Abstract
We describe our management of an immunocompetent individual who developed obstructive uropathy and candidemia as a result of a fungal bezoar in the kidney. These sequelae arose from candiduria, provoked after several courses of antibiotics. Successful treatment included therapy with both culture-appropriate intravenous antifungals and operative intervention, including direct irrigation of the affected kidney with amphotericin B, relief of renal obstruction with a ureteral stent, a percutaneous nephrostomy tube, and ultimately endoscopic removal of the fungal bezoar. Our patient was successfully treated as evidenced by negative urine culture and lack of ongoing symptomatology.
Candiduria is a common problem. Risk factors include the use of Foley catheters, antibiotics, and immunosuppression, including diabetes mellitus (1). Candiduria can be complicated by ascending infection and the formation of fungal bezoars (2). The Infectious Diseases Society of America guidelines for management of fungal bezoars are predominantly based on case reports in infants with structural abnormalities of the genitourinary tract (2). In this case report, we describe the complex management of a woman with normal genitourinary anatomy treated in a multimodal fashion.
CASE REPORT
A 57-year-old woman with diabetes mellitus, a hemoglobin A1c of 6.7%, and a history of nephrolithiasis presented with emphysematous pyelonephritis due to Klebsiella pneumoniae. She was treated with intravenous antibiotics and placement of a right ureteral stent. The patient returned over the following month with nausea, vomiting, and flank pain, for which she received various intravenous and oral antibiotics. On her third presentation, she was admitted for acute kidney injury (creatinine of 4.73 mg/dL) and started on broad-spectrum antibiotics. Urinalysis showed white blood cells >50 × 103/μL, nitrites negative, leukocyte esterase 2+, blood 2+, and protein 100 mg/dL. Additionally, yeast was identified on the urine culture. She was started empirically on fluconazole. A computed tomography (CT) scan obtained at that time was suspicious for a stone adjacent to the right ureteral stent. The yeast on urine culture was later identified as Candida glabrata resistant to fluconazole with a minimum inhibitory concentration (MIC) of 256 μg/mL. She was switched to micafungin, which had an MIC of 0.06 μg/mL.
The patient underwent right ureteroscopy and laser lithotripsy with ureteral stent exchange. During the procedure, a lesion of unusual appearance was suspicious for a fungal bezoar. After the procedure, she became septic with a temperature of 39.2°C, blood pressure of 75/39 mm Hg, and heart rate of 134 beats per minute. Blood cultures grew C. glabrata, which was now resistant to micafungin with an MIC of 1.0 μg/mL. Intravenous liposomal amphotericin B 5.0 mg/kg was initiated. A repeat CT confirmed a bezoar in the renal pelvis, as well as an obstructed collecting system, prompting placement of a right percutaneous nephrostomy (PCN). An antegrade nephrostogram demonstrated no extravasation of contrast. Administration of deoxycholate amphotericin B via PCN was initiated at 10 mg per liter of 5% dextrose in water infused by gravity.
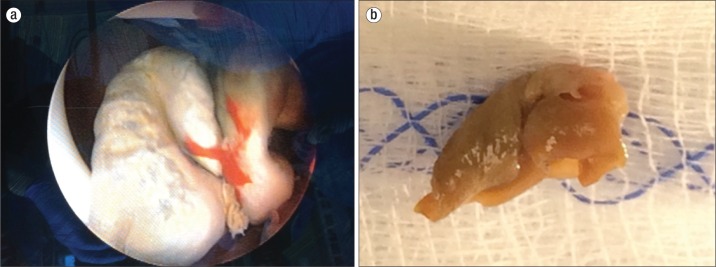
After 5 days of combination therapy, the patient underwent endoscopic removal of the fungal bezoar via a percutaneous approach and ureteral stent exchange (Figure 1a). Intraoperatively, the bezoar was described as leathery and round (Figure 1b). On postoperative day 1, the PCN was removed at bedside, and the patient was transitioned to systemic deoxycholate amphotericin B 0.5 mg/kg for an additional 14 days. She was discharged to a long-term acute care hospital to complete this course of antifungals. She had microbiological cure with a negative urine culture at day 17 and no evidence of residual hydronephrosis.
Figure 1.
(a) Fungal bezoar identified with nephroscope in right renal pelvis. (b) Fungal bezoar after endoscopic removal.
DISCUSSION
To our knowledge, this is the first multidrug-resistant C. glabrata fungal bezoar reported in an adult with normal genitourinary anatomy. Although candiduria is a common problem, renal fungal bezoars are rare. Judicious and nonjudicious use of urinary catheters and antibiotics have led to an increase in candiduria and fungal bezoars. Patients with any degree of immunosuppression are at particular risk for developing complications associated with candiduria.
The most common causes of fungal bezoars are C. albicans and C. tropicalis (3–7). C. glabrata may have inherent resistance to azoles, which are typically first-line agents for treatment of candidal urinary tract infections. In this particular case, micafungin was chosen as first-line therapy because of fungemia. Two cultures obtained 14 days apart demonstrated increasing micafungin resistance, leading to the use of amphotericin B.
The Infectious Diseases Society of America treatment guidelines recommend selecting antimicrobials depending on sensitivities of the cultured organism and including different modalities of therapy, including systemic antifungal therapy and local irrigation through PCN. Scerpella and Alhalel used intravenous and direct irrigation with amphotericin B as well as urologic intervention with success (4). In regards to the irrigation of the kidney with amphotericin B, a 10 mg/L dose was used as detailed above. There are reports of using a dose of 50 mg/L for irrigation (8, 9), but in vitro studies demonstrate that most strains of Candida are susceptible to <1 μg/mL (8). The optimal duration of irrigation is unknown. One series mentioned an average duration of 6 days (range, 4–14) (10).
Surgical debridement and removal of the fungal bezoar is a key element in eradicating infection. While the exact timing of surgical intervention is unclear, endoscopic removal of the bezoar should be considered when irrigation fails to result in either clinical or radiographic improvement (8). We successfully treated our patient with a combination of three modalities: systemic amphotericin B, local renal irrigation with amphotericin B, and endoscopic removal of the renal bezoar.
References
- 1.Lundstrom T, Sobel J. Nosocomial candiduria: a review. Clin Infect Dis. 2001;32(11):1602–1607. doi: 10.1086/320531. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Paola G, Mogorovich A, Fiorini G, Cuttano MG, Manassero F, Selli C. Candida bezoars with urinary tract obstruction in two women without immunocompromising conditions. Sci World J. 2011;11:1168–1172. doi: 10.1100/tsw.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scerpella EG, Alhalel R. An unusual cause of acute renal failure: bilateral ureteral obstruction due to Candida tropicalis fungus balls. Clin Infect Dis. 1994;18(3):440–442. doi: 10.1093/clinids/18.3.440. [DOI] [PubMed] [Google Scholar]
- 5.Jiang SH, Myers RL, Walters GD. Candida tropicalis bezoar as a cause of obstructive nephropathy. Kidney Int. 2011;79(6):690. doi: 10.1038/ki.2010.518. [DOI] [PubMed] [Google Scholar]
- 6.Shimada S, Nakagawa H, Shintaku I, Saito S, Arai Y. Acute renal failure as a result of bilateral ureteral obstruction by Candida albicans fungus balls. Int J Urol. 2006;13(8):1121–1122. doi: 10.1111/j.1442-2042.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 7.Sadegi BJ, Patel BK, Wilbur AC, Khosla A, Shamim E. Primary renal candidiasis: importance of imaging and clinical history in diagnosis and management. J Ultrasound Med. 2009;28(4):507–514. doi: 10.7863/jum.2009.28.4.507. [DOI] [PubMed] [Google Scholar]
- 8.Wise GJ. Amphotericin B in urological practice. J Urol. 1990;144(2 Pt 1):215–223. doi: 10.1016/s0022-5347(17)39415-6. [DOI] [PubMed] [Google Scholar]
- 9.Irby PB, Stoller ML, McAninch JW. Fungal bezoars of the upper urinary tract. J Urol. 1990;143(3):447–451. doi: 10.1016/s0022-5347(17)39987-1. [DOI] [PubMed] [Google Scholar]
- 10.Wise GJ, Kozinn PJ, Goldberg P. Amphotericin B as a urologic irrigant in the management of noninvasive candiduria. J Urol. 1982;128(1):82–84. doi: 10.1016/s0022-5347(17)52765-2. [DOI] [PubMed] [Google Scholar]