Abstract
We conducted an observational, longitudinal prospective study in which we measured the diameters of the inferior vena cava (IVC) of 47 patients using ultrasonography. The aim of our study was to assess the state of blood volume and to determine the percentage of patients who responded to intravascular volume expansion. Only 17 patients (36%) responded to fluid management. A higher number of responding patients had cardiovascular failure compared with nonresponders (82% vs. 50%, P = 0.03). Among the patients with cardiovascular failure, the probability of finding responders was 4.6 times higher than that of not finding responders (odds ratio, 4.66; 95% confidence interval, 1.10–19.6; P = 0.04). No significant difference was observed in the mortality rate between the two groups (11% vs. 23%, P = 0.46). In conclusion, responding to intravascular volume expansion had no impact on patient survival in the intensive care unit.
The main objective of this study was to use ultrasonography to determine the percentage of patients who responded to fluid administration. As secondary objectives, we determined the mortality rates and the differences in hemodynamic variables between responders and nonresponders among the patients admitted to the intensive care unit (ICU) of the Instituto Nacional de Cancerología, México.
METHODS
We performed an observational, longitudinal, prospective study on the oncologic patients admitted to the ICU of Instituto Nacional de Cancerología, México, between January 2015 and June 2015. During this period, we measured the patients' inferior vena cava (IVC) diameters using ultrasonography before beginning fluid reanimation. The sample size was calculated using the proportion formula n = Za · p0 · q0 / d2, where p is the possibility of finding a patient who responds to volume expansion (50%), and q = p − 1, with a significance level of 0.05% that corresponds to a Z value of 1.96 and has a 12% accuracy (d). Using this formula, the required sample size for the study was determined to be at least 34 patients.
The IVC diameter was measured in the subxiphoid window using transthoracic echocardiography operating in the M mode, 2 to 3 cm from the junction of the IVC and the right atrium. We determined the maximum and minimum diameters of the IVC during the patients' respiratory cycles. In patients with spontaneous ventilation, the collapsibility index of the IVC (cIVC) values were calculated using the following formula: [(maximum diameter − minimum diameter / maximum diameter) × 100], and in patients with positive-pressure mechanical ventilation, the distensibility index of IVC (dIVC) values were calculated using the formula [(maximum diameter − minimum diameter / minimum diameter) × 100]. If the dIVC index was >18% or the cIVC index was >40%, the patient was determined to be responsive to intravascular volume expansion. Doctors trained in basic critical care ultrasonography performed the measurements to reduce bias and decrease the error margins.
We followed the guidelines outlined in the Declaration of Helsinki and its modifications as outlined in the Declaration of Tokyo for biomedical research in humans, along with the ethical considerations formulated in the “Ley General de Salud de los Estados Unidos Mexicanos” (General Law of Health in United Mexican States) for health research. The investigation is classified under the 17th article of the health research regulations as category I. This was a research study involving minimal risk because ultrasonography is a noninvasive diagnostic method with minimal risks for the participants.
This study included patients over 18 years old with positive-pressure mechanical ventilation whose dIVC indices were measured. This study also included patients over 18 years old with spontaneous ventilation whose cIVC indices were measured. We excluded patients who were treated with fluid reanimation at the time of ICU admission prior to IVC measurements. Patient data were collected immediately after the patients were admitted to the ICU. We collected information regarding patients' demographic characteristics, comorbidities, hemodynamic variables, type of oncological illness, and diagnosis at the time of admission. We calculated APACHE II (Acute Physiology and Chronic Health Evaluation II), SOFA (Sequential Organ Failure Assessment), and MEXSOFA (Mexican Sequential Organ Failure Assessment) scores to assess the severity of patients' organ failure. APACHE II, SOFA, and MEXSOFA scores were calculated using each patient's worst clinical and laboratory values during the first 24 hours of their stay in the ICU. The rate of organ failure was determined using the SOFA score, and severity of organ failure was determined using the patients' laboratory and clinical data, as well as information about their vasopressor or inotropic doses (1–4). We documented the number of volume-responsive patients.
The numerical values were expressed as the mean ± standard deviation if the distribution was normal or as the median ± the interquartile range if the distribution was nonnormal. Data distribution was evaluated using the Kolmogorov-Smirnov test. Nominal variables were expressed using percentages. Numerical variables were compared using a Student's t test, Mann-Whitney U test, and chi-square test. Fisher's exact test was used to analyze the nominal variables. To establish an association between cardiovascular failure and volume responsiveness, we used the X2 value from the Mantel-Haenszel test and expressed it as an odds ratio with a confidence interval of 95%. A P value < 0.05 was considered statistically significant. The heart rate needed to predict the response to intravascular volume expansion was calculated using the area under the receiver operating characteristic curve. We also calculated the values for specificity, sensitivity, positive predictive value, and negative predictive value. All data were analyzed using SPSS version 22.0 for Windows.
RESULTS
This study included 47 critically ill oncologic patients who had been previously administered intravenous crystalloids. We measured the IVC diameters and calculated the dIVC or cIVC for each patient. Table 1 shows the general characteristics of the patient population. Less than half of the patients admitted to the ICU responded to the administration of intravascular volume expansion (n = 17, 36.2%). Table 2 shows the clinical characteristics of the critically ill oncologic patients admitted to the ICU who were classified according to their volume expansion responsiveness. In patients with heart rates >88 beats per minute, the minimum parameters required to predict volume responsiveness were a sensitivity of 82.4%, a specificity of 53.3%, a positive predictive value of 50%, and a negative predictive value of 84.2%. In patients with heart rate >88 beats per minute, the rate of unnecessary crystalloid administration was 15.8% (Figure 1).
Table 1.
General characteristics of the 47 oncologic patients in the ICU whose inferior vena cava diameters were measured using ultrasonography
| Variable | Results |
|---|---|
| Age (years)* | 51 (30.5–60.5) |
| Women | 26 (55.3%) |
| APACHE II (points)* | 12 (10–20) |
| SOFA (points)** | 6.7 ± 3.7 |
| MEXSOFA (points)** | 7.7 ± 4.6 |
| Type of oncologic disease | |
| Solid tumor | 26 (55%) |
| Hematologic | 21 (45%) |
| Organ failure | |
| ≤2 | 24 (51%) |
| ≥3 | 23 (49%) |
| Comorbidities | |
| Systemic arterial hypertension | 12 (26%) |
| Diabetes mellitus | 8 (17%) |
| Chronic kidney disease | 2 (4%) |
| Other | 25 (53%) |
| Causes of admission to ICU | |
| Hypovolemic shock | 16 (34%) |
| Septic shock | 16 (34%) |
| Acute heart failure | 6 (13%) |
| Acute respiratory failure | 3 (6%) |
| Other | 6 (13%) |
| Norepinephrine requirement | 28 (60%) |
| Norepinephrine dose (mcg/kg/min)* | 0.2 (0.1–0.7) |
| Inotropic requirement | 1 (2%) |
| Volume responders | 17 (36%) |
Median and interquartile range.
Mean ± standard deviation.
APACHE II indicates Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; MEXSOFA, Mexican Sequential Organ Failure Assessment; SOFA, Sequential Organ Failure Assessment.
Table 2.
Clinical and hemodynamic characteristics of critically ill oncologic patients who were admitted to the ICU classified according to their volume response
| Variable | Responder (n = 17) | Nonresponder (n = 30) | P value |
|---|---|---|---|
| Age (years)** | 46.2 ± 18.9 | 48.8 ± 16.1 | 0.63 |
| SOFA (points)** | 7 ± 3.5 | 6.6 ± 3.9 | 0.72 |
| APACHE (points)* | 16 (10–20) | 12 (10–19) | 0.63 |
| MEXSOFA (points)** | 8.3 ± 5.6 | 7.4 ± 4.0 | 0.52 |
| SAP (mm Hg)* | 92 (90–108) | 100 (100–110) | 0.05 |
| DAP (mm Hg)* | 60 (50–70) | 67.5 (60–70) | 0.18 |
| Heart rate (bpm)* | 99 (90–120) | 88 (78–100) | 0.047 |
| Central venous saturation (%)* | 76 (69–80) | 74 (69–79) | 0.59 |
| Lactate (mmol/L)* | 2.7 (1.2–6.2) | 1.8 (1–3.7) | 0.31 |
| Base excess (mmol/L)* | –5.7 (−2.9 to −9) | –4.5 (−2 to −6.3) | 0.71 |
| Bicarbonate (mmol/L)** | 19.8 ± 5.51 | 19.9 ± 6.68 | 0.97 |
| Organ failures ≤ 2 | 10 (58.8) | 14 (46.6) | 0.42 |
| Organ failures ≥ 3 | 7 (41.2) | 16 (53.4) | |
| Cardiovascular failure | 14 (82.3%) | 15 (50%) | 0.03 |
| Respiratory failure | 13 (76.4%) | 27 (90%) | 0.21 |
| Hematologic failure | 8 (47%) | 22 (73.3%) | 0.72 |
| Hepatic failure | 4 (23.5%) | 8 (26.6%) | 0.55 |
| Renal failure | 5 (29.4%) | 9 (30%) | 0.97 |
| Neurologic failure | 3 (17.6%) | 2 (6.6%) | 0.33 |
| Mechanical ventilation requirement | 10 (58.8%) | 16 (53.3%) | 0.73 |
| Hypovolemic shock | 8 (47%) | 8(26%) | 0.16 |
| Septic shock | 6 (35.2%) | 10 (33.3%) | 0.89 |
| Death in ICU | 2 (11%) | 7(23.3%) | 0.46 |
Median and interquartile range.
Mean ± standard deviation.
DAP indicates diastolic arterial pressure; ICU, intensive care unit; SAP, systolic arterial pressure.
Figure 1.
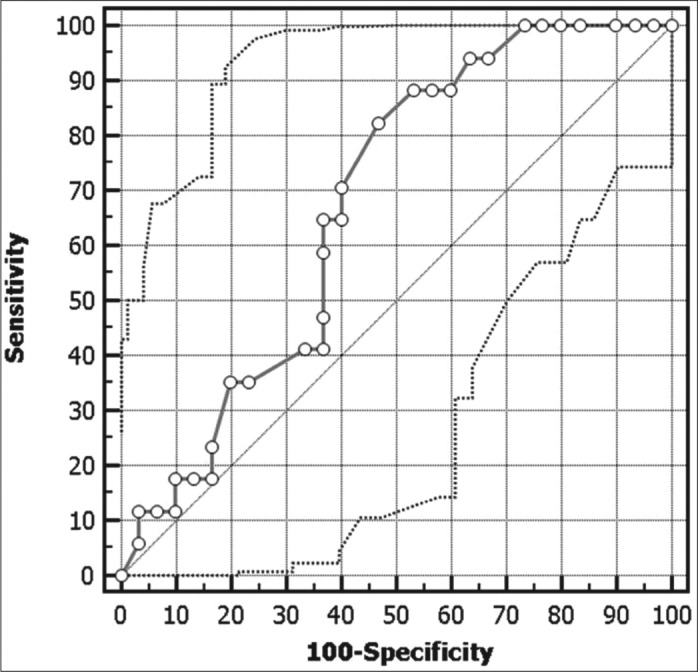
Determination of the heart rate for predicting the response to intravascular volume expansion.
DISCUSSION
This study had three main findings:
Only 36% of the critically ill oncologic patients who were admitted to the ICU responded to intravascular volume expansion.
Patients with cardiovascular failure and tachycardia were more likely to respond to volume expansion.
There was no significant difference in mortality rates between responders and nonresponders.
In patients with circulatory shock, fluid reanimation with crystalloids is one of the main treatment options to increase cardiac output and improve tissue perfusion (5–9). The clinical parameters used to evaluate blood volume in patients experiencing shock have poor specificity (10–12). Currently, the recommended procedure is to determine dynamic variables such as IVC measurements using ultrasonography in patients with mechanical ventilation during the respiratory cycle to predict their responsiveness to volume expansion. This procedure is also useful in patients with spontaneous ventilation (13). Muller et al reported that in patients with spontaneous ventilation, a cIVC index >40% is usually associated with volume responsiveness (14).
According to Michard et al, up to 72% of critically ill patients will respond to volume expansion with a significant increase in stroke volume or cardiac output (15). This contrasts significantly with our study, because only 36% of the patients admitted to the ICU in our institution were volume responders. VASST study data demonstrated that maintaining a positive water balance for 12 hours to 4 days after beginning the fluid reanimation can increase mortality in critically ill patients (16). Therefore, it is important to evaluate the volume responsiveness prior to the administration of intravenous liquids to determine if patients would benefit from it (17). We are not aware of any clinical studies that compare the mortality rates of volume responders and nonresponders. In our study, we did not find statistically significant differences in mortality rates between these two groups of critically ill patients. With respect to organ failure, we observed that the patients with cardiovascular failure, as determined by SOFA scores, were more likely to respond to the administration of intravenous fluids. With the above data, we can infer that the patients admitted to the ICU with hemodynamic instability and evaluated for volume responsiveness using their clinical and laboratory data of tissue hypoperfusion are those who can benefit from fluid reanimation, which results in increased cardiac output and, thus, increased oxygen supply to the tissues (3).
In the hemodynamic evaluation and monitoring described by Pinsky, he reported that the heart rate increases in patients with shock due to the increase of sympathetic tone; in short, “tachycardia is never good” (5). In our study, we found that a heart rate of 88 beats per minute was a threshold that helps determine the responsiveness of a patient to fluid administration, with a sensitivity of 88.4% and a specificity of 53.3%.
In the present study, only a third of the patients evaluated at the time of ICU admission using ultrasonography responded to volume expansion. One limitation of our study is that the measurements taken using ultrasonography can be operator dependent. It is important to evaluate who will benefit from fluid administration to avoid complications related to fluid overload. It must also be stressed that finding a volume responder is not the only parameter to be considered when deciding if fluid reanimation is needed, unless there are clinical and laboratory data of hypovolemic and tissue hypoperfusion that warrant intravascular volume expansion.
References
- 1.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 2.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 4.Namendys-Silva SA, Silva-Medina MA, Vásquez-Barahona GM, Baltazar-Torres JA, Rivero-Sigarroa E, Fonseca-Lazcano JA, Domínguez-Cherit G. Application of a modified sequential organ failure assessment score to critically ill patients. Braz J Med Biol Res. 2013;46(2):186–193. doi: 10.1590/1414-431X20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest. 2007;132(6):2020–2029. doi: 10.1378/chest.07-0073. [DOI] [PubMed] [Google Scholar]
- 6.Finfer S, Delaney A. Pulmonary artery catheters. BMJ. 2006;333(7575):930–931. doi: 10.1136/bmj.39017.459907.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trottier SJ, Taylor RW. Physicians' attitudes toward and knowledge of the pulmonary artery catheter: Society of Critical Care Medicine membership survey. New Horiz. 1997;5(3):201–206. [PubMed] [Google Scholar]
- 8.Michard F, Teboul JL, Vincent JL. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer; 2002. Detection of fluid responsiveness; pp. 553–563. [Google Scholar]
- 9.Monnet X, Teboul JL. Volume responsiveness. Curr Opin Crit Care. 2007;13(5):549–553. doi: 10.1097/MCC.0b013e3282ec68b2. [DOI] [PubMed] [Google Scholar]
- 10.Connors AF, Jr, McCaffree DR, Gray BA. Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N Engl J Med. 1983;308(5):263–267. doi: 10.1056/NEJM198302033080508. [DOI] [PubMed] [Google Scholar]
- 11.Shippy CR, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med. 1984;12(2):107–112. doi: 10.1097/00003246-198402000-00005. [DOI] [PubMed] [Google Scholar]
- 12.McGee S, Abernethy WB, III, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 13.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 14.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY AzuRea group. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 16.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]


