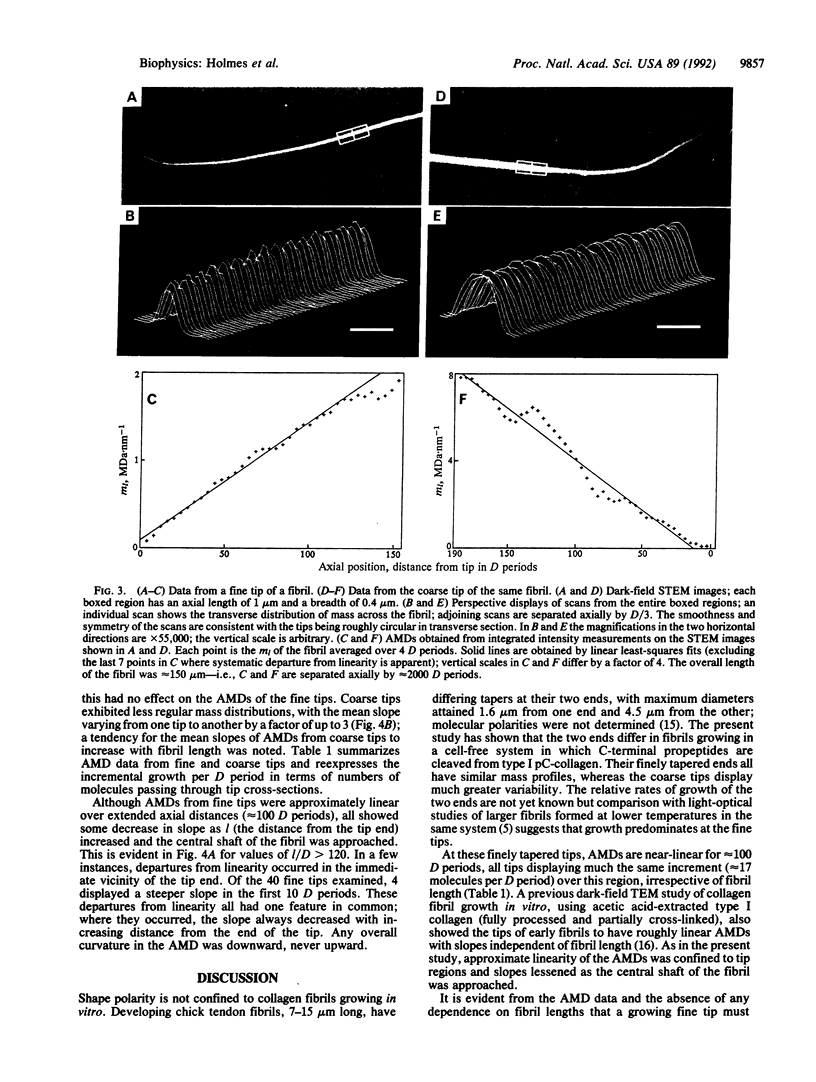
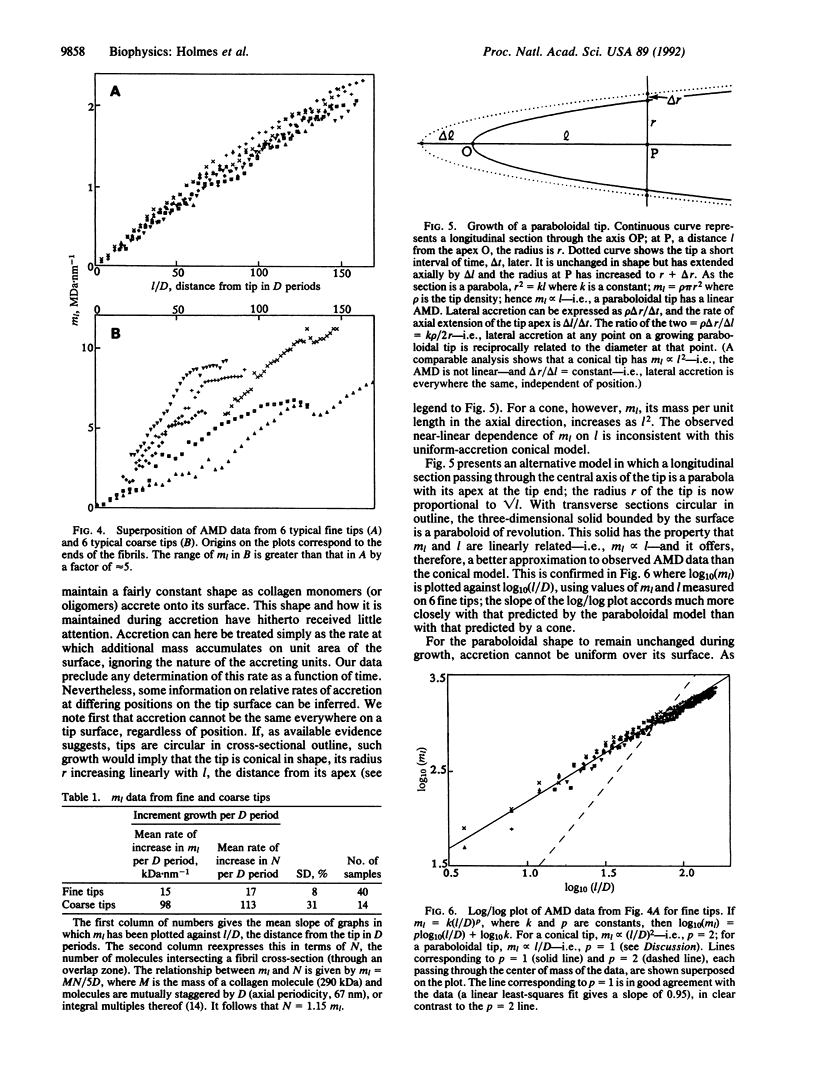
Abstract
Collagen fibrils generated in vitro at 37 degrees C by enzymic removal of C-terminal propeptides from type I pC-collagen (an intermediate in the normal processing of type I procollagen to collagen containing the C-terminal propeptides but not the N-terminal propeptides) display shape polarity, with one tip fine tapered and the other coarse tapered. Mass measurements by scanning transmission electron microscopy show that the mass per unit length along both kinds of tip increases roughly linearly over distances of approximately 100 D periods from the fibril end [D (axial periodicity) = 67 nm]. The fine tips of fibrils of widely differing lengths exhibit near-identical mass distributions, the mass in all cases increasing at the rate of approximately 17 molecules per D period, irrespective of fibril length. Coarse tips display less regular behavior. These results show that (i) the shape of a fine tip is not conical but resembles more closely a paraboloid of revolution, and (ii) for this shape to be maintained throughout growth, accretion (rate of mass uptake per unit area) cannot everywhere be the same on the surface of the tip but must decrease as the diameter increases. To a first approximation, accretion alpha (diameter)-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bard J. B., Chapman J. A. Diameters of collagen fibrils grown in vitro. Nat New Biol. 1973 Nov 21;246(151):83–84. doi: 10.1038/newbio246083a0. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Zycband E. I., Winkelmann D. A., Trelstad R. L. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler-Nagy C., Bruckner P., Hayashi T., Prockop D. J. Isolation of unhydroxylated type I procollagen folding of the protein in vitro. Arch Biochem Biophys. 1981 Dec;212(2):668–677. doi: 10.1016/0003-9861(81)90411-2. [DOI] [PubMed] [Google Scholar]
- Hojima Y., McKenzie J. A., van der Rest M., Prockop D. J. Type I procollagen N-proteinase from chick embryo tendons. Purification of a new 500-kDa form of the enzyme and identification of the catalytically active polypeptides. J Biol Chem. 1989 Jul 5;264(19):11336–11345. [PubMed] [Google Scholar]
- Hojima Y., van der Rest M., Prockop D. J. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985 Dec 15;260(29):15996–16003. [PubMed] [Google Scholar]
- Holmes D. F., Chapman J. A. Axial mass distributions of collagen fibrils grown in vitro: results for the end regions of early fibrils. Biochem Biophys Res Commun. 1979 Apr 27;87(4):993–999. doi: 10.1016/s0006-291x(79)80005-4. [DOI] [PubMed] [Google Scholar]
- Holmes D. F., Mould A. P., Chapman J. A. Morphology of sheet-like assemblies of pN-collagen, pC-collagen and procollagen studied by scanning transmission electron microscopy mass measurements. J Mol Biol. 1991 Jul 5;220(1):111–123. doi: 10.1016/0022-2836(91)90385-j. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Kadler K. E., Mould A. P., Hojima Y., Holmes D. F., Cummings C., Chapman J. A., Prockop D. J. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J Mol Biol. 1989 Nov 20;210(2):337–345. doi: 10.1016/0022-2836(89)90335-5. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987 Nov 15;262(32):15696–15701. [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Collagen fibrils in vitro grow from pointed tips in the C- to N-terminal direction. Biochem J. 1990 Jun 1;268(2):339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler K. E., Hulmes D. J., Hojima Y., Prockop D. J. Assembly of type I collagen fibrils de novo by the specific enzymic cleavage of pC collagen. The fibrils formed at about 37 degrees C are similar in diameter, roundness, and apparent flexibility to the collagen fibrils seen in connective tissue. Ann N Y Acad Sci. 1990;580:214–224. doi: 10.1111/j.1749-6632.1990.tb17930.x. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C., Makowski L. Twisting of fibrin fibers limits their radial growth. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8991–8995. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]





