Sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved in Canada for use in the management of type 2 diabetes (T2DM) since May 2014. Three agents from this class are licensed in Canada (canagliflozin, dapagliflozin, and empagliflozin). These agents are likely to be used commonly in family practice because they are once-daily oral medications that lower blood glucose levels and they are associated with weight loss, lower blood pressure, and a low risk of hypoglycemia. Furthermore, recent evidence showed reduced cardiovascular mortality with empagliflozin.1
The Canadian Diabetes Association clinical practice guidelines were updated in 2015 to include this class. They note a risk of “rare diabetic ketoacidosis [DKA] (may occur with no hyperglycemia),”2 a potentially life-threatening condition that has been observed in some postmarketing reports. Here we present a case of euglycemic DKA associated with use of an SGLT2 inhibitor, with discussion of the potential mechanism of progression to DKA, considerations for prevention and recognition of this complication, and safe prescribing of this class.
Case
In February 2015, a 42-year-old woman presented to a community emergency department (ED) with shortness of breath. Her past history included T2DM since age 28, hypertension, obesity, psoriasis, hypothyroidism, and polycystic ovary syndrome. Her medications included 1000 mg of metformin twice daily, multidose insulin (MDI) with glargine twice daily and rapid-acting insulin 3 times daily, 10 mg of ramipril once daily, 25 mg of hydrochlorothiazide once daily, 10 mg of rosuvastatin once daily, 150 mg of medroxyprogesterone every 3 months, and 88 mg of levothyroxine once daily. Her diabetes had been initially treated with metformin and sulfonylureas; she started taking insulin 5 years after diagnosis when planning a pregnancy. Post partum she continued taking MDI but resumed metformin. Two months before presentation, 100 mg of canagliflozin daily was added to improve glycemic control and facilitate weight loss. This was associated with a marked improvement in blood glucose level (hemoglobin A1c concentration went from 10% to 8.7%), despite a substantial reduction in insulin dose (90 units per day [1.0 unit/kg/d] to 50 units per day [0.63 units/kg/d]), and considerable weight loss (89 to 80 kg). Six days before presentation her canagliflozin dose was increased to 150 mg daily and her insulin dose was 40 units per day (0.5 units/kg/d). The day before admission she felt unwell with some shortness of breath accompanied by nausea and vomiting, which she attributed to a flulike illness. Concerned about hypoglycemia, she omitted her insulin and monitored her capillary blood glucose levels, which were all below 12 mmol/L. She became increasingly short of breath and was brought to the ED. On examination, she was agitated but conscious and was afebrile, with an increased respiratory rate of 25 breaths/min. There were no physical findings of infection or abnormalities on cardiorespiratory examination, nor were there signs to suggest pulmonary embolus. Findings from a chest x-ray scan and electrocardiogram were normal. Her capillary blood glucose level was 15.2 mmol/L, and initial bloodwork showed a high anion gap and metabolic acidosis (pH of 7.07, anion gap of 30 mmol/L, PCO2 of 0.9 kPa, and bicarbonate level < 3 mmol/L). A diagnosis of DKA was made and standard treatment was initiated with intravenous insulin and fluids. Although blood glucose levels fell quickly below 10 mmol/L, her acidosis was somewhat resistant. Because she remained acidotic (pH of 6.9), a bolus of bicarbonate was administered before transfer to a tertiary centre where she recovered with continued fluids and intravenous insulin. No obvious precipitating cause of DKA was identified, except for insulin omission. C-peptide levels were undetectable indicating no endogenous insulin secretion. The SGLT2 inhibitor was discontinued and the patient returned to her original therapy with MDI and metformin. Since discontinuing canagliflozin her glycemic control is worse despite increased insulin doses (90 units per day), and she has regained the weight she had lost.
Discussion
This report illustrates an atypical case of DKA with only minor hyperglycemia in a patient taking an SGLT2 inhibitor for what appeared to be T2DM. There are several factors that could easily have resulted in the diagnosis of DKA being missed or delayed. Diabetic ketoacidosis is most commonly associated with type 1 diabetes (T1DM), while her clinical history and phenotype were consistent with a clinical diagnosis of T2DM. Her “shortness of breath” due to Kussmaul breathing as a result of her acidemia is a rare cause of respiratory distress presenting to the ED, which most commonly is due to cardiac or respiratory causes. Finally, hyperglycemia (glucose level > 14 mmol/L) is generally a cardinal feature in most common definitions of DKA.
Thus, the absence of marked hyperglycemia is striking. Her blood glucose had not been elevated the day before admission and was only 15 mmol/L on admission. The DKA was likely due to insulin omission in a patient with unrecognized insulin deficiency. It is unclear whether her nausea was due to a concomitant viral illness precipitating DKA or a consequence of early DKA resulting from excessive reductions in insulin dosing after addition of the SGLT2 inhibitor.
Although this patient had transitioned to insulin after a relatively short period, she had several clinical features commonly associated with insulin resistance (obesity, hypertension, and polycystic ovary syndrome), and her insulin requirements were more typical of T2DM. However, the absence of C-peptide suggests that in fact she did have T1DM. The presence of positive anti–glutamic acid decarboxylase autoantibodies would be supportive, but a negative result does not exclude a diagnosis of T1DM, as this test can have negative results in up to 20% of adults.3
Diabetic ketoacidosis is most commonly seen in T1DM but it does occur in T2DM, albeit at a very low rate (0.32 to 2.0 per 1000 patient-years, according to data from a database collected before SGLT2 approval [unpublished data from Y. Wang et al]). Diabetic ketoacidosis can develop if insulin requirements exceed the insulin available, particularly if this mismatch is marked or prolonged. Such mismatches will develop if insulin is omitted in T1DM, but can arise in both T1DM and T2DM when insulin requirements are increased, for example during intercurrent illnesses, largely as a result of increased counterregulatory hormones (epinephrine, cortisol, glucagon, and growth hormone), which oppose the actions of insulin. This might happen particularly when there is long-standing T2DM, or “burnt-out diabetes,” with very little in the way of insulin production.
This deficiency of insulin, absolute or relative, leads to unrestrained lipolysis, which overwhelms the liver’s capacity to metabolize free fatty acids, resulting in ketogenesis and ultimately ketoacidosis. As only relatively small amounts of insulin are required to restrain lipolysis and ketogenesis, DKA is uncommon in T2DM, in which endogenous insulin production generally persists. The risk is much greater in T1DM if insulin is omitted, as there is no endogenous insulin production to prevent lipolysis.
Because insulin’s main role is glucose regulation, it is not surprising that in situations of insulin deficiency, hyperglycemia and ketonemia are closely coupled. Furthermore, treatment with insulin will lead to resolution of both.
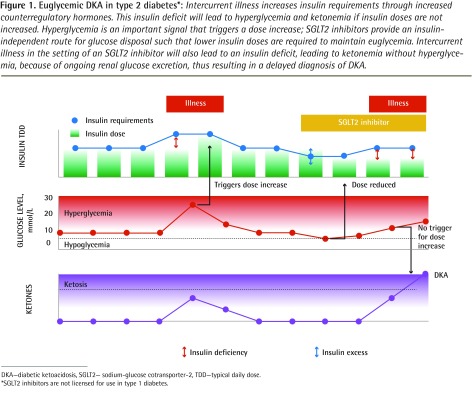
The unique feature illustrated by this case of DKA associated with SGLT2 inhibitor use is the apparent uncoupling of ketonemia from hyperglycemia. The SGLT2 inhibitors lower blood glucose levels through an insulin-independent means of glucose disposal by preventing reabsorption of filtered glucose in the proximal convoluted tubule. Those using SGLT2 inhibitors need less insulin to control blood glucose levels. As a result there will be less insulin available to restrain lipolysis. This increases the risk of DKA, particularly during intercurrent illness, when counterregulatory hormone levels increase. Unrestrained lipolysis will lead to ketonemia, but without any marked rise in blood glucose levels because of ongoing glucosuria (Figure 1).
Figure 1.
Euglycemic DKA in type 2 diabetes*: Intercurrent illness increases insulin requirements through increased counterregulatory hormones. This insulin deficit will lead to hyperglycemia and ketonemia if insulin doses are not increased. Hyperglycemia is an important signal that triggers a dose increase; SGLT2 inhibitors provide an insulin-independent route for glucose disposal such that lower insulin doses are required to maintain euglycemia. Intercurrent illness in the setting of an SGLT2 inhibitor will also lead to an insulin deficit, leading to ketonemia without hyperglycemia, because of ongoing renal glucose excretion, thus resulting in a delayed diagnosis of DKA.
DKA—diabetic ketoacidosis, SGLT2— sodium-glucose cotransporter-2, TDD—typical daily dose.
*SGLT2 inhibitors are not licensed for use in type 1 diabetes.
This uncoupling of hyperglycemia and ketonemia with insulin deficiency in those taking SGLT2 inhibitors is especially dangerous because hyperglycemia is an important trigger for patients and health care professionals to administer insulin. Unrecognized cases or delayed diagnosis of DKA can be fatal.
Other case reports of DKA with SGLT2 inhibitors seem to suggest several important risk factors.4,5 Most, but not all, cases occurred in insulin users, some with long-standing T2DM, who likely had diminished β-cell function.5,6 However, in many cases patients had been misclassified as having T2DM and were insulin deficient, as shown by negative findings for C-peptide levels (as in our case), and several had positive findings for autoantibodies (markers of T1DM).5 Although T1DM is most common in children and adolescents, it can present later in life as latent autoimmune diabetes of adults (LADA); LADA often has a more indolent course, and patients are initially assumed to have T2DM, but might have other autoimmune diseases, and progress to needing insulin after a relatively short period (1 to 5 years). The diagnosis is generally made in retrospect.
In most DKA cases associated with an SGLT2 inhibitor, there was a precipitating event such as an intercurrent illness, pancreatitis, or insulin omission. Dietary restriction and alcohol ingestion, which are often associated with a reduction in insulin doses, also seemed to be precipitants in a series of cases in individuals with T1DM taking SGLT2 inhibitors. Very low intake of carbohydrates (such as the Atkins diet) while taking SGLT2 inhibitors likely increases the risk of DKA because the diet is ketogenic and because reduced insulin requirements to maintain euglycemia might be insufficient to control lipolysis.7
In 2015, Health Canada8 reported that it had initiated a safety review of SGLT2 inhibitors and ketoacidosis, and an alert was subsequently issued in 2016 highlighting the connection.9 Similarly, the US Food and Drug Administration has issued warnings about SGLT2 inhibitors after reports of DKA in patients taking these agents, emphasizing that DKA might arise without a substantial increase in blood glucose levels.10
The SGLT2 inhibitors are approved for use in T2DM and are not recommended in T1DM. The patient in our case had no endogenous insulin production and, despite the presence of insulin resistance, was more typical of someone with T1DM; she likely has LADA. An important point to reduce the risk of DKA with SGLT2 inhibitor use is to be sure that the patients for whom these agents are prescribed definitely have T2DM; LADA can easily be overlooked in primary care, as it can develop slowly and, indeed, oral agents might be effective for a period of time. Other clues for a diagnosis of LADA are the presence of a normal body mass index, presentation at a younger age than might be typical for T2DM, early failure of oral agents, low insulin requirements, and a family or personal history of other autoimmune diseases, such as thyroid disease in this case. The SGLT2 inhibitors should not be prescribed off label for people with T1DM because of an increased risk of euglycemic DKA. The safety and efficacy of these agents in T1DM is currently being tested.11
Current data examining whether SGLT2 inhibitors are associated with increased risk of DKA in T2DM are conflicting: both increased incidence5 and no increase1 have been reported. Some caution might be necessary when the type of diabetes is unclear, particularly in patients taking long-standing insulin believed to have T2DM, as they might have diminished β-cell function. In such cases, simultaneous testing for C-peptide and glucose levels might be helpful, although interpretation of results can be challenging and referral to a diabetes specialist would be recommended before initiation of SGLT2 inhibitors. Ketone level testing is not usually necessary when prescribing these agents in T2DM.
Conclusion
Sodium-glucose cotransporter-2 inhibitors might be safely used in patients with T2DM who have endogenous insulin production. However, in situations where there is little or no insulin production—such as T1DM, LADA, pancreatitis, or in very long-standing T2DM treated with insulin with little pancreatic insulin production—DKA, and especially undiagnosed DKA, is a possibility. The diagnosis of DKA for patients taking SGLT2 inhibitors should not be ruled out because of glucose levels in the normal range.
EDITOR’S KEY POINTS
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are not indicated for use in type 1 diabetes. However, some patients with apparent type 2 diabetes (T2DM) might in fact have latent autoimmune diabetes of adults (LADA).
To reduce the risk of diabetic ketoacidosis (DKA) with SGLT2 inhibitor use, ensure that the patients for whom these agents are prescribed definitely have T2DM; LADA can easily be overlooked in primary care, as it can develop slowly and oral agents might be effective for a period of time.
In patients with little or no insulin production—such as in type 1 diabetes, LADA, pancreatitis, or very long-standing T2DM treated with insulin with little pancreatic insulin production—DKA is a possibility. The diagnosis of DKA for patients taking SGLT2 inhibitors should not be ruled out because of a glucose level in the normal range.
Footnotes
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de septembre 2016 à la page e518.
Competing interests
Dr Clement has received honoraria for delivering continuing medical education from the following companies: AstraZeneca (dapagliflozin), Boehringer Ingelheim and Eli Lilly (empagliflozin), and Janssen (canagliflozin). Dr Senior has received research support as a local principal investigator for clinical trials of sodium-glucose cotransporter-2 inhibitors sponsored by Bristol Myers Squibb (dapagliflozin) and Boehringer Ingelheim (empagliflozin). He has received consulting fees and speaker fees for delivering continuing medical education to physicians and other health care professionals from AstraZeneca (dapagliflozin), Boehringer Ingelheim and Eli Lilly (empagliflozin), and Janssen (canagliflozin).
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. Epub 2015 Sep 17. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, editor. Canadian Diabetes Association clinical practice guidelines. 2015 Update. Toronto, ON: Canadian Diabetes Association; 2015. Table 1. Antihyperglycemic agents for use in type 2 diabetes. Available from: guidelines.diabetes.ca. Accessed 2015 Oct 15. [Google Scholar]
- 3.McDonald TJ, Colclough K, Brown R, Shields B, Shepherd M, Bingley P, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from type 1 diabetes. Diabet Med. 2011;28(9):1028–33. doi: 10.1111/j.1464-5491.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 4.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JB, Hirsch IC. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–93. doi: 10.2337/dc15-0843. Epub 2015 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680–6. doi: 10.2337/dc15-1251. Epub 2015 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa W, Kazuhiko S. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135–8. doi: 10.1111/jdi.12401. Epub 2015 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638–42. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 8.Health Canada [website] Forxiga, Invokana. Health Canada begins safety review of diabetes drugs known as SGLT2 inhibitors and risk of ketoacidosis. Ottawa, ON: Health Canada; 2015. Available from: www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2015/53892a-eng.php. Accessed 2016 Jul 26. [Google Scholar]
- 9.Health Canada [website] SGLT2 inhibitors [INVOKANA (canagliflozin), FORXIGA (dapagliflozin), XIGDUO (dapagliflozin/metformin), JARDIANCE (empagliflozin)]—risk of diabetic ketoacidosis. Ottawa, ON: Health Canada; 2016. Available from: www.healthycanadians.gc.ca/recall-alert-rappel-avis/hcsc/2016/58404a-eng.php#related-connexe. Accessed 2016 Jul 25. [Google Scholar]
- 10.US Food and Drug Administration [website] FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Silver Spring, MD: US Food and Drug Administration; 2016. Available from: www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed 2016 Jul 26. [Google Scholar]
- 11.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38(3):412–9. doi: 10.2337/dc13-2955. Epub 2014 Sep 30. [DOI] [PubMed] [Google Scholar]