Abstract
Objective
Upregulation of the receptor for advanced glycation end products (RAGE) has been proposed as a pathophysiological mechanism underlying the development of atrial fibrillation (AF). We sought to investigate if soluble RAGE levels are associated with AF in Caucasian patients.
Methods
Patients (n = 587) were prospectively recruited and serum levels of soluble RAGE (sRAGE) and endogenous secretory RAGE (esRAGE) measured. The patients included 527 with sinus rhythm, 32 with persistent AF (duration >7 days, n = 32) and 28 with paroxysmal AF (duration <7 days, n = 28).
Results
Patients with AF were older and had a greater prevalence of heart failure than patients in sinus rhythm. Circulating RAGE levels were higher in patients with persistent AF [median sRAGE 1190 (724–2041) pg/ml and median esRAGE 452 (288–932) pg/ml] compared with paroxysmal AF [sRAGE 799 (583–1033) pg/ml and esRAGE 279 (201–433) pg/ml, p ≤ 0.01] or sinus rhythm [sRAGE 782 (576–1039) pg/ml and esRAGE 289 (192–412) pg/ml, p < 0.001]. In multivariable logistic regression analysis, independent predictors of persistent AF were age, heart failure, sRAGE [odds ratio 1.1 per 100 pg/ml, 95% confidence interval (CI) 1.0–1.1, p = 0.001] and esRAGE [odds ratio 1.3 per 100 pg/ml, 95% CI 1.1–1.4, p < 0.001]. Heart failure and age were the only independent predictors of paroxysmal AF. In AF patients, sRAGE [odds ratio 1.1 per 100 pg/ml, 95% CI 1.1–1.2, p = 0.007] and esRAGE [odds ratio 1.3 per 100 pg/ml, 95% CI 1.0–1.5, p = 0.017] independently predicted persistent compared with paroxysmal AF.
Conclusions
Soluble RAGE is elevated in Caucasian patients with AF, and both sRAGE and esRAGE predict the presence of persistent AF.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is found in 1% of the population, with a prevalence that increases as the population ages. [1] The precise molecular mechanisms involved in atrial remodeling remain incompletely understood, but it is clear that pro-fibrotic and pro-inflammatory pathways increase the vulnerability to AF. [2, 3] One such pathway that occurs with aging involves the non-enzymatic binding of proteins and lipids leading to the formation of advanced glycation end-products (AGEs). [4, 5] AGEs may play a role in the pathophysiology of AF through their interaction with the cellular receptor for AGE (RAGE), [6] which causes inflammation and fibrosis via sustained activation of nuclear factor-kappaB [7] and upregulation of cytokines and adhesion molecules. [8] In addition to its presence on cells, isoforms of RAGE (sRAGE) exist in the circulation including full-length cell-surface RAGE which results from proteolytic cleavage (cleaved RAGE), and endogenous secretory RAGE (esRAGE), a splice variant that binds RAGE ligands but lacks the cytosolic tail critical for signal transduction. [9]
Although there is some evidence that circulating forms of soluble RAGE may have clinical utility as biomarkers of AF, [10–12] the 3 studies to date have produced varying results. This may be due to the biomarker measured (sRAGE or esRAGE), the type of AF patient studied (paroxysmal or persistent AF), or the ethnicity of the patient (Chinese, [10, 12] European [11]). This study aimed to clarify the possible association between levels of soluble RAGE and AF through the measurement of both sRAGE and esRAGE in Caucasian patients with sinus rhythm (SR), persistent AF and paroxysmal AF. We hypothesized that RAGE signaling plays a role in the maintenance of AF, and therefore that serum levels of sRAGE and esRAGE may differ according to whether AF was persistent or paroxysmal.
Materials and Methods
Study sample
We prospectively enrolled patients presenting for cardiac investigations between 2008 and 2011 at Austin Health, Melbourne, Australia. For the purpose of this study, patients of non-Caucasian ethnicity and those with a paced rhythm were excluded. The research was carried out according to the Declaration of Helsinki (2000) of the World Medical Association, and was approved by the Human Research Ethics Committee at Austin Health, Melbourne. All participants provided written consent.
Clinical measurements and definitions
A standardized medical questionnaire was completed and verified with the hospital medical record. Blood pressure was measured in the supine position and anthropometric measurements were taken. A 12-lead ECG was recorded to determine heart rate and rhythm. Paroxysmal AF was defined as having paroxysms of AF that terminated within 7 days of onset. [13] All patients with a documented history of AF who were in SR at the time of recruitment were considered to have paroxysmal AF. Persistent AF was defined as AF lasting >7 days and included patients with long-standing persistent and permanent AF. Diabetes was diagnosed based on a documented history, treatment with anti-diabetic medication or when the fasting blood glucose was >7 mmol/L. Hypertension was diagnosed as present if previously diagnosed by a physician and/or there was current use of anti-hypertensive medication. Dyslipidemia was defined as present if previously diagnosed by a physician and/or there was current use of lipid lowering agents. Cigarette smoking was defined as smoking within the preceding 12 months. Obesity was diagnosed when the body mass index was >30 kg/m2. Renal impairment was diagnosed when the estimated glomerular filtration rate (eGFR) was <60 ml/min/1.73m2. Left ventricular (LV) ejection fraction was calculated with the modified Simpson method or quantitative LV angiography. LV dysfunction was defined as a LV ejection fraction <50%. Heart failure was diagnosed when there was evidence of previous dyspnea, fluid retention or low cardiac output secondary to cardiac dysfunction, or documented rales, jugular venous distension or pulmonary edema prior to the current admission. Valvular heart disease was diagnosed when left sided valve dysfunction was severe on recent echocardiography. Previous myocardial infarction was diagnosed when there was evidence of a typical rise and gradual fall of troponin I associated with either ischemic symptoms or ECG changes according to standard criteria. [14]
Biochemical Analyses
Blood samples were collected at enrollment and serum was obtained by centrifuging blood at 3000 rpm at 4°C for 10 minutes. Specimens were stored at –80°C. Plasma electrolytes were measured on a Hitachi 911 automatic analyzer (Roche Diagnostics, Mannheim, Germany). The eGFR was calculated using the four-component abbreviated Modification of Diet in Renal Disease equation. Glycated hemoglobin (HbA1c) was measured by automated high-performance liquid chromatography (Biorad Diamat, CA, USA), and fasting lipids by enzymatic colorimetry. Total cholesterol was measured by enzymatic colorimetric methods and low-density lipoprotein cholesterol was calculated using the Friedewald equation. Serum C-reactive protein levels were measured using the Synchron LX® system on a Beckman Coulter analyser.
Serum sRAGE (R&D Systems, Minneapolis, MN) and esRAGE (B-Bridge International, Cupertino, CA) were measured using a sandwich ELISA according to the manufacturer’s instructions. The limit of detection was 4.1 pg/ml and 25 pg/ml respectively. The intra-assay and inter-assay coefficients of variation values for sRAGE were 6.0% and 7.2% respectively and for esRAGE were 1.5% and 10.0% respectively. Samples were run in duplicate and the mean values used for analysis.
Statistical analyses
Analysis was performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Categorical variables are expressed as n (%) and assessed using Fisher’s exact tests. Continuous variables are expressed as mean ± standard deviation and assessed using one-way between-group analysis of variance. Post-hoc comparisons were conducted using Fisher’s least significant difference. Serum RAGE and C-reactive protein were non-normally distributed and were compared using the Kruskal-Wallis test. Median values and 25th-75th percentiles are presented in the results. Subgroup comparisons were conducted using the Mann-Whitney U test. Spearman’s correlation coefficient (rs) was used to determine the relationship between sRAGE and esRAGE. As diabetes accelerates glycation and is an independent risk factor for AF, [4, 15, 16] we specifically examined whether diabetes influenced the relationship between AF and serum RAGE using generalized linear model analysis, with serum RAGE as the dependent variable and persistent AF and diabetes as explanatory variables. The interaction term for AF and diabetes was examined in this model. Multivariable logistic regression analysis was performed to determine independent predictors of paroxysmal and persistent AF compared with SR using clinical variables potentially associated with AF, with a p-value <0.1 in univariable analysis. Variables considered included age, gender, obesity, hypertension, diabetes, smoking, renal impairment, myocardial infarction, heart failure, valvular heart disease, insulin use, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker use, statin use, systolic blood pressure, low density lipoprotein cholesterol, HbA1c, C-reactive protein and circulating sRAGE and esRAGE. The same variables were considered when predicting persistent versus paroxysmal AF in addition to antiarrhythmic use. Serum sRAGE and esRAGE were highly correlated and were analyzed in separate models to avoid issues with multicollinearity. Two-tailed p-values <0.05 were considered significant.
Results
Clinical and biochemical characteristics according to heart rhythm
We recruited 681 patients and excluded 87 patients of non-Caucasian ethnicity and 7 patients with a cardiac pacemaker. In the remaining 587 patients, 60 (10.2%) had AF, of whom 32 (5.5%) had persistent AF and 28 (4.8%) had paroxysmal AF. The clinical and biochemical characteristics of the study population are presented in Table 1. Patients with AF (either persistent or paroxysmal) were older and more likely to have heart failure than patients in SR (both p ≤ 0.001). Prevalence of diabetes and other cardiac risk factors including hypertension, dyslipidemia, obesity and smoking were similar across the groups. Patients with persistent AF were more likely to have renal impairment and a prior stroke and were less likely to be taking statins than patients in SR (all p < 0.05). Warfarin use was significantly higher in patients with AF compared with SR (p < 0.001), and was greatest in those with persistent compared with paroxysmal AF. No patients were taking novel oral anticoagulant agents. Antiarrhythmic agents were commonly used in patients with paroxysmal and persistent AF. Over two-thirds of the total cohort were taking renin-angiotensin system blockers, with similar use in all 3 groups. Heart rate was higher in patients with persistent AF compared with the other groups while blood pressure was similar. Estimated GFR was lower in patients with persistent AF compared with SR (p = 0.009) and C-reactive protein was higher in patients with paroxysmal AF compared with SR (p = 0.03); levels of both markers were similar in patients with paroxysmal and persistent AF. Low density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides levels were similar in all 3 groups (all p > 0.05). Glycemic control was similar in diabetic patients with SR (HbA1c = 7.4 ± 1.2%), paroxysmal AF (HbA1c = 7.8 ± 1.6%) and persistent AF (HbA1c = 7.3 ± 1.2%), p = 0.47.
Table 1. Clinical characteristics of study patients.
| Sinus Rhythm | Paroxysmal AF | Persistent AF | P Value | |
|---|---|---|---|---|
| (n = 527) | (n = 28) | (n = 32) | ||
| Age, years | 64 ± 11 | 70 ± 10† | 70 ± 10† | 0.001 |
| Female | 178 (34%) | 10 (36%) | 9 (28%) | 0.80 |
| Obesity | 237 (45%) | 16 (57%) | 15 (47%) | 0.45 |
| Diabetes mellitus | 294 (56%) | 16 (57%) | 16 (50%) | 0.82 |
| Hypertension | 426 (80%) | 25 (89%) | 27 (84%) | 0.50 |
| Dyslipidemia | 434 (82%) | 20 (71%) | 22 (69%) | 0.06 |
| Myocardial infarction | 111 (21%) | 10 (36%) | 5 (16%) | 0.13 |
| Heart failure | 29 (6%) | 10 (36%)† | 12 (38%)† | <0.001 |
| Valvular heart disease | 28 (5%) | 4 (14%) | 4 (13%) | 0.03 |
| Renal impairment | 110 (21%) | 10 (36%) | 12 (38%)† | 0.02 |
| Cigarette smoking | 75 (14%) | 3 (11%) | 1 (3%) | 0.19 |
| Stroke | 20 (4%) | 2 (7%) | 5 (16%)† | 0.01 |
| Medications | ||||
| Warfarin | 10 (2%) | 5 (18%)† | 24 (75%)†‡ | <0.001 |
| Antiplatelet | 392 (74%) | 20 (71%) | 10 (31%)†‡ | <0.001 |
| Beta-blocker | 245 (47%) | 18 (64%) | 22 (69%)† | 0.01 |
| Antiarrhythmic | 3 (0.6%) | 11 (39%)† | 5 (16%)†‡ | <0.001 |
| ACEi or ARB | 366 (69%) | 24 (86%) | 21 (66%) | 0.15 |
| Diuretic | 183 (35%) | 17 (61%)† | 21 (66%)† | <0.001 |
| Statin | 387 (73%) | 20 (71%) | 17 (53%)† | 0.05 |
| Insulin | 145 (28%) | 4 (14%) | 6 (19%) | 0.20 |
| Physical Parameters | ||||
| Heart rate, beats/min | 67 ± 13 | 65 ± 14 | 79 ± 21†‡ | <0.001 |
| Systolic BP, mmHg | 133 ± 23 | 139 ± 21 | 132 ± 23 | 0.43 |
| LV dysfunction | 77 (15%) | 7 (26%) | 9 (29%) | 0.05 |
| Biochemical Parameters | ||||
| Renal impairment | 80 ± 28 | 74±29 | 67±24† | 0.02 |
| LDL cholesterol, mmol/L | 2.4 ± 1.0 | 2.2 ± 1.0 | 2.2 ± 1.0 | 0.36 |
| C-reactive protein, mg/L | 2.7 (1.2, 5.3) | 7.3 (2.0, 11.2)† | 4.6 (1.3, 8.3) | 0.03 |
| Serum sRAGE, pg/ml | 782 (576, 1039) | 799 (583, 1033) | 1190 (724, 2041)†‡ | 0.001 |
| Serum esRAGE, pg/ml | 289 (192, 412) | 279 (201, 433) | 452 (288, 932)†‡ | 0.001 |
Subgroup analysis:
† p <0.05 vs. sinus rhythm
‡ p <0.05 vs. paroxysmal AF.
Values are total numbers and proportion (%), mean ± SD, or median (25, 75th percentile). ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; BP = blood pressure; LDL = low-density lipoprotein; LV = left ventricular; RAGE = receptor for advanced glycation end products.
Serum RAGE levels
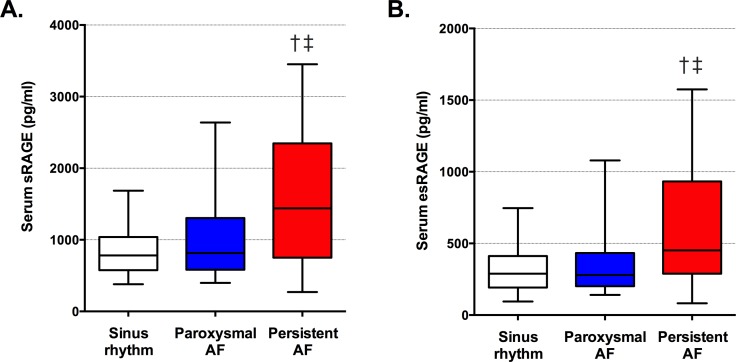
Serum RAGE levels in the study cohort are shown (S1 File). Serum sRAGE and esRAGE were highly correlated in patients with SR (rs = 0.87, p<0.001), paroxysmal AF (rs = 0.89, p<0.001) and persistent AF (rs = 0.94, p<0.001). Median serum sRAGE and esRAGE levels were significantly higher in patients with persistent AF compared with SR (both p<0.001) and paroxysmal AF (both p≤0.01) (Table 1 and Fig 1).
Fig 1.
Serum sRAGE (A) and esRAGE (B) levels were significantly elevated in patients with persistent AF. Median and interquartile ranges are shown. Error bars indicate 5th to 95th percentiles. † Sinus rhythm vs. persistent AF, both p < 0.001; ‡ paroxysmal AF vs. persistent AF, both p ≤ 0.01.
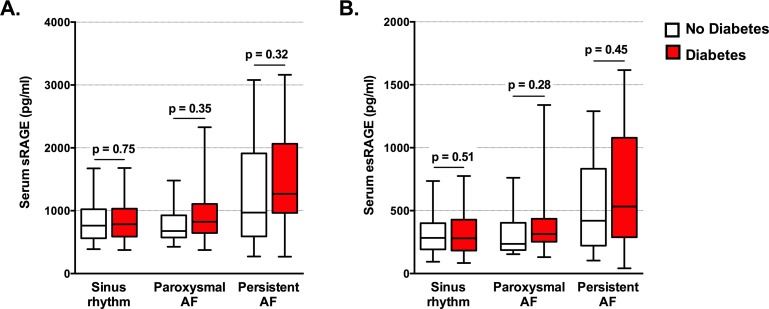
We also examined sRAGE and esRAGE levels according to diabetes status, given that diabetes is a condition of accelerated glycation (Fig 2). There were 326 patients with diabetes of whom 294 were in SR, 16 had paroxysmal AF and 16 had persistent AF. Serum sRAGE and esRAGE levels did not differ according to the presence or not of diabetes. Furthermore, diabetes did not influence the relationship between persistent AF and serum RAGE, with no interaction between persistent AF and diabetes for sRAGE (p = 0.20) or esRAGE (p = 0.24).
Fig 2.
Serum sRAGE (A) and esRAGE (B) levels did not differ according to the presence of diabetes. Median and interquartile ranges are shown. Error bars indicate 5th to 95th percentiles. P values are shown.
Predictors of atrial fibrillation compared with sinus rhythm
The independent predictors of persistent AF compared with SR are shown in Table 2. Heart failure, age, serum sRAGE (model 1) and serum esRAGE (model 2) independently predicted persistent AF, while C-reactive protein and renal impairment did not. Heart failure was the strongest predictor of persistent AF, but even after exclusion of patients with heart failure, both sRAGE [OR 1.1 per 100 pg/ml, 95% CI 1.1, 1.2, p < 0.001] and esRAGE [OR 1.3 per 100 pg/ml, 95% CI 1.2, 1.5, p < 0.001] remained significantly associated with persistent AF. The independent predictors of paroxysmal AF compared with SR were heart failure [OR 9.1, 95%CI 3.7, 22.5, p<0.001] and age [OR 1.6 per decade, 95%CI 1.1, 2.5, p = 0.028], while C-reactive protein did not reach statistical significance.
Table 2. Multivariable logistic regression analysis demonstrating predictors of persistent atrial fibrillation compared with sinus rhythm.
| Variables | Model 1: sRAGE | Model 2: esRAGE | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (per decade) | 1.63 (1.10, 2.43) | 0.02 | 1.56 (1.05, 2.31) | 0.03 |
| Heart failure | 7.12 (2.92, 17.40) | <0.001 | 6.15 (2.45, 15.45) | <0.001 |
| Renal impairment | 0.78 (0.32, 1.89) | 0.58 | 0.72 (0.29, 1.79) | 0.72 |
| C-reactive protein (per 1mg/L) | 1.01 (1.00, 1.02) | 0.09 | 1.01 (1.00, 1.02) | 0.07 |
| Serum RAGE (per 100 pg/ml) | 1.08 (1.03, 1.13) | 0.001 | 1.25 (1.11, 1.40) | <0.001 |
CI = confidence intervals; OR = odds ratio; RAGE = receptor for advanced glycation end products
Predictors of persistent compared with paroxysmal atrial fibrillation
The independent predictors of persistent compared with paroxysmal AF are shown in Table 3. Both serum sRAGE (OR 1.3 per 100 pg/ml, 95%CI 1.0, 1.2, p = 0.007) and esRAGE (1.26 95%CI 1.04, 1.51, p = 0.017) were associated with an increased risk of persistent compared with paroxysmal AF. Antiarrhythmic use and renin-angiotensin system blockers were not statistically significant.
Table 3. Multivariable logistic regression analysis demonstrating predictors of persistent compared with paroxysmal atrial fibrillation.
| Variables | Model 1: sRAGE | Model 2: esRAGE | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | P Value | |
| Antiarrhythmic agent | 0.38 (0.10, 1.18) | 0.16 | 0.16 (0.10, 1.48) | 0.16 |
| ACEi or ARB | 0.31 (0.08, 1.25) | 0.10 | 0.30 (0.08, 1.17) | 0.08 |
| Serum RAGE (per 100 pg/ml) | 1.13 (1.03, 1.24) | 0.007 | 1.26 (1.04, 1.51) | 0.02 |
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CI = confidence intervals; OR = odds ratio; RAGE = receptor for advanced glycation end products
Discussion
This is the first study in Caucasian patients to measure serum levels of both sRAGE and esRAGE in patients with SR, persistent AF and paroxysmal AF. Serum sRAGE and esRAGE were significantly higher in patients with persistent AF and predicted the presence of persistent AF compared with SR. An important finding was that both elevated serum sRAGE and esRAGE independently predicted persistent compared with paroxysmal AF.
Our results confirm and extend the work of others that increased serum sRAGE levels predict persistent AF compared with SR [10, 11] and persistent AF compared with paroxysmal AF. [10] By contrast to our finding that elevated esRAGE predicted persistent compared with paroxysmal AF, lower esRAGE was associated with AF in patients of Chinese ethnicity [10, 12] and Chinese patients with persistent AF had lower levels of esRAGE compared to patients with paroxysmal AF. [10] Although there have been limited studies examining RAGE in AF, soluble RAGE has been extensively studied in other forms of cardiovascular disease. The strong correlation in this study between serum sRAGE and esRAGE levels is in agreement with the work of Colhoun et al [17] who measured sRAGE and esRAGE in patients with diabetes (predominantly Caucasian), and reported that the 2 markers were highly correlated (r = 0.88), with elevations predicting incident coronary artery disease events. In Caucasian patients with end-stage renal failure, sRAGE and esRAGE were also highly correlated (r = 0.95). [18] By contrast, studies in patients of Chinese or Japanese ethnicity have reported that lower levels of esRAGE were associated with coronary artery disease severity, [19] increased carotid intima-media thickness [20] and heart failure [21], and an inverse association has been noted between esRAGE and sRAGE levels. [21]
Ethnic differences in serum sRAGE and esRAGE levels have been noted in multiple previous studies. [17, 22, 23] Interestingly, the G82S SS polymorphism of the RAGE gene within the ligand-binding domain of the receptor is associated with reduced serum esRAGE levels in Chinese patients and reduced serum sRAGE levels in Korean patients, yet this same polymorphism was not related to sRAGE or esRAGE levels in Caucasian patients. [18, 24, 25] In other studies, the G82S SS polymorphism is associated with an increased prevalence of coronary artery disease and inflammation in Chinese patients, an increased prevalence of rheumatoid arthritis in Caucasian patients and enhanced ligand binding and cytokine generation in tissue culture studies. [26, 27] Taken together with our own findings, we speculate that differences in ethnicity and gene polymorphisms could explain much of the discordant findings from previous studies of these soluble receptors. This raises the question of whether RAGE gene polymorphisms need to be considered when interpreting levels of these soluble receptors, particularly when multiple ethnic groups are being studied together.
Clinical predictors of atrial fibrillation
Both heart failure and age were independently associated with AF. Although heart failure was the strongest predictor of persistent AF compared with SR, serum RAGE independently predicted AF in multivariable analysis. When heart failure patients were excluded from analysis, sRAGE and esRAGE remained significantly associated with persistent AF. These results are supported by the observations of Raposeiras-Roubin et al, [28] who noted a positive association between persistent AF and serum sRAGE in patients with chronic heart failure. Furthermore, although heart failure was a risk factor for developing AF in the present study, it did not differentiate paroxysmal from persistent AF, suggesting that other factors (possibly RAGE) may be of importance in the maintenance of AF. Glycation may be particularly relevant in elderly subjects, where the abundance of glycated protein is increased by virtue of age. [5]
Diabetes is associated with accelerated formation of AGEs, [4, 5] which is of interest as patients with diabetes share a similar risk factor profile to AF and diabetes independently contributes to AF development. [15, 16] However, we found no independent association between AF and diabetes. Our finding that serum RAGE levels were similar in patients with and without diabetes is in agreement with findings from other high-risk cohorts. [11, 29]
Potential mechanisms of atrial fibrillation–from cell to serum
AGEs contribute to myocardial remodelling directly via collagen cross-linking and indirectly via interaction with RAGE. [4] Patients with AF have elevated levels of AGEs in atrial tissue and serum compared to those in SR and myocardial AGEs positively correlate with fibrosis. [11, 30] High fat diets promote myocardial AGE and RAGE accumulation, which enhances cardiac inflammation and AGE formation is accelerated by hyperglycaemia and may represent a mechanistic link between diabetes and AF. [16, 31] The receptor for AGEs, RAGE may contribute to tissue repair in response to acute cellular stress, but in chronic disease states such as diabetes and heart failure, excessive ligand accumulation upregulates RAGE expression, perpetuating sustained inflammation and fibrosis—an environment that is conducive to AF. [2–4] Circulating ligands of RAGE are increased in patients with persistent AF compared with paroxysmal AF [10] and SR. [10, 11] RAGE is widely expressed on cardiomyocytes, vascular cells and inflammatory cells and its activation leads to augmented nuclear factor-kappaB activity, which is increased in the atrial tissue of patients with AF compared with SR. [6, 32]
In the present study, the total pool of soluble RAGE and the novel splice variant esRAGE were highest in patients with persistent AF. As esRAGE comprised less than 40% of total soluble RAGE, this would suggest that cleaved RAGE is increased in patients with persistent AF. Both cleaved RAGE and esRAGE are truncated versions of cellular RAGE, capable of binding RAGE ligands without initiating an inflammatory response. The precise relationship between tissue RAGE and serum RAGE levels in man remains unclear, but limited experimental data has shown that serum RAGE levels reflect tissue RAGE levels. [33] It has been suggested that circulating RAGE isoforms may act as decoy molecules by competing with cell-surface RAGE for ligand binding to attenuate vascular damage. [19] However in this study, we speculate that elevated serum levels of sRAGE and esRAGE reflect increased cellular /atrial RAGE, which would amplify inflammation and fibrosis [34] and serve as an enduring substrate for AF maintenance. In agreement with this hypothesis, the majority of circulating sRAGE is generated from membrane cleavage of cellular RAGE, and sRAGE levels positively correlate with AGEs and other inflammatory biomarkers. [35]
Experimental data would also suggest that activation of the AGE/RAGE axis is associated with AF. Diabetic atria exhibit increased arrhythmogenicity and diffuse interstitial fibrosis, which is suppressed by AGE inhibition. [36, 37] Alagebrium (an AGE-inhibitor) reduces myocardial inflammation and collagen cross-linking in association with a reduction in cardiac AGEs and RAGE. [31, 34] Recombinant soluble RAGE also attenuates RAGE-mediated myocardial fibrosis. [38] Angiotensin II receptor blockers reduce both cellular and soluble RAGE, [33] and a meta-analysis reported that renin-angiotensin system blockade prevents incident AF. [39] In a small clinical study of newly identified AF, diabetic patients randomized to pioglitazone showed reduced progression to permanent AF compared with placebo (30% vs. 49%, p = 0.028), in parallel with a reduction in serum AGE levels. [40] Specific anti-RAGE therapies are under development, [41] but have yet to be tested in experimental models of AF or in patients with AF.
Study limitations
There are study limitations that should be acknowledged. Firstly, AF may be asymptomatic and remain undetected for many years. We may not have captured every patient with paroxysmal AF, although this is unlikely to have impacted on the main study findings. Secondly, the sample sizes for the paroxysmal and persistent AF groups were relatively small, but were adequately powered to detect significant differences in sRAGE and esRAGE. The power to detect a difference with a type 1 error of 5% (two-tailed) was 86% and 85% respectively for the persistent AF versus paroxysmal AF group and 92% and 91% respectively for comparing the persistent AF group with the SR group. Thirdly, this was a cross-sectional study and although we have demonstrated an association between circulating RAGE and persistent AF, direct causation and risk could not be assessed.
Conclusions
The molecular mechanisms that promote atrial remodeling and maintenance of AF are poorly defined. We report that serum sRAGE and esRAGE are significantly elevated in Caucasian patients with persistent AF. Further clinical studies in larger cohorts are now warranted to establish the potential value of sRAGE levels in predicting the natural history of AF. The therapeutic implications of interrupting the AGE-RAGE axis in patients with AF are unknown, but warrant further investigation.
Supporting Information
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; RAGE = receptor for advanced glycation end products.
(XLSX)
Acknowledgments
The authors would like to gratefully acknowledge Mr. Michael Bassett-Smith, Mr. Peter Gleeson and Ms. Michelle Ord for their assistance with data collection for this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Heart Foundation Clinical Research Scholarship (http://heartfoundation.org.au) to TL and the Pfizer Cardiovascular Lipid grant (https://www.pfizergrants.com.au/grants/CardiovascularLipid.aspx) to TL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–32. 10.1016/j.jacc.2011.05.061 [DOI] [PubMed] [Google Scholar]
- 3.Chen MC, Chang JP, Liu WH, Yang CH, Chen YL, Tsai TH, et al. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am J Cardiol. 2008;102:861–5. 10.1016/j.amjcard.2008.05.038 [DOI] [PubMed] [Google Scholar]
- 4.Ramasamy R, Vannucci SJ, Yan SSD, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. [DOI] [PubMed] [Google Scholar]
- 5.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- 7.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–808. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D, Wang Y, Xu Y. Decreased serum endogenous secretory receptor for advanced glycation endproducts and increased cleaved receptor for advanced glycation endproducts levels in patients with atrial fibrillation. Int J Cardiol. 2012;158:471–2. 10.1016/j.ijcard.2012.05.042 [DOI] [PubMed] [Google Scholar]
- 11.Raposeiras-Roubin S, Rodino-Janeiro BK, Grigorian-Shamagian L, Seoane-Blanco A, Moure-Gonzalez M, Varela-Roman A, et al. Evidence for a role of advanced glycation end products in atrial fibrillation. Int J Cardiol. 2012;157:397–402. 10.1016/j.ijcard.2011.05.072 [DOI] [PubMed] [Google Scholar]
- 12.Yan X, Shen Y, Lu L, Sano M, Fukuda K, Shen W. Decreased endogenous secretory RAGE and increased hsCRP levels in serum are associated with atrial fibrillation in patients undergoing coronary angiography. Int J Cardiol. 2013;166:242–5. 10.1016/j.ijcard.2012.08.055 [DOI] [PubMed] [Google Scholar]
- 13.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96 e21. 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38:2114–30. [DOI] [PubMed] [Google Scholar]
- 15.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. 10.1016/j.amjcard.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–68. 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 17.Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–85. 10.2337/db11-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalousova M, Jachymova M, Mestek O, Hodkova M, Kazderova M, Tesar V, et al. Receptor for advanced glycation end products—soluble form and gene polymorphisms in chronic haemodialysis patients. Nephrol Dial Transplant. 2007;22:2020–6. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, et al. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2009;206:540–5. 10.1016/j.atherosclerosis.2008.12.045 [DOI] [PubMed] [Google Scholar]
- 20.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005;28:2716–21. [DOI] [PubMed] [Google Scholar]
- 21.Wang LJ, Lu L, Zhang FR, Chen QJ, De Caterina R, Shen WF. Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. Eur J Heart Fail. 2011;13:440–9. 10.1093/eurjhf/hfq231 [DOI] [PubMed] [Google Scholar]
- 22.Hudson BI, Moon YP, Kalea AZ, Khatri M, Marquez C, Schmidt AM, et al. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS). Atherosclerosis. 2011;216:192–8. 10.1016/j.atherosclerosis.2011.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvin E, Halushka M, Rawlings A, Hoogeveen RC, Ballantyne CM, Coresh J, et al. sRAGE and risk of diabetes, cardiovascular disease and death. Diabetes. 2013;62:2116–21. 10.2337/db12-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng WH, Lu L, Wang LJ, Yan XX, Chen QJ, Zhang Q, et al. RAGE gene polymorphisms are associated with circulating levels of endogenous secretory RAGE but not with coronary artery disease in Chinese patients with type 2 diabetes mellitus. Arch Med Res. 2009;40:393–8. 10.1016/j.arcmed.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 25.Jang Y, Kim JY, Kang SM, Kim JS, Chae JS, Kim OY, et al. Association of the Gly82Ser polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating levels of soluble RAGE and inflammatory markers in nondiabetic and nonobese Koreans. Metabolism. 2007;56:199–205. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Shao Y, Lai W, Ren H, Xu D. Association of polymorphisms in the RAGE gene with serum CRP levels and coronary artery disease in the Chinese Han population. J Hum Genet. 2010;55:668–75. 10.1038/jhg.2010.85 [DOI] [PubMed] [Google Scholar]
- 27.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–35. [DOI] [PubMed] [Google Scholar]
- 28.Raposeiras-Roubin S, Rodino-Janeiro BK, Grigorian-Shamagian L, Moure-Gonzalez M, Seoane-Blanco A, Varela-Roman A, et al. Soluble receptor of advanced glycation end products levels are related to ischaemic aetiology and extent of coronary disease in chronic heart failure patients, independent of advanced glycation end products levels: New Roles for Soluble RAGE. Eur J Heart Fail. 2010;12:1092–100. 10.1093/eurjhf/hfq117 [DOI] [PubMed] [Google Scholar]
- 29.Koyama Y, Takeishi Y, Niizeki T, Suzuki S, Kitahara T, Sasaki T, et al. Soluble Receptor for advanced glycation end products (RAGE) is a prognostic factor for heart failure. J Card Fail. 2008;14:133–9. 10.1016/j.cardfail.2007.10.019 [DOI] [PubMed] [Google Scholar]
- 30.Begieneman MP, Rijvers L, Kubat B, Paulus WJ, Vonk AB, van Rossum AC, et al. Atrial fibrillation coincides with the advanced glycation end product N(epsilon)-(carboxymethyl)lysine in the atrium. Am J Pathol. 2015;185:2096–104. 10.1016/j.ajpath.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 31.Tikellis C, Thomas MC, Harcourt BE, Coughlan MT, Pete J, Bialkowski K, et al. Cardiac inflammation associated with a Western diet is mediated via activation of RAGE by AGEs. Am J Physiol Endocrinol Metab. 2008;295:E323–30. 10.1152/ajpendo.00024.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu YC, Du YM, Wu SL, Chen QX, Wu HL, Zhou SF. Activated nuclear factor-kappaB and increased tumor necrosis factor-alpha in atrial tissue of atrial fibrillation. Scand Cardiovasc J. 2009;43:292–7. 10.1080/14017430802651803 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Yamagishi S, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res. 2005;70:137–41. [DOI] [PubMed] [Google Scholar]
- 34.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–92. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, et al. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc Res. 2008;76:52–6. 10.1016/j.mvr.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 36.Kato T, Yamashita T, Sekiguchi A, Sagara K, Takamura M, Takata S, et al. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol. 2006;17:890–4. [DOI] [PubMed] [Google Scholar]
- 37.Kato T, Yamashita T, Sekiguchi A, Tsuneda T, Sagara K, Takamura M, et al. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol. 2008;19:415–20. 10.1111/j.1540-8167.2007.01037.x [DOI] [PubMed] [Google Scholar]
- 38.Lu L, Zhang Q, Xu Y, Zhu Z-b, Geng L, Wang L-j, et al. Intra-coronary administration of soluble receptor for advanced glycation end-products attenuates cardiac remodeling with decreased myocardial transforming growth factor-beta1 expression and fibrosis in minipigs with ischemia-reperfusion injury. Chin Med J. 2010;123:594–8. [PubMed] [Google Scholar]
- 39.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–307. 10.1016/j.jacc.2010.01.043 [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Wang J, Wang G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in diabetes mellitus patients. Int Heart J. 2014;55:499–505. [DOI] [PubMed] [Google Scholar]
- 41.Walker D, Lue LF, Paul G, Patel A, Sabbagh MN. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert Opin Investig Drugs. 2015;24:393–9. 10.1517/13543784.2015.1001490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ACEi = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; RAGE = receptor for advanced glycation end products.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.