and neck surgery we found that the evidence concerning this topic is weak. After analysis of the current available evidence, we found no significant protective effect of anticoagulation regimens on overall FRFF-survival. Also, in this study, flap failure was not related to smoking or alcohol use.
Remarkably, heparin and LMWH use were significantly associated with an increased risk of flap failure in univariate analyses. However, these drugs were often administered in patients who had undergone anastomosis revision during primary surgery, or when the surgeon decided that the flap was at higher risk of failure for any other reason. This negative association of heparin or LMWHs could not be detected after correction for other covariates in multivariate analysis.
Overall completeness, quality and applicability of evidence
We approached the literature in a broad fashion, by including all studies that compared anticoagulative regimens. All potentially relevant studies were obtained and could be appraised for directness of evidence and risk of bias. To avoid missing important, relevant publications, only after the critical appraisal we excluded studies that did not describe the FRFF. The most relevant and highest quality studies were considered for meta-analysis. Of the studies finally selected, data from the four most recent studies were available for analysis. Unfortunately, the data from Sun et al could not be retrieved25. Moreover, the quality of the evidence on this subject is generally low, for instance because of a lack of randomization, which is prone to introduce selection bias as addressed below. However, the direction of the evidence of each of the different studies is similar, giving value to the outcome to support our daily practice.
Comparison with other reviews
While the success rate of free flap reconstruction is already high, free flap failure does occur and can be a serious complication, resulting in loss of function and cosmesis2. Therefore, the comparison of anticoagulative regimens to optimize free flap survival has been a focus of many, mostly retrospective, trials and reviews5, 6, 11–13. Unfortunately, in these trials no significant benefit was found from treatment with anticoagulants (including aspirin, (low-molecular weight) heparins, dextran-40 and prostaglandin E1) versus no additional treatment. Studies on flap patency have always described heterogeneous groups of flaps from different donor- and recipient sites. As the size of the flap, the vessel diameter and the pedicle length vary between flaps from various donor sites, it can be assumed that the donor site of the flap may be of influence on flap outcomes. We therefore investigated the protective effect of anticoagulants in a single-flap, single recipient site population. Our individual patient data meta-analysis is the first to show that administration of anticoagulative drugs does not result in higher FRFF patency in head and neck in head and neck reconstruction in particular.
Potential biases in review
Several aspects of this meta-analysis should be addressed. First, data were received from only four of the five studies that met the criteria to participate in the meta-analysis, all studies were retrospective in design and treatment allocation was not randomized, with exception of the study by Disa21, but always according to the preference of the operating surgeon, i.e selection bias was introduced. Selection bias in general can lead to an overestimation of effect of a treatment. In this meta-analysis we found no such beneficial effect supporting the conclusion that anticoagulants have no additional protective effect on flap failure.
Second, we aimed to correct for factors that might influence flap outcomes, including smoking, drinking, tumor TNM staging (in the case of reconstruction after cancer resections) and ASA-score. However, due to the low number of flap failures and the limited availability of several of these variables, it was not possible to correct for all these factors in a single multivariable model. In addition, critically important variables, such as history of previous radiation therapy, history of previous neck surgery, history of previous free flap surgery, use of couplers for the venous anastomoses or the use of loupes versus operative microscopes for the anastomoses were not recorded and could not be commented on.
Third, it is known that cancer causes a hypercoagulable state due to expression of various procoagulants35. Unfortunately, data were not always clear whether patients had undergone reconstruction after cancer resection, or for a different indication, such as trauma. Therefore, it was not possible to compare flap outcomes in oncology versus non-oncology patients.
Implications for clinical practice
Since our meta-analysis revealed no beneficial effect of anticoagulants on the occurrence of flap failure of the FRFF, the use in daily practice seems not justifiable. Moreover, since some anticoagulants, such as dextrans, have shown a higher risk of systemic complications, including anaphylaxis, renal failure and adult respiratory distress syndrome, the risk of harm of these anticoagulants simply outweighs the lack of benefit21, 36, 37. In addition, because of the lack of effect, administering anticoagulants to prevent flap failure will probably not be cost-effective. It should be noted that this study concerns only those cases where anticoagulants are administered solely to prevent FRFF failure. This meta-analysis does not comprise other purposes to administer anticoagulative drugs like an expected duration of immobilization and the standard prophylaxis to prevent a hypercoagulable state in head and neck oncology patients.
Conclusion
The current review suggests that there is weak evidence that administering anticoagulative drugs to prevent failure of FRFFs in the head and neck region has no beneficial effect. In addition, literature has shown that the use of postoperative anticoagulants can result in a higher rate of systemic complications. Since the evidence is weak, and certain possible confounding factors were not analyzed, a surgeon may still have clinical arguments to administer anticoagulants.
Key points.
Aspirin, low-molecular weight dextran and (low-molecular weight) heparin are among the most frequently used anticoagulants to improve FRFF survival.
Unfortunately, evidence towards the effectiveness of postoperative anticoagulant us on FRFF survival is generally of low quality; there is a need for randomized controlled studies.
Despite the low quality of the individual studies, all studies describe that administration of anticoagulants did not significantly improve flap survival.
Combination of these results in the present meta-analysis supports the hypothesis that anticoagulants may not contribute to increased flap patency.
This meta-analysis did not comprise anticoagulant use for other indications, such as DVT prophylaxis. Postoperative administration of anticoagulants may still be indicated for these reasons.
Acknowledgments
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Appendices
Appendix A. Search strategy
| Database | Search string | Results |
|---|---|---|
| PubMed | (“tissue transfer”[tiab] OR “donor flap”[tiab] OR “skin flap”[tiab] OR “skin transplant”[tiab] OR “forearm flap”[tiab] OR “tissue transplant”[tiab] OR “microvascular surgery”[tiab] OR “anterolateral thigh flap”[tiab] OR “fibula flap”[tiab] OR “fibular flap”[tiab] OR “latissimus dorsi flap”[tiab] OR “head and neck reconstruction”[tiab] OR “free flap”[tiab] OR “thoracodorsal artery perforator”[tiab] OR “tram flap”[tiab] OR “deep inferior epigastric artery perforator”[tiab] OR “dieap”[tiab] OR “rectus abdominis”[tiab] OR “free tissue flaps”[MeSH terms]) AND (“acenocoumarol”[Tiab] OR “anticoagulant”[Tiab] OR “anticoagulation”[Tiab] OR “antithrombogenic”[Tiab] OR “antithrombotic”[Tiab] OR “anti-thrombus”[Tiab] OR “Ascal”[Tiab] OR “Aspirin”[Tiab] OR “Dalteparine”[Tiab] OR “Dextran”[Tiab] OR “Fragmin”[Tiab] OR “Fraxiparine”[Tiab] OR “Heparin”[Tiab] OR “Ketorolac”[Tiab] OR “LMWH”[Tiab] OR “Milrinone”[Tiab] OR “Prostaglandin”[Tiab] OR “Pharmacotherapy”[Tiab] OR “Rheomacrodex”[Tiab] OR “Ticlopidine”[Tiab] OR “Warfarin”[Tiab] OR “Anticoagulants”[MeSH Terms] OR “Fibrinolytic Agents”[MeSH Terms]) |
367 |
| EMBASE | ('free tissue transfer'/syn OR 'tissue transfer':ti,ab OR 'donor flap':ti,ab OR 'skin flap'/syn OR 'skin transplant'/syn OR 'forearm flap':ti,ab OR 'tissue transplant':ti,ab OR 'microvascular surgery'/syn OR 'anterolateral thigh flap'/syn OR 'fibula flap'/syn OR 'fibular flap'/syn OR 'latissimus dorsi flap'/syn OR 'head and neck reconstruction':ti,ab OR 'free flap'/syn OR 'thoracodorsal artery perforator'ti,ab OR 'tram flap'/syn OR 'deep inferior epigastric artery perforator':ti,ab OR 'dieap':ti,ab) AND ('acenocoumarol'/syn OR 'anticoagulant'/syn OR 'anticoagulation'/syn OR 'antithrombogenic':ti,ab OR 'antithrombotic'/syn OR 'anti-thrombus':ti,ab OR 'ascal'/syn OR 'aspirin'/syn OR 'dalteparine':ti,ab OR 'dextran'/syn OR 'fragmin'/syn OR 'fraxiparine'/syn OR 'heparin'/syn OR 'ketorolac'/syn OR 'lmwh':ti,ab OR 'milrinone'/syn OR 'prostaglandin'/syn OR 'rheomacrodex'/syn OR 'ticlopidine'/syn OR 'warfarin'/syn) |
4393 |
| CinAHL | See PubMed | 11 |
| Web of Science | See PubMed | 311 |
Footnotes
Conflict of interest: none declared
References
- 1.Saint-Cyr M, Wong C, Buchel EW, Colohan S, Pederson WC. Free tissue transfers and replantation. Plast Reconstr Surg. 2012;130:858e–878e. doi: 10.1097/PRS.0b013e31826da2b7. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao HT, Leu YS, Lin CC. Primary closure versus radial forearm flap reconstruction after hemiglossectomy: functional assessment of swallowing and speech. Ann Plast Surg. 2002;49:612–616. doi: 10.1097/00000637-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lam L, Samman N. Speech and swallowing following tongue cancer surgery and free flap reconstruction--a systematic review. Oral Oncol. 2013;49:507–524. doi: 10.1016/j.oraloncology.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hurvitz KA, Kobayashi M, Evans GR. Current options in head and neck reconstruction. Plast Reconstr Surg. 2006;118:122e–133e. doi: 10.1097/01.prs.0000237094.58891.fb. [DOI] [PubMed] [Google Scholar]
- 5.Brands MT, van den Bosch SC, Dieleman FJ, Berge SJ, Merkx MA. Prevention of thrombosis after microvascular tissue transfer in the head and neck. A review of the literature and the state of affairs in Dutch Head and Neck Cancer Centers. Int J Oral Maxillofac Surg. 2010;39:101–106. doi: 10.1016/j.ijom.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Kruse AL, Luebbers HT, Gratz KW, Obwegeser JA. Factors influencing survival of free-flap in reconstruction for cancer of the head and neck: a literature review. Microsurgery. 2010;30:242–248. doi: 10.1002/micr.20758. [DOI] [PubMed] [Google Scholar]
- 7.Glicksman A, Ferder M, Casale P, Posner J, Kim R, Strauch B. 1457 Years of Microsurgical Experience. Plast Reconstr Surg. 1997;100:355–363. doi: 10.1097/00006534-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Ketchman LD. Pharmacological alterations in the clotting mechanism: Use in microvascular surgery. J Hand Surg. 1978;3:407–415. doi: 10.1016/s0363-5023(78)80133-6. [DOI] [PubMed] [Google Scholar]
- 9.Davies DM. A world survey of anticoagulation practice in clinical microvascular surgery. Br J Plast Surg. 1982;35:96–99. doi: 10.1016/0007-1226(82)90095-9. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel JH, Polat JK. Microvascular flap reconstruction by otolaryngologists: Prevalence, postoperative care, and monitoring techniques. Laryngoscope. 2007;117:485–490. doi: 10.1097/MLG.0b013e31802d6e66. [DOI] [PubMed] [Google Scholar]
- 11.Askari M, Fisher C, Weniger FG, Bidic S, Lee WPA. Anticoagulation Therapy in Microsurgery: A Review. J Hand Surg (USA) 2006;31:836–846. doi: 10.1016/j.jhsa.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Stephan B, Schenk JF, Nemeh A, Pindur G. The use of antithrombotic agents in microvascular surgery. Clin Hemorheol Microcirc. 2009;43:51–56. doi: 10.3233/CH-2009-1220. [DOI] [PubMed] [Google Scholar]
- 13.Jokuszies A, Herold C, Niederbichler AD, Vogt PM. Anticoagulative strategies in reconstructive surgery--clinical significance and applicability. Ger Med Sci. 2012;10 doi: 10.3205/000152. Doc01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salemark L. International survey of current microvascular practices in free tissue transfer and replantation surgery. Microsurgery. 1991;12:308–311. doi: 10.1002/micr.1920120415. [DOI] [PubMed] [Google Scholar]
- 15.Lee KT, Jeon BJ, Lim SY, et al. The effects of ketorolac on microvascular thrombosis in lower extremity reconstruction. Plast Reconstr Surg. 2012;129:1322–1327. doi: 10.1097/PRS.0b013e31824ec33f. [DOI] [PubMed] [Google Scholar]
- 16.Pugh CM, Dennis RH, 2nd, Massac EA. Evaluation of intraoperative anticoagulants in microvascular free-flap surgery. J Natl Med Assoc. 1996;88:655–657. [PMC free article] [PubMed] [Google Scholar]
- 17.Biemer E, Jaeger K. Drug treatment, after care and secondary interventions following lower leg reconstruction by free tissue transfer. Chirurg. 1986;57:140. [PubMed] [Google Scholar]
- 18.Davar D, Gimbel ML, Smith RE. Prophylactic anticoagulation is safe in patients undergoing flap reconstruction post mastectomy: A single-center single-surgeon retrospective review. J Gen Intern Med. 2011;26:S9. [Google Scholar]
- 19.Riberti C, Costa P, Lefevre JC, Chassagne JF. Clinical experience with the combination of dextran 40, dihydroergotoxine, lidocaine in intravenous perfusion in the prevention of postoperative ischemia of skin flaps. Minerva Chir. 1984;39:819–823. [PubMed] [Google Scholar]
- 20.Ashjian P, Chen CM, Pusic A, Disa JJ, Cordeiro PG, Mehrara BJ. The effect of postoperative anticoagulation on microvascular thrombosis. Ann Plast Surg. 2007;59:36–39. doi: 10.1097/01.sap.0000264837.15110.2f. [DOI] [PubMed] [Google Scholar]
- 21.Disa JJ, Polvora VP, Pusic AL, Singh B, Cordeiro PG. Dextran-related complications in head and neck microsurgery: do the benefits outweigh the risks? A prospective randomized analysis. Plast Reconstr Surg. 2003;112:1534–1539. doi: 10.1097/01.PRS.0000083378.58757.54. [DOI] [PubMed] [Google Scholar]
- 22.Gerressen M, Pastaschek CI, Riediger D, et al. Microsurgical free flap reconstructions of head and neck region in 406 cases: a 13-year experience. J Oral Maxillofac Surg. 2013;71:628–635. doi: 10.1016/j.joms.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Lighthall JG, Cain R, Ghanem TA, Wax MK. Effect of postoperative aspirin on outcomes in microvascular free tissue transfer surgery. Otolaryngol Head Neck Surg. 2013;148:40–46. doi: 10.1177/0194599812463320. [DOI] [PubMed] [Google Scholar]
- 24.Riva FM, Chen YC, Tan NC, et al. The outcome of prostaglandin-E1 and dextran-40 compared to no antithrombotic therapy in head and neck free tissue transfer: analysis of 1,351 cases in a single center. Microsurgery. 2012;32:339–343. doi: 10.1002/micr.21958. [DOI] [PubMed] [Google Scholar]
- 25.Sun T, Chien S, Lee J, Cheng L, Hsu L, Chen P. Is dextran infusion as an antithrombotic agent necessary in microvascular reconstruction of the upper aerodigestive tract? J Reconstr Microsurg. 2003;19:463–466. doi: 10.1055/s-2003-44634. [DOI] [PubMed] [Google Scholar]
- 26.Jayaprasad K, Mathew J, Thankappan K, et al. Safety and efficacy of low molecular weight dextran (dextran 40) in head and neck free flap reconstruction. J Reconstr Microsurg. 2013;29:443–448. doi: 10.1055/s-0033-1343950. [DOI] [PubMed] [Google Scholar]
- 27.Kroll SS, Miller MJ, Reece GP, et al. Anticoagulants and hematomas in free flap surgery. Plast Reconstr Surg. 1995;96:643–647. doi: 10.1097/00006534-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn TK, Java KR, Lowe D, Brown JS, Rogers SN. Safety of a regimen for thromboprophylaxis in head and neck cancer microvascular reconstructive surgery: non-concurrent cohort study. Br J Oral Maxillofac Surg. 2012;50:227–232. doi: 10.1016/j.bjoms.2011.03.265. [DOI] [PubMed] [Google Scholar]
- 29.Chen CM, Ashjian P, Disa JJ, Cordeiro PG, Pusic AL, Mehrara BJ. Is the use of intraoperative heparin safe? Plast Reconstr Surg. 2008;121:49e–53e. doi: 10.1097/01.prs.0000299267.84139.2a. [DOI] [PubMed] [Google Scholar]
- 30.Deutinger M, Rath T, Constantinou E, Schneider B. The influence of postoperative medical treatment and type of microvascular anastomosis on free tissue transfer. Eur J Plast Surg. 1998;21:273–276. [Google Scholar]
- 31.Jones SJ, Scott DA, Watson R, Morrison WA. Milrinone does not improve free flap survival in microvascular surgery. Anaesth Intensive Care. 2007;35:720–725. doi: 10.1177/0310057X0703500510. [DOI] [PubMed] [Google Scholar]
- 32.Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 33.Khouri RK, Sherman R, Buncke HJ, Jr, et al. A phase II trial of intraluminal irrigation with recombinant human tissue factor pathway inhibitor to prevent thrombosis in free flap surgery. Plast Reconstr Surg. 2001;107:408–415. doi: 10.1097/00006534-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Okochi M, Okazaki M, Asato H. Oral antithrombotic treatment and postoperative thrombotic complications after head and neck reconstruction using free flaps. J Plast Surg Hand Surg. 2012;46:163–166. doi: 10.3109/2000656X.2012.697374. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JA, Weitz JI. Hypercoagulable states. Clin Chest Med. 2010;31:659–673. doi: 10.1016/j.ccm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 36.van der Klauw MM, Wilson JH, Stricker BH. Drug-associated anaphylaxis: 20 years of reporting in The Netherlands (1974–1994) and review of the literature. Clin Exp Allergy. 1996;26:1355–1363. doi: 10.1046/j.1365-2222.1996.d01-300.x. [DOI] [PubMed] [Google Scholar]
- 37.Vos SCB, Hage JJ, Woerdeman LAE, Noordanus RP. Acute renal failure during dextran-40 Antithrombotic prophylaxis: Report of two microsurgical cases. Ann Plast Surg. 2002;48:193–196. doi: 10.1097/00000637-200202000-00014. [DOI] [PubMed] [Google Scholar]