Abstract
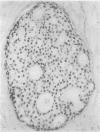
The reliability of an immunohistological method, applied to paraffin wax sections, was assessed for determination of oestrogen receptor content of biochemically oestrogen receptor negative breast carcinomata. Sixty consecutive tumours with oestrogen receptor concentrations of less than 10 fmol/mg cytosol protein, as estimated by dextran-coated charcoal biochemical assay, were examined. Paraffin wax sections were treated with DNAse before applying a peroxidase-anti-peroxidase method using ER-ICA monoclonal antibodies. Fifty one cases (85%) were negative, six (10%) weakly positive, and three (5%) were moderately positive. No strongly positive cases were seen. It is suggested that cases with weakly positive staining, especially when localised to a small area, should be regarded as negative. On the other hand, as the three moderately stained cases included two small tubular carcinomas and an invasive ductal carcinoma with high progesterone receptor concentrations, it is more likely that the biochemical assay in these cases represented false negative results due to sampling error or inclusion of fibrous or other non-neoplastic tissue in the assayed samples. It is concluded that the immunohistological method used here is fairly reliable and would be especially valuable for determination of oestrogen receptor content in small, mammographically detected tumours from which no tissue would be available for biochemical assay or frozen section examination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J., Orntoft T. F., Poulsen H. S. Immunohistochemical demonstration of estrogen receptors (ER) in formalin-fixed, paraffin-embedded human breast cancer tissue by use of a monoclonal antibody to ER. J Histochem Cytochem. 1988 Dec;36(12):1553–1560. doi: 10.1177/36.12.2461414. [DOI] [PubMed] [Google Scholar]
- Cheng L., Binder S. W., Fu Y. S., Lewin K. J. Demonstration of estrogen receptors by monoclonal antibody in formalin-fixed breast tumors. Lab Invest. 1988 Mar;58(3):346–353. [PubMed] [Google Scholar]
- Cudahy T. J., Boeryd B. R., Franlund B. K., Nordenskjöld B. A. A comparison of three different methods for the determination of estrogen receptors in human breast cancer. Am J Clin Pathol. 1988 Nov;90(5):583–590. doi: 10.1093/ajcp/90.5.583. [DOI] [PubMed] [Google Scholar]
- Hiort O., Kwan P. W., DeLellis R. A. Immunohistochemistry of estrogen receptor protein in paraffin sections. Effects of enzymatic pretreatment and cobalt chloride intensification. Am J Clin Pathol. 1988 Nov;90(5):559–563. doi: 10.1093/ajcp/90.5.559. [DOI] [PubMed] [Google Scholar]
- Said J., Shintaku I. P. Detection of estrogen receptors with monoclonal antibodies in paraffin sections. Am J Clin Pathol. 1988 Jul;90(1):120–120. doi: 10.1093/ajcp/90.1.120a. [DOI] [PubMed] [Google Scholar]
- Shintaku I. P., Said J. W. Detection of estrogen receptors with monoclonal antibodies in routinely processed formalin-fixed paraffin sections of breast carcinoma. Use of DNase pretreatment to enhance sensitivity of the reaction. Am J Clin Pathol. 1987 Feb;87(2):161–167. doi: 10.1093/ajcp/87.2.161. [DOI] [PubMed] [Google Scholar]
- Shousha S., Coady A. T., Stamp T., James K. R., Alaghband-Zadeh J. Oestrogen receptors in mucinous carcinoma of the breast: an immunohistological study using paraffin wax sections. J Clin Pathol. 1989 Sep;42(9):902–905. doi: 10.1136/jcp.42.9.902. [DOI] [PMC free article] [PubMed] [Google Scholar]