Abstract
Gene therapy development has been limited by our inability to target multifocal cancer with systemic delivery. We developed a systemically administered, tumor-targeted liposomal nanodelivery complex (SGT-94) carrying a plasmid encoding RB94, a truncated form of the RB gene. In preclinical studies, RB94 showed marked cytotoxicity against tumor but not normal cells. SGT-94 was administered intravenously in a first-in-man study in metastatic genitourinary cancer. Minimal side effects were observed; dose-limiting toxicity (DLT) has not been reached in 11 evaluable patients. There was evidence of clinical activity at the 2.4 mg dose with one complete remission (CR) and one partial remission (PR). The patient in CR was retreated upon progression and had a second PR. Furthermore, there was tumor-specific targeting of the SGT-94 complex. One patient had wedge resections of two lung metastases which demonstrated RB94 expression at the DNA level by polymerase chain reaction (PCR) and at the protein level by Western blotting, with no RB94 present in normal contiguous lung. In conclusion, systemically delivered SGT-94 showed evidence of selective tumor targeting and was well tolerated with evidence of clinical activity. Additional studies are warranted to explore the activity of this drug as a single agent and in combination therapy.
Introduction
Translation of the RB gene starting from the second in-frame AUG codon produces a truncated protein, RB94, which lacks the 112 N-terminal amino acids present in the full length RB110 wild-type protein. In vitro, transfection of plasmids coding for RB94 leads to marked cytotoxicity against all tumor cell lines tested, irrespective of genetic makeup and status of native RB expression.1,2,3 Remarkably, such expression of RB94 in nontransformed cells does no apparent harm.3 To date, there has been no documented development of tolerance to RB94 expression. This is in contrast to the full-length transcript, for which numerous long-term clones can be obtained after RB110 transfection in RB-negative cancer cell lines.1
In vivo, we have demonstrated considerable regression of subcutaneous xenograft tumor derived from human bladder and small-cell lung carcinomas by means of direct injection into these tumors of an adenoviral vector coding for RB94.2 While these results established proof-of-principle for use of RB94 as a novel cytotoxic therapy, the lack of a systemic delivery system represents a fundamental barrier to use of this (or any other) gene-therapy agent in the context of disseminated human cancers.
In response to this limitation, a tumor-targeted complex for systemic gene delivery has been developed. In this nanoscale complex, the payload is encapsulated within a cationic liposome. The surface of the liposome is decorated with an anti-transferrin receptor (TfR) single-chain antibody fragment. This arrangement targets cancer cells by binding to the TfR which is highly expressed on tumor cells and effectively internalized via receptor-mediated endocytosis.4,5,6
The prototype of this nanocomplex encapsulates a plasmid encoding the wild-type p53 gene (SGT-53). Systemic administration of SGT-53 effectively and selectively delivers the p53 gene to both primary and metastatic tumor cells, sensitizing them to radiation and chemotherapy.7,8,9,10,11,12 In preclinical studies, the combination of SGT-53 and conventional therapies resulted in significant tumor growth inhibition and even complete tumor regression in animal models of various tumor types.7,8,9,10,11 A phase l study has been completed using SGT-53 for advanced solid tumors.12 Minimal side effects and preliminary evidence for efficacy and tumor-targeted delivery were reported.12 Two phase 2 studies of SGT-53 are currently ongoing.
In this first-in-man phase l study, a plasmid containing the RB94 cDNA rather than p53 was used with the identical SGT delivery system, to form an agent termed SGT-94. Preclinical studies using intravenously administered SGT-94 showed tumor-specific targeting of bladder cancer cells in subcutaneous tumors, as well as antitumor activity in combination with chemotherapy.13 Genitourinary tumors were treated in this study with a focus on metastatic bladder cancer, since high levels of TfR are present in human bladder cancers, especially in recurrent high-grade disease14,15,16 making them a potential target for the SGT nanodelivery system. The plasmid within SGT-94 also included a modified cytomegalovirus (CMV) promoter which results in high expression of RB94 protein allowing RB94 protein to be detected in the cytoplasm of the cancer cell by immunochemical staining, even though RB is a nuclear protein.13 Here we report the results of a phase 1 trial which demonstrate the safety of SGT-94 as a therapeutic agent, the ability of the SGT-94 nanocomplex to express RB94 in metastatic tumor cells, and the potential of SGT-94 for the treatment of bladder cancer.
Results
Patient characteristics
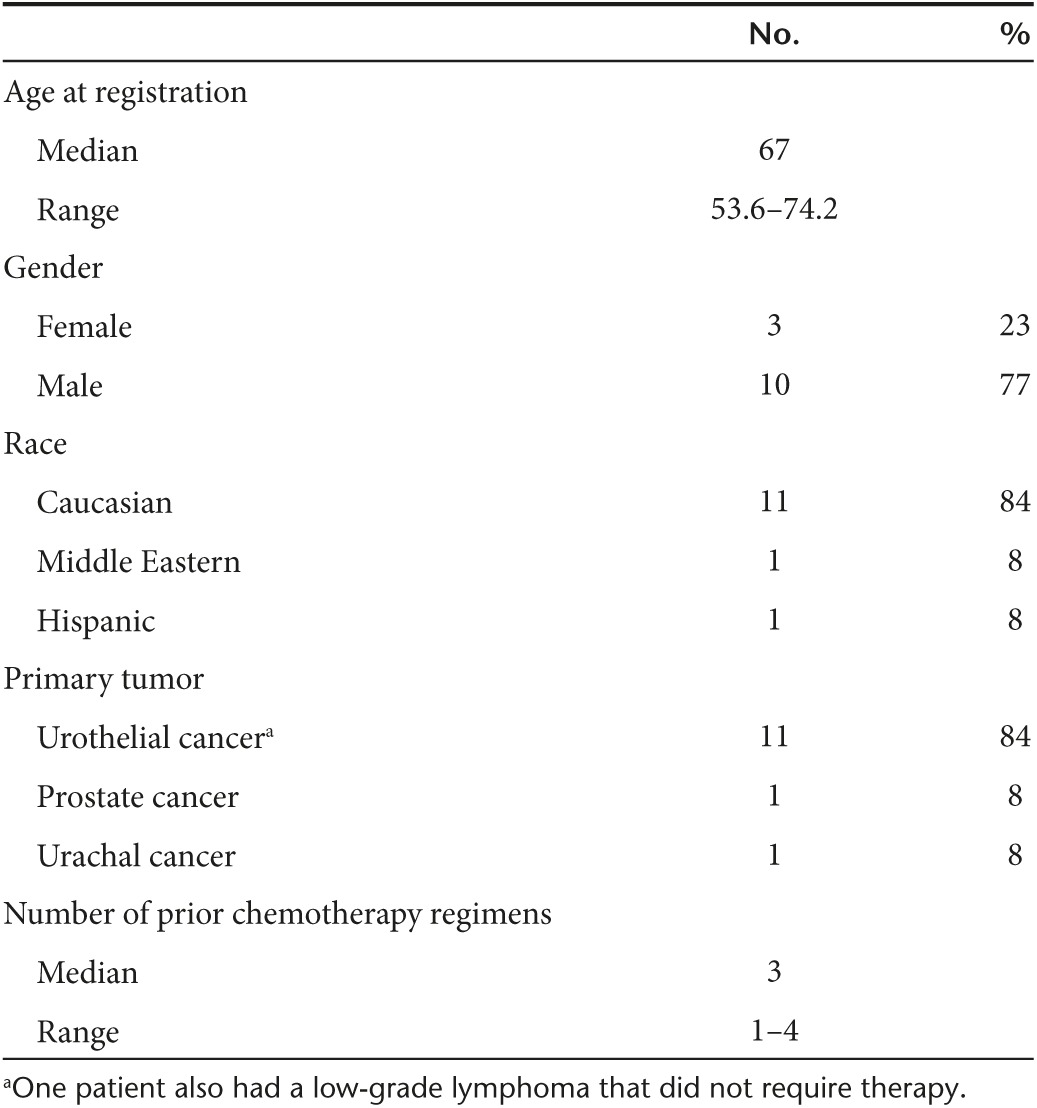
Between February 2012 and July 2014, 13 previously treated patients with metastatic incurable genitourinary cancers were consented and treated on this clinical trial. Eleven patients had urothelial cancer and one patient each had prostate and urachal cancer. Demographic and clinical characteristics are given in Table 1. All patients had prior standard of care and received at least one dose of SGT-94. As described below in the discussion, 11 patients were considered evaluable for activity and DLT.
Table 1. Patient characteristics.
Therapy-related toxicity
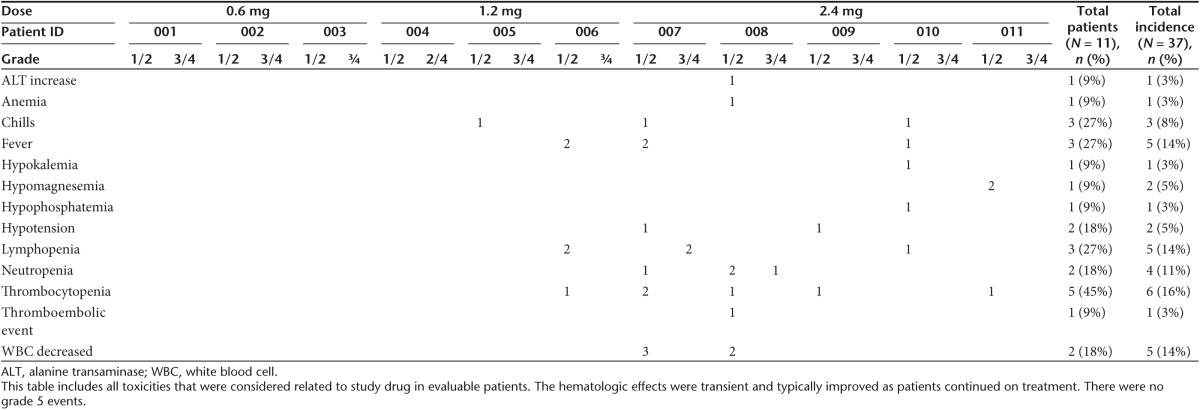
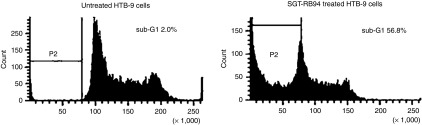
The adverse events experienced by the 11 evaluable subjects are given in Table 2. Subjects 12 and 13 were administered a malformed SGT-94 nanocomplex which had been prepared with liposomes that were biologically inactive which we suspect may be related to an inadvertent freeze/thaw of the liposome. Each complex mix had to be tested for biological activity as shown in Figure 1. The defective product did not give cytotoxicity above baseline. Further evaluation of the separate components confirmed it was related to the liposome. All complex mixes for subjects 1–11 showed similar excellent biological activity as seen in Figure 1. While the adverse events associated with these subjects have been included in a separate table (Table 3) for completeness, they are not considered evaluable for DLT or activity.
Table 2. Toxicity in evaluable patients.
Figure 1.
Flow cytometry showing typical marked increase in number of sub-G1 cells 24 hours after treatment with 2 µg of SGT-94 following mixing in the Cell Therapy Laboratory for systemic i.v. injection to the patient. This was done posttreatment and was required for every mix that was infused.
Table 3. Toxicity (at least possibly related) in nonevaluable patients.

The treatment was very well tolerated (Table 2). The majority of the adverse events observed in the evaluable patients were grade 1/2, with the most frequent toxicity recorded being grade 1/2 thrombocytopenia (45% of subjects). The hematologic effects were typically transient and improved as patients continued therapy. Fever and chills were experienced by 27% of the patients and were expected based upon previous studies with liposomes and the SGT-53 nanocomplex.12 In addition two subjects (18%) experienced grade1/2 lymphopenia or a decrease in white blood cell count. Of the grade 3/4 adverse events observed in the trial only three were deemed even possibly related to SGT-94, two instances (1 grade 3 and 1 grade 4) of lymphopenia in subject 7, and one instance of grade 3 neutropenia in subject 8. All of these of the adverse events, including grade 3/4 events, were transient and resolved while continuing treatment. There were no grade 5 events.
There was no DLT at the 0.6 mg DNA or 1.2 mg DNA dose levels. The protocol initially required decreasing the dose of the drug in the setting of any myelosuppression. Thus, the first two patients treated at the 2.4 mg DNA dose initially were not able to complete the first cycle, one due to grade 2 thrombocytopenia (subject 7) and the second due to transient grade 3 neutropenia (subject 8). Transient grade 1/2 neutropenia had been observed in three patients in a phase la gene therapy trial using the SGT nanocomplex to deliver the wtp53 gene rather than RB94.12 However, in the SGT-53 trial this was not considered DLT and the patients continued on treatment with no further occurrences of neutropenia.
Due to this observation, the protocol was amended allowing continuing treatment if transient decreases in platelet count or neutrophil count were observed. Patients were then allowed to continue on full-dose therapy for platelet count at a level of ≥75,000 and absolute neutrophil count (ANC) >1,000, with a DNA dose reduction by 1 level for a platelet count between 50,000–75,000 or ANC between 500–999. If, while continuing therapy, the absolute platelet count and the neutrophil count improved, patients were retreated at the original DNA dose level.
Three additional patients who were treated at the 2.4 mg dose did not experience a DLT using the new criteria. In addition, subject 8, who had to discontinue therapy based upon the earlier criteria, but had a response, chose to resume treatment upon progression. This patient was able to complete four full rounds of therapy using the new toxicity criteria without any DLTs (Table 4).
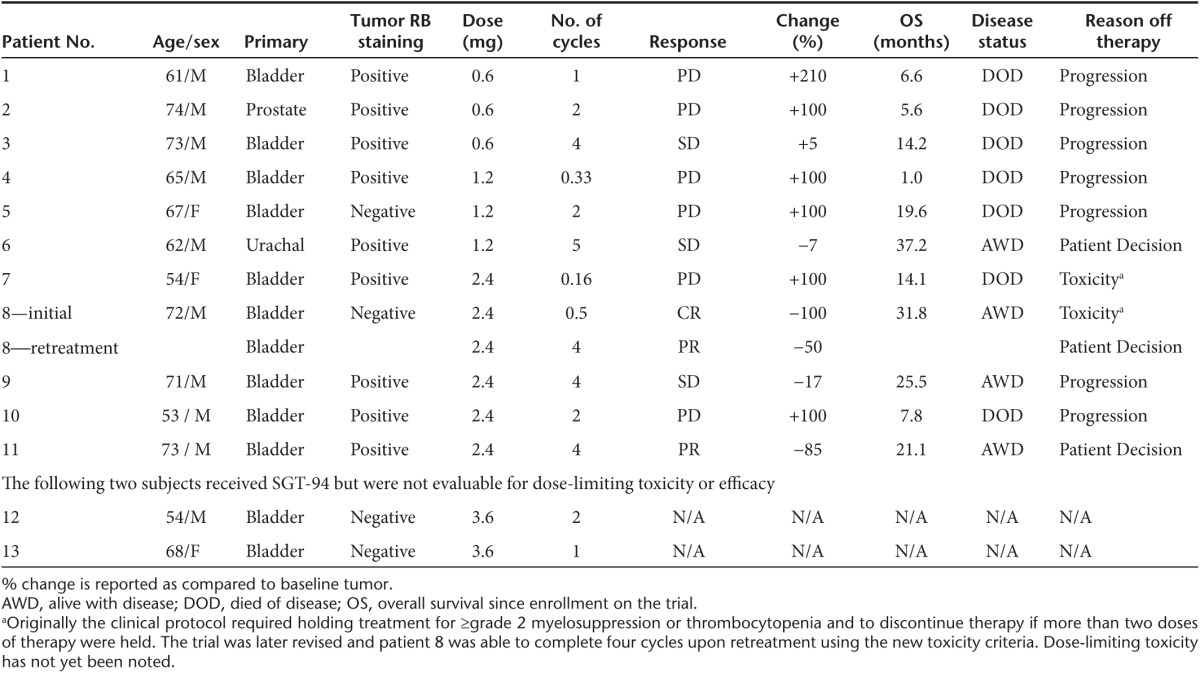
Table 4. Primary tumor with dose, response, and survival outcome.
Clinical activity
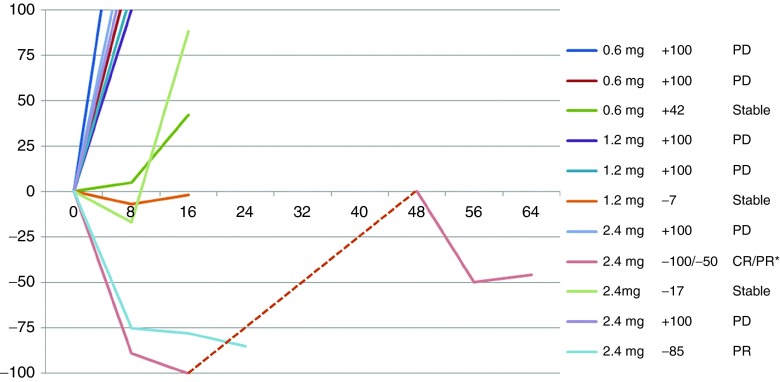
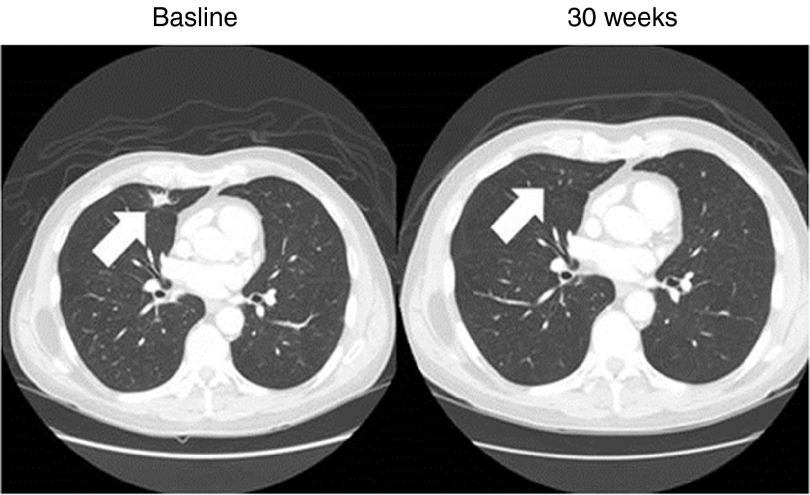
Evidence of clinical activity was observed in patients treated on this phase 1 trial in a dose-dependent manner. A summary of the SGT-94 dose, primary tumor response, and survival outcome for the 11 evaluable subjects is given in Table 4. In addition, a spider plot of the observed responses is shown in Figure 2. At the lowest dose of SGT-94 (0.6 mg DNA), one individual (subject 3) demonstrated stable disease after four cycles of treatment. Similarly, one subject treated at the 1.2 mg DNA dose (subject 6) also demonstrated stable disease at the end of five cycles. More significant responses were observed when the dose administered was increased to 2.4 mg DNA/infusion. Despite receiving only three initial doses of SGT-94 due to grade 2 neutropenia, one of the five subjects treated at this dose level (subject 8) had a clinical complete remission of lung metastasis (Figure 3). After having been off treatment for 4 months, this individual experienced progression with a biopsy-proven peritoneal seeding and was retreated at the 2.4 mg dose completing four cycles of therapy without interruption. Again, the subject experienced transient side effects including neutropenia and thrombocytopenia (Table 2) that resolved and they achieved a partial remission following this retreatment. A second subject at the 2.4 mg DNA dose (subject 11) experienced a partial remission after four cycles of treatment, and stable disease was reported in a third subject (subject 9). Most significantly, with the exception of subject 3 (SD at the 0.6 mg DNA dose, who survived ~14 months), all of the subjects who exhibited a clinical response are still surviving. The longest survival to date is ˃3 years since entering the trial (Table 4).
Figure 2.
Spider chart of clinical responses for evaluable patients. *This patient had an initial CR, and then upon progression was retreated achieving a PR upon retreatment.
Figure 3.
CT scan of a bladder cancer metastasis to the lung in subject 8. He had received four prior cycles of chemotherapy (gemcitabine and cisplatin; dose-dense methotrexate, vinblastine, doxorubicin, with cisplatin; gemcitabine, paclitaxel, and doxorubicin; and cisplatin, gemcitabine, with ifosfamide). The baseline scan shows a 1.6 cm lung tumor metastasis from a bladder cancer present at baseline (arrow) which is no longer present at 30 weeks after three treatments with 2.4 mg of SGT-94. He had other nonmeasurable lung metastases at baseline which also resolved. He later progressed with biopsy proven peritoneal implants measuring 2.1 cm in size and had a partial response after completing four full cycles of therapy.
Tumor-specific targeting
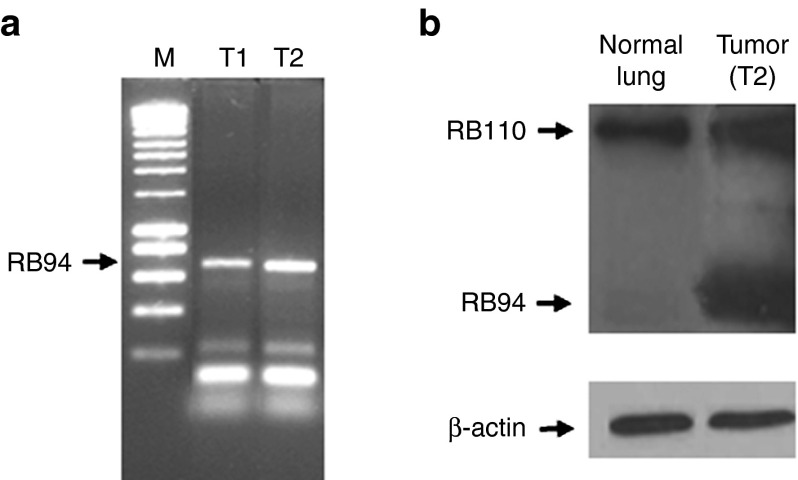
A key component of the SGT-94 nanocomplex is the anti-TfR single-chain antibody fragment that is utilized as the tumor-targeting moiety. The specificity of the tumor targeting has been established in extensive preclinical studies.7,8,9,10,11 Strong evidence for tumor-specific targeting was also observed in this trial in two individual subjects. After experiencing stable disease on treatment, subject 6 had wedge resections of lung metastases. His tumor carried the wild-type RB gene. Two metastatic urachal lung metastases were removed 48 hours after the third infusion of SGT-94 (1.2 mg dose). To determine if the SGT-94 delivered RB94 gene was present in these tumors, DNA polymerase chain reaction (PCR) was performed using RB94-specific primers (Figure 4a). A strong band is evident after PCR in both of the tumors, demonstrating the presence of the transgene in these lung metastases. Moreover, significant RB94 protein expression was documented in both tumors by Western analysis, whereas no RB94 protein expression was evident in the contiguous normal lung (Figure 4b). Since the tumors contained wild-type RB110, normal RB110 protein expression was observed in both tumor and normal lung (Figure 4b).
Figure 4.
Evidence for SGT-94 tumor-specific targeting. (a) Two metastatic bladder cancers (T1 and T2) were removed following a wedge resection of lung metastases in subject 6 ~48 hours after treatment with a 1.2 mg dose of SGT-94. Each tumor showed the presence of RB94 using an RB94-specific polymerase chain reaction primers (arrow) but not in normal lung tissue (not shown). (b) RB94 protein expression was also seen in each tumor (only T2 shown), whereas no RB94 was found in the contiguous normal lung. Since the tumors contained wild-type RB110, it was present in both tumor and normal lung as shown.
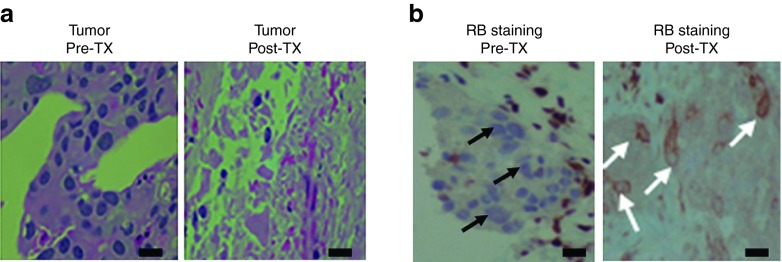
In addition, a pretreatment lymph node biopsy obtained from subject 5 was found to be an RB-negative metastasis. An additional biopsy was taken from the same lymph node ~36 hours after the third treatment with the 1.2 mg dose of SGT-94. The initial biopsy contained a large amount of viable tumor cells with no necrotic areas present. In contrast, the posttreatment biopsy showed numerous necrotic tumor cells (Figure 5a). These necrotic tumor cells demonstrated strong RB cytoplasmic staining, suggesting that the observed cancer cell death likely resulted from the SGT-94-mediated delivery of RB94 into the tumor cells (Figure 5b). Although RB94 is expressed normally in the nucleus, SGT-94 has been shown to produce large amounts of RB94 protein that can also be seen in the cytoplasm.13
Figure 5.
Immunostaining evidence for SGT-94-induced tumor cell cytotoxicity and gene transfer. (a) Detection of tumor cell death. The two panels show paraffin sections of a metastatic bladder cancer lymph node from subject 5 before SGT-94 treatment (Left) (few if any dead cells seen) and in the same lymph node 36 hours after the third treatment with a 1.2 mg dose of SGT-94 (right) (marked tumor cell necrosis is apparent). (b) Immunostaining for presence of RB94. The left panel demonstrates the absence of RB wild-type staining in this RB-negative lymph node metastasis (black arrows) before treatment. The right panel is an area in the same biopsy shown in a after systemic SGT-94 treatment showing only necrotic cancer cells that have RB94 cytoplasmic staining (white arrows) indicative of SGT-94 transfer and cancer cell death. Bars = 20 nm.
Discussion
Intravenously administered SGT-94 showed tumor-specific delivery of the gene and had evidence of clinical activity. These results overcome a major limitation in gene therapy development which has been severely constrained by the need for direct tumor injection. The need for direct tumor injection limits its use to a rather small subset of cancer patients who may be treated by other local measures including surgery, radiation, or percutaneous ablation. A systemic delivery system overcomes these limitations, opening up the field to a large number of cancer patients with multifocal systemic metastases who lack any further standard of care therapies.
Although the potential of RB94 as a potent human cancer-specific cytotoxic agent which had no effect on normal human cells was identified over 20 years ago,1 the full potential of RB94 could not be realized without such a systemic delivery system to target and treat both primary and metastatic disease. SynerGene Therapeutics (Potomac, MD) has developed a nanocomplex, for the systemic delivery of molecular medicines, including plasmid DNA for use in gene therapy. In these nanomedicines (which use the SGT prefix), the payload is encapsulated within a cationic liposome, the surface of which is decorated with an anti-TfR single-chain antibody fragment designed to target cancer cells via the TfR, which is highly expressed on surface of tumor cells. The TfR-bound nanocomplex is efficiently internalized via receptor-mediated endocytosis. The SGT nanocomplex has been shown to specifically deliver various payloads, including plasmid DNA,7,8,9,10,11,13 siRNA/miRNA,17,18 small molecules,19 and even imaging agents,20,21,22 to both primary and metastatic tumor cells in vivo. This nanocomplex also crosses the blood–brain barrier.23 The SGT nanocomplex carrying the wtp53 gene (SGT-53) has completed phase 1a and b clinical trials where it was shown to be well tolerated at the therapeutic doses tested and demonstrated anticancer effect.12 This nanomedicine has now moved into phase 2 trials (NCT02340156, NCT02340117). Thus, the SGT nanodelivery system was employed as a means to efficiently deliver RB94 (in the product termed SGT-94).
While SGT-94 was administered to a total of 13 subjects, only 11 were considered evaluable as it was discovered that subjects 12 and 13 had unintentionally been administered less biologically active SGT-94. However, this did not result in any SGT-94 related serious adverse events or lead to a DLT. Upon discovery of this, the components of the SGT-94 nanocomplex were analyzed. Cationic liposomes are one of the three components that comprise the SGT-94 nanocomplex. It was determined that the loss of biological activity arose from the use of damaged liposomes in preparation of SGT-94, which we suspect was due to inadvertent freezing/thawing of the liposome. Another sample from the same lot of liposomes stored elsewhere was found to produce fully active SGT-94. The damaged liposomes had been used for preparing SGT-94 for all six of the infusions in cycle 2 for subject 12 and for all five of the infusions for subject 13. Thus, these two subjects were not considered evaluable for DLT or for clinical activity. In a different trial involving wtp53 as the therapeutic gene in an analogous nanocomplex using the exact same lot of liposome,12 six patients were treated at a 3.6 mg dose for a total of 48 doses and no malformation of the nanocomplexes were evident. Use of lyophilized material for future trials will overcome any difficulties in preparing and storing fresh liposome which was used in this trial.
The 11 evaluable subjects received SGT-94 over three DNA dose ranges, three subjects at 0.6 mg, three at 1.2 mg, and five at 2.4 mg DNA/infusion. In this trial, SGT-94 was very well tolerated with no DLT at any of the DNA doses tested. Moreover, the majority of the patients received multiple cycles of SGT-94, up to five cycles (30 infusions in one case), with no increase in toxicity thus demonstrating the safety of the nanomedicine. No serious adverse events were classified as definitely related to SGT-94. Only one serious adverse event, hypotension experienced by subject 7, was classified as probably related to the study drug. However, in the phase 1 trial with SGT-53 grade1/2 hypotension occurring within 24 hours after the first infusion was one of the most frequently occurring adverse events and was considered related to the SGT-53. Thus, since the adverse event with SGT-94 occurred during the 23 hours observation period after the first infusion of this subject, this event was expected. Increasing the dose of steroid premedication appeared to be helpful in controlling some of these affects, including the fevers and chills some subjects experienced. DLT was not observed at the 2.4 mg dose, suggesting that future trials may explore further dose escalation.
Although the objective of this trial was to assess the safety of SGT-94, evidence of clinical activity was also observed. Three of five patients (60%) treated at the 2.4 mg dose showed some clinical effect. One subject had a complete remission of a lung metastasis and an additional partial response upon retreatment when new tumors arose in a different location. Moreover, a second subject treated at this dose level also displayed a partial response with 85% reduction in tumor size. Stable disease was produced in a third subject treated at the 2.4 mg DNA dose level. However, in subjects treated at the 0.6 or 1.2 mg DNA dose levels, only one stable disease, and no partial or complete response, was observed at each dose.
It is likely that the tumor-targeting nature of the SGT nanodelivery system plays a role in this observed clinical activity. To confirm that systemically administered SGT-94 targets tumors and delivers the RB-94 gene into the tumor cells, the presence of both the transgene and the RB-94 protein was evaluated in metastases from subjects in the trial. Evidence of selective targeting to tumor tissue was found by both PCR and Western analysis in two metastatic bladder nodules from the lungs of a patient. Since SGT-94 can be given at least twice a week with relatively low toxicity, numerous doses can be administered with the expectation that this tumor-specific targeting will occur with each treatment.
The results discussed above serve as proof-of-concept that systemically administered SGT-94 is safe and that the RB94 gene payload can be efficiently delivered to metastases, resulting in clinical activity. The benefits of developing a systemic delivery system for genes are readily apparent. This would avoid intratumoral injections and the risks associated with them in patients with tumors with altered vasculature, as well as avoiding potential toxicities associated with nontargeted administration. In addition, the ability to target multifocal areas of tumor including much smaller, earlier metastases that may not be discernable easily on CT scan or amenable to needle placement is of potential great clinical benefit.
The tumor-targeted activity and lack of cell death in normal urothelial cells in preclinical models also indicate that SGT-94 has a therapeutic index that may be beneficial when used in combination therapy.13 The lack of prolonged myelosuppression and potential for novel antigen exposure with a novel gene product also may make these therapies an attractive partner for immune checkpoint inhibitors. The subject who experienced a repeat response upon retreatment with SGT-94 was subsequently treated with an immune checkpoint inhibitor upon progression and is once again in a complete remission with long-term immunotherapy.
Additionally, preclinical studies suggested RB94 was cytotoxic to multiple tumor types irrespective of their genetic makeup, suggesting that SGT-94 could be expanded to other tumor types regardless of RB status. Although this approach is contrary to personalized medicine in which specific gene mutations are sought as a basis for treatment, a therapeutic modality that can actually be effective without the necessity, time, and cost of determining the genetic makeup of a particular tumor would have significant clinical potential.
In conclusion, treatment with SGT-94 was shown to produce tumor-specific targeting. It was well tolerated and no DLT was observed. There was also evidence of clinical activity. Further studies at a higher dose of SGT-94 are warranted along use of SGT-94 in combination with chemotherapy and/or immune checkpoint inhibitors.
Materials and Methods
Patient selection. Between 23 February 2012 and 15 July 2014, 13 patients were enrolled in this MD Anderson Institutional Review Board approved clinical trial. This trial (trial registration ID: NCT01517464, https://clinicaltrials.gov) was an open label, single center, sequential dose-escalating phase 1 trial, evaluating the safety and potential activity of SGT-94. The patient population consisted of those with confirmed histological diagnosis of genitourinary tumors, with incurable metastatic disease for which no additional standard therapy was available. All patients signed informed consents to participate. Patients had adequate lung function with spirometry showing at least 70% of predicted volumes of FEV1 and adequate cardiac function with a left ventricular ejection fraction ≥45%. Patients were required to have adequate physiologic reserve as shown as by Zubrod performance status ≤2–3. Patients had adequate laboratory values including an ANC ≥1,200 mm3, a platelet count ≥100,000 mm3, and AST/ALT ≤3 times the upper limit of normal. Patients needed either normal kidney function or a creatinine clearance ≥40 ml/min as calculated by Cockcroft–Gault. The patients required an adequate hemoglobin ≥10 g/dl and PT/PTT <1.5 times than the upper limit of normal. Patients were excluded if they had other experimental therapy in the past 30 days or a prior radiation within 4 weeks. In addition, patients were excluded if they had a myocardial infarction within the past 6 months or history of a cerebrovascular accident (CVA) or transient ischemic attack (TIA) in the past 6 months, New York Heart Associate ≥grade 2 congestive heart failure, unstable angina, or evidence of uncontrolled hypertension defined as a systolic blood pressure >140 or a diastolic blood pressure ≥90 despite therapy.
Patient treatment. The RB94 plasmid used (pSCMV-RB94) for treatment has been previously described13 and was encapsulated with the SGT systemic tumor-targeted liposomal nanodelivery system (SGT-94). The cGMP produced liposome component and sucrose excipient were provided by SynerGene Therapeutics. The RB94 plasmid and TfR were manufactured under cGMP conditions by the Waisman Clinical BioManufacturing Facility (Madison, WI). All three components and excipient were combined in the MD Anderson Cancer Center Cell Therapy Laboratory for each patient dose. Final testing of each complex preparation included: gram stain, visual inspection for lack of aggregates, and particle sizing (done preinfusion), with postinfusion testing for biological activity in cell cultures zeta potential and a 14-day sterility testing (aerobic and anaerobic).
Study agent administration to each subject was planned in a sequential manner, starting at 0.6 mg DNA per infusion with escalation based on the continuous reassessment method statistical design to assign dose levels on the basis of toxicities. Dose levels used were 0.6, 1.2, and 2.4 mg DNA/infusion. New dose levels were only opened after all subjects completed one full cycle of therapy at the previous dose level. At each new dose level, the first subject must have completed one full cycle before enrolling additional subjects at the same dose. After this, patients in the same cohort could be treated concurrently. There was no intrapatient dose escalation.
In the phase 1 trial with the SGT nanocomplex delivering the wt p53 plasmid DNA (SGT-53), the primary toxicities noted were grade 1 and 2 transient fever starting 6–8 hours after infusion and lasting 12–16 hours (5 of 11 patients, 46%) and transient hypotension over a similar time frame in 7 patients (7 of 11 patients, 64%).12 Based upon these findings all patients in this SGT-94 study were administered premedication with dexamethasone (8–20 mg per oral (PO)) 60 minutes prior to therapy; diphenhydramine (25 mg i.v.) and famotidine (20 mg i.v.) 30 minutes prior to the infusion; and acetaminophen 650 mg PO prior to each infusion. In addition, the patients in this study were administered their first dose of SGT-94 in the hospital and were monitored for fever, chills, hypotension, and rigors for ~24 hours and were allowed to receive additional dexamethasone (20 mg i.v.) for suspected acute hypersensitivity reactions including hypotension or bronchospasm. During this initial 24-hour time period, the patients received i.v. fluid (at least 0.5 NS osmolarity) running at 100 ml/hour.
Subsequent infusions were given in the outpatient setting. Study drug was administered twice weekly for 3 weeks followed by a 1 week break (4 weeks = 1 cycle). The infusions typically occurred over 1–2.5 hours depending upon the dose and volume of the drug. All infusions were completed within 8 hours of preparing a given dose. Blood pressure and pulse were monitored approximately every 15 minutes during study drug infusion and for 2 hours after each dose. Patients received an EKG ~2 hours after administration of the study drug. Patients were allowed to receive antiemetics, analgesics, and other supportive measures as indicated by the treating physician.
Assessments, follow-up, and monitoring. Patients were seen by a physician or their designee prior to each cycle of treatment. During the first infusion, the treating physician was present to assess toxicity. Prior to each dose patients received a physical examination with documentation of adverse events. Laboratory studies, including a CBC, platelet count differential, calcium albumin, glucose phosphorus, magnesium, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, bun, creatinine, prothrombin time (PT), partial thromboplastin time (PTT), and d-dimer were performed on a weekly basis during therapy. Patients were restaged with CT or MRI after every two cycles of treatment. Eleven patients who were evaluable in that they received at least one dose of correctly prepared reagent were included for toxicity assessment.
Criteria for discontinuing or altering therapy. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events v4.0 (http://ctep.cancer.gov/forms/). Patients continued on therapy until evidence of progressive disease or unacceptable DLT which was defined as any acute allergic reaction ≥grade 3, or as any other adverse events ≥grade 3 that was felt to be a least possibly related to study drug (with the exception of fevers or chills that respond promptly to treatment) and that did not resolve to ≤grade 1 within 2 weeks of holding drug. In the initial version of the trial, treatment was held for any ≥grade 3 related therapy, with therapy discontinued if it did not improve to <grade 1 by the time the next dose was due. However, in a clinical trial of the combination of SGT-53 and docetaxel, a transient decrease in platelet count was observed in a few patients. Although likely due to docetaxel treatment, this was seen to improve while continuing therapy. Based upon this, the protocol was then amended so that transient decreases in platelet count and neutrophil count were not considered DLTs unless treatment was delayed for ˃2 weeks. Patients experiencing a platelet nadir <20,000 or an ANC <500 were counted as a DLT, requiring dose reduction. Patients were able to continue full dose of the drug for ANC ≥1,000 and a platelet count >75,000. Patients were able to have a transient reduction in DNA dose for an ANC ≥500 to 999 or platelet count between 50,000 and 75,000. The infusion was held for an ANC <500 or platelet count <50,000.
Dose-escalation process. Patients were enrolled in cohorts of three. Each patient in the cohort beyond the first was enrolled only after the previous patient had completed one cycle of therapy without experiencing unacceptable toxicity. An independent committee of experts comprised an outside data and safety monitoring board that evaluated the data from each cohort prior to starting the next cohort at a higher dose.
Statistics. The primary purpose of this phase 1 dose finding trial is to define a practically attainable and clinically tolerable dose. The target rate of dose-limiting adverse events was 33%. There were five dose levels considered, with one additional lower dose level in case of unexpected DLT in the first dose. Using the continuous reassessment method design, a maximum of 24 patients could be treated, with 3 patients per cohort.
Tumor analysis. Although the primary endpoint of this trial was to establish a maximal tolerated dose (MTD) of SGT-94, a secondary objective was to document evidence of tumor-specific RB94 expression within tumor tissue after systemic administration of SGT-94 and possible efficacy. Patients were consented for biopsy to be performed in cycle 1 most commonly the day after infusion 3 was administered. Clinical response was assessed according to standard criteria for the particular solid tumor. In general, Response Evaluation Criteria in Solid Tumors criteria was used where applicable (http://ctep.cancer.gov/guidelines/recist.html).
Quantification of sub-G1 produced by SGT-94 using flow cytometry. As the percentage of cells in a population at a late stage of apoptosis is reflected by the percentage of cells present as a sub-G1 peak on flow cytometry, this assay can be used to assess the level of apoptosis induced by RB94 plasmid DNA, and thus its potency to drive cell death. After each SGT-94 complex for infusion was prepared in the Cell Therapy laboratory, a small sample was obtained and tested. The percentage of cells in sub-G1 present 24 hours after transfection with SGT-94 at a plasmid DNA dose of 2 µg was assessed. HTB-9 (5,637) RB-negative bladder cancer cells were plated into six well-plates at 1 × 105 cells/well on the day before transfection. The cells were washed with serum-free minimum essential media (MEM) prior to addition of the complex. Four to six wells were transfected with complex. After 4–6 hours of transfection, an equal volume of MEM containing 20% fetal bovine serum (FBS) was added to the wells. Twenty-four hours after transfection, the cells were removed from the plate with trypsin, washed with phosphate-buffered saline (PBS), treated with a hypotonic propidium iodide solution, and subjected to flow cytometry. A typical result showing a marked sub-G1 population following SGT-94 treatment compared to controls indicative of the biological integrity of each mix is shown in Figure 1.
Evidence for tumor-specific delivery of SGT-94 using DNA PCR. The presence of RB94 in patients' tumor was assessed using DNA PCR primers specific for the RB94 gene construct as has been previously published.13 RB94 is not normally expressed in any normal tissue or tumor. Tumor tissue was obtained between 24 and 48 hours after SGT-94 treatment. When possible, adjacent normal tissue was obtained simultaneously. DNA for PCR was obtained using High Pure PCR Template Preparation Kit from Roche (Indianapolis, IN). Approximately 25 mg of solid tissue was frozen on dry ice, 200 µl lysis buffer and 40 µl Proteinase K were added, and immediately mixed using a pestle on dry ice. The mixture was then incubated at 50 °C for 1 hour, 200 µl binding buffer added, and incubated at 72 °C for 10 minutes. Subsequently, 100 µl of isopropanol was added, transferred to a high filter tube, and centrifuged for 1 minute at 8,000× g. It was then washed with inhibitor removal buffer once and with wash buffer twice. Finally, 200 μl of prewarmed (70 °C) elution buffer was added and was centrifuged for 1 minute at 8,000× g. A 0.2 µg aliquot was used for DNA PCR as previously described.13
Western blotting. Each patient's normal or cancer tissue at surgery was cut, suspended in 0.5 ml of lysis buffer (150 mmol/l NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/l Tris pH 8.0, and protease inhibitors), manually homogenized in a Dounce homogenizer, and left on ice for 30 minutes. The tissue lysate was then transferred into a fresh tube, sonicated for 3 minutes, and then centrifuged at 12,000 rpm for 20 minutes at 4 °C. It was then stored at −80 °C. Subsequently the tissue lysate samples were run on a 4–20% Tris-glysine gradient gel and transferred to 0.2 µm polyvinylidene difluoride (PVDF) membrane. RB94 protein was identified using an anti-RB monoclonal antibody (QED Biosciences, San Diego, CA), followed by a peroxidase-conjugated anti-mouse IgG antibody (Santa Cruz Biotechnology, Paso Robles, CA), chemiluminescence using the SuperSignal West Dura kit (Pierce Thermo, Rockford, IL) and exposure to X-ray film. Equal loading was confirmed by striping and probing with anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO).
Immunostaining. Formalin-fixed, paraffin-embedded tissues were stained for RB in the Pathology Department at the MD Anderson Cancer Center using Clinical Laboratory Improvement Amendments (CLIA)-approved methodology and reagents. The anti-RB antibody stains both wild-type RB110 and RB94 and therefore was only useful to detect RB94-specific staining in tumors that were negative for RB wild type. Only two patients with an RB-negative tumor were documented in this trial.
Acknowledgments
These studies were supported by National Cancer Institute Specialized Programs of Excellence grant P50 CA091846 (to A.S-R. and W.F.B.), the Genitourinary Cancers Program of the CCSG shared resources at the MD Anderson Cancer Center (P30CA016672) (to W.F.B. and A.S-R.), the Marcus Foundation (to W.F.B.) from the MD Anderson Cancer Center, the Andrea Hovsepian and Arsen Sohigian Fund for Urothelial Cancer Research (to A. S-R.), a Hi-CRIS Award (to A.S-R.) from the MD Anderson Cancer Center, and SynerGene Therapeutics. E.H.C. and K.F.P. are inventors of the delivery system described for which several patents owned by Georgetown University have been issued. The patents have been licensed to SynerGene Therapeutics for commercial development. E.H.C. owns equity interests in SynerGene. E.H.C. also serves as a nonpaid scientific consultant to SynerGene. The other authors declare no conflict of interest.
References
- Xu, HJ, Xu, K, Zhou, Y, Li, J, Benedict, WF and Hu, SX (1994). Enhanced tumor cell growth suppression by an N-terminal truncated retinoblastoma protein. Proc Natl Acad Sci USA 91: 9837–9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, HJ, Zhou, Y, Seigne, J, Perng, GS, Mixon, M, Zhang, C et al. (1996). Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res 56: 2245–2249. [PubMed] [Google Scholar]
- Zhang, X, Multani, AS, Zhou, JH, Shay, JW, McConkey, D, Dong, L et al. (2003). Adenoviral-mediated retinoblastoma 94 produces rapid telomere erosion, chromosomal crisis, and caspase-dependent apoptosis in bladder cancer and immortalized human urothelial cells but not in normal urothelial cells. Cancer Res 63: 760–765. [PubMed] [Google Scholar]
- Daniels, TR, Bernabeu, E, Rodríguez, JA, Patel, S, Kozman, M, Chiappetta, DA et al. (2012). The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta 1820: 291–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, TR, Delgado, T, Helguera, G and Penichet, ML (2006). The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol 121: 159–176. [DOI] [PubMed] [Google Scholar]
- Daniels, TR, Delgado, T, Rodriguez, JA, Helguera, G and Penichet, ML (2006). The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 121: 144–158. [DOI] [PubMed] [Google Scholar]
- Xu, L, Huang, CC, Huang, W, Tang, WH, Rait, A, Yin, YZ et al. (2002). Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther 1: 337–346. [PubMed] [Google Scholar]
- Xu, L, Pirollo, KF, Tang, WH, Rait, A and Chang, EH (1999). Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther 10: 2941–2952. [DOI] [PubMed] [Google Scholar]
- Xu, L, Frederik, P, Pirollo, KF, Tang, WH, Rait, A, Xiang, LM et al. (2002). Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther 13: 469–481. [DOI] [PubMed] [Google Scholar]
- Xu, L, Tang, WH, Huang, CC, Alexander, W, Xiang, LM, Pirollo, KF et al. (2001). Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med 7: 723–734. [PMC free article] [PubMed] [Google Scholar]
- Yu, W, Pirollo, KF, Rait, A, Yu, B, Xiang, LM, Huang, WQ et al. (2004). A sterically stabilized immunolipoplex for systemic administration of a therapeutic gene. Gene Ther 11: 1434–1440. [DOI] [PubMed] [Google Scholar]
- Senzer, N, Nemunaitis, J, Nemunaitis, D, Bedell, C, Edelman, G, Barve, M et al. (2013). Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther 21: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollo, KF, Rait, A, Zhou, Q, Zhang, XQ, Zhou, J, Kim, CS et al. (2008). Tumor-targeting nanocomplex delivery of novel tumor suppressor RB94 chemosensitizes bladder carcinoma cells in vitro and in vivo. Clin Cancer Res 14: 2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar, I, Ayhan, A, Bircan, K, Ergen, A and Taşar, C (1991). Transferrin receptor activity as a marker in transitional cell carcinoma of the bladder. Br J Urol 67: 165–168. [DOI] [PubMed] [Google Scholar]
- Rahman, SA, Yokoyama, M, Nishio, S and Takeuchi, M (1997). Flow cytometric evaluation of transferrin receptor in transitional cell carcinoma. Urol Res 25: 325–329. [DOI] [PubMed] [Google Scholar]
- Smith, NW, Strutton, GM, Walsh, MD, Wright, GR, Seymour, GJ, Lavin, MF et al. (1990). Transferrin receptor expression in primary superficial human bladder tumours identifies patients who develop recurrences. Br J Urol 65: 339–344. [DOI] [PubMed] [Google Scholar]
- Pirollo, KF, Zon, G, Rait, A, Zhou, Q, Yu, W, Hogrefe, R et al. (2006). Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther 17: 117–124. [DOI] [PubMed] [Google Scholar]
- Pirollo, KF, Rait, A, Zhou, Q, Hwang, SH, Dagata, JA, Zon, G et al. (2007). Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res 67: 2938–2943. [DOI] [PubMed] [Google Scholar]
- Hwang, SH, Rait, A, Pirollo, KF, Zhou, Q, Yenugonda, VM, Chinigo, GM et al. (2008). Tumor-targeting nanodelivery enhances the anticancer activity of a novel quinazolinone analogue. Mol Cancer Ther 7: 559–568. [DOI] [PubMed] [Google Scholar]
- Pirollo, KF, Dagata, J, Wang, P, Freedman, M, Vladar, A, Fricke, S et al. (2006). A tumor-targeted nanodelivery system to improve early MRI detection of cancer. Mol Imaging 5: 41–52. [PubMed] [Google Scholar]
- Freedman, M, Chang, EH, Zhou, Q and Pirollo, KF (2009). Nanodelivery of MRI contrast agent enhances sensitivity of detection of lung cancer metastases. Acad Radiol 16: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C, Rait, A, Pirollo, KF, Dagata, JA, Farkas, N and Chang, EH (2008). Nanoimmunoliposome delivery of superparamagnetic iron oxide markedly enhances targeting and uptake in human cancer cells in vitro and in vivo. Nanomedicine 4: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, SS, Rait, A, Kim, E, Pirollo, KF, Nishida, M, Farkas, N et al. (2014). A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 8: 5494–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]