Abstract
Adoptive T-cell transfer is a promising treatment approach for metastatic cancer, but efficacy in solid tumors has only been achieved with toxic pre- and postconditioning regimens. Thus, adoptive T-cell therapies would benefit from complementary modalities that enable their full potential without excessive toxicity. We aimed to improve the efficacy and safety of adoptive T-cell transfer by using adenoviral vectors for direct delivery of immunomodulatory murine cytokines into B16.OVA melanoma tumors with concomitant T-cell receptor transgenic OT-I T-cell transfer. Armed adenoviruses expressed high local and low systemic levels of cytokine when injected into B16.OVA tumors, suggesting safety of virus-mediated cytokine delivery. Antitumor efficacy was significantly enhanced with adenoviruses coding for murine interleukin-2 (mIL-2) and tumor necrosis factor-α (mTNFα) when compared with T-cell transfer alone or viruses alone. Further improvement in efficacy was achieved with a triple combination of mIL-2, mTNFα, and OT-I T-cells. Mechanistic studies suggest that mIL-2 has an important role in activating T-cells at the tumor, while mTNFα induces chemokine expression. Furthermore, adenovirus treatments enhanced tumor-infiltration of OT-I T-cells as demonstrated by SPECT/CT imaging of 111In-labeled cells. Our results suggest the utility of cytokine-coding adenoviruses for improving the efficacy of adoptive T-cell therapies.
Introduction
Cancer immunotherapy with the adoptive transfer of tumor-infiltrating lymphocytes (TIL), T-cell receptor (TCR) or chimeric antigen receptor engineered T-cells has gained momentum in recent years.1 Durable responses have been observed with CD19-targeted chimeric antigen receptor in B-cell malignancies,2 autologous TIL in metastatic melanoma3 as well as TCR targeted against melanoma antigens.4,5 Yet, the efficacy of chimeric antigen receptor against solid tumors remains unsatisfactory6,7 and toxicities are associated with systemic high-dose chemotherapy preconditioning and interleukin-2 (IL-2) postconditioning frequently used in TIL/TCR regimens. Importantly, the infused cells face an immunosuppressive tumor microenvironment that impedes their effector functions.8 This issue must be resolved for successful and safe adoptive cell therapy (ACT) of solid tumors.
Immunomodulatory cytokines can induce antitumor immune responses when used as single agents9 or encoded by adenoviral vectors.10,11,12,13,14 The latter approach has the advantage of high local versus low systemic concentrations, with obvious relevance to both safety and efficacy, keeping in mind that it is normal organs that are responsible for adverse events while efficacy occurs at the tumor. Apart from the well-established concomitant use of IL-2 with TIL transfer, only few other cytokines have been studied in combination with adoptive T-cell transfer. In a recent phase 1/2 clinical trial, intralesional injections of adenovirus expressing interferon gamma (IFNg) combined with TIL infusion demonstrated the feasibility of the combination approach.15 Preclinically, immunomodulatory cytokines (not vectored) have been used to enable effective TCR transfer in murine melanoma.10,11,12,16
In this study, we constructed nonreplicating cytokine-coding adenoviruses and tested their ability to enhance adoptive T-cell transfer for melanoma. We hypothesized that the local production of immunostimulatory cytokines from adenoviral vectors could promote the function of the adoptively transferred T-cells for improved therapeutic outcome. mTNFα and mIL-2 emerged as effective cytokines when coupled with OT-I TCR transgenic T-cells for the treatment of murine B16.OVA melanoma, with indications that the dual cytokine combination countered tumor immunosuppression in the context of T-cell transfer. Our results support the further development of cytokine-armed adenoviruses as enhancers of adoptive T-cell therapies for solid tumors. Specifically, these results lay the groundwork for a human clinical trial which is in development by TILT Biotherapeutics.
Results
Armed adenoviruses express biologically active murine cytokines in vitro
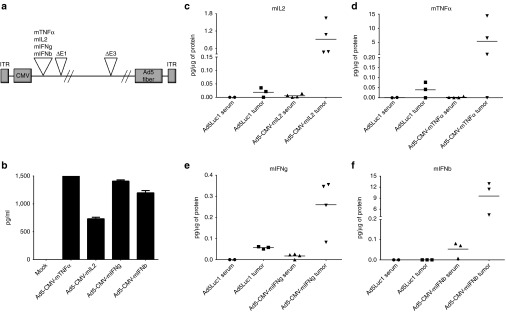
Transgenes were placed under the human cytomegalovirus promoter for constitutive expression of murine cytokines (Figure 1a). To confirm expression, supernatants from virus-infected cell cultures were analyzed for cytokines by enzyme-linked immunosorbent assay (ELISA) after a 48-hour incubation period (Figure 1b). Concentrations of in vitro-produced cytokines varied from 750 pg/ml (mIL-2) to 1,500 pg/ml (mTNFα). Functionality of the cytokines was confirmed with bioassays (Supplementary Figure S1). In conclusion, all viruses produced biologically active murine cytokines in vitro.
Figure 1.
Cytokine expression from adenoviral vectors in vitro and in vivo. (a) Schematic of adenovirus construct containing murine cytokine genes under the cytomegalovirus (CMV) promoter. (b) Cytokine concentrations in cell culture supernatants 48 hours after infection. Tumor and serum levels of (c) mIL2, (d) mTNFα, (e) mIFNg, and (f) mIFNb were measured from B16.OVA tumor-bearing mice 72 hours after intratumoral injection of cytokine-coding viruses or unarmed control virus Ad5Luc1. Horizontal lines, mean values.
Intratumoral injection of virus results in high local and low systemic levels of cytokine
For the assessment of in vivo transgene expression, B16.OVA tumors were injected intratumorally with cytokine-armed adenoviruses (1 × 109 VP/tumor). Tumor and serum were analyzed 72 hours later for cytokine content (Figure 1c–f). For all viruses, local expression of transgene was observed. From a safety perspective, it is noteworthy that very low levels of cytokines were detected in the serum.
Cytokine-armed adenoviruses combined with adoptive T-cell transfer inhibit the growth of B16.OVA tumors
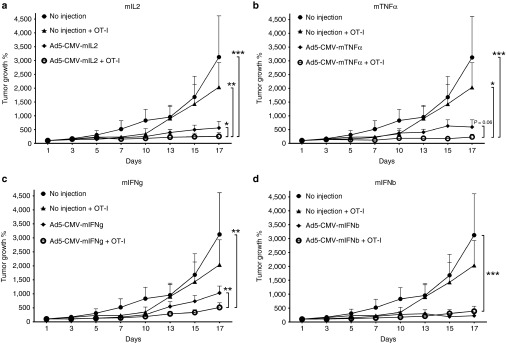
Antitumor efficacy of adenovirus combined with OT-I cells was studied in C57BL/6 mice with established B16.OVA tumors (Figure 2). The animals received a single administration of 1.5 × 106 CD8+ enriched OT-I T-cells and weekly injections of adenoviruses (1 × 109 VP/injection). OT-I T-cell treatment only moderately suppressed tumor growth16 but when combined with Ad5-CMV-mIL2, significantly improved tumor growth control was observed when compared with both single agents and untreated control (combination versus Ad5-CMV-mIL2, P < 0.05; combination versus OT-I, P < 0.01; combination versus mock, P < 0.001) (Figure 2a). The combination of Ad5-CMV-mTNFα and OT-I T-cells inhibited tumor growth significantly over OT-I and mock alone (combination versus OT-I, P < 0.05; combination versus mock, P < 0.001), and a trend for improved efficacy was observed over virus alone (combination versus Ad5-CMV-mTNFα, P = 0.06) (Figure 2b). Ad5-CMV-mIFNg combined with OT-I transfer resulted in tumor growth suppression that was significant compared with the virus alone and mock (combination versus Ad5-CMV-mIFNg, P < 0.01; combination versus mock, P < 0.01), but not over OT-I alone (Figure 2c). For Ad5-CMV-IFNb, the addition of OT-I T-cells did not improve antitumor efficacy (combination versus Ad5-CMV-mIFNb, P = ns) but was superior to mock (combination versus mock, P < 0.001) (Figure 2d).
Figure 2.
Antitumor efficacy of cytokine-armed adenoviruses combined with adoptive T-cell transfer. B16-OVA tumor-bearing C57BL/6 mice were administered 1.5 × 106 CD8-enriched OT-I T-cells intraperitoneally on Day 1 with concurrent intratumoral injections of 1 × 109 viral particles of adenoviruses armed with (a) mIL2, (b) mTNFα, (c) mIFNg, and (d) mIFNb. Virus treatments continued every 7 days. Error bars, mean + SEM, n = 5–6. *P < 0.05, **P < 0.01, ***P < 0.001.
Double combination of Ad5-CMV-mTNFα and Ad5-CMV-mIL2 with T-cell transfer is superior to monotherapies against B16.OVA
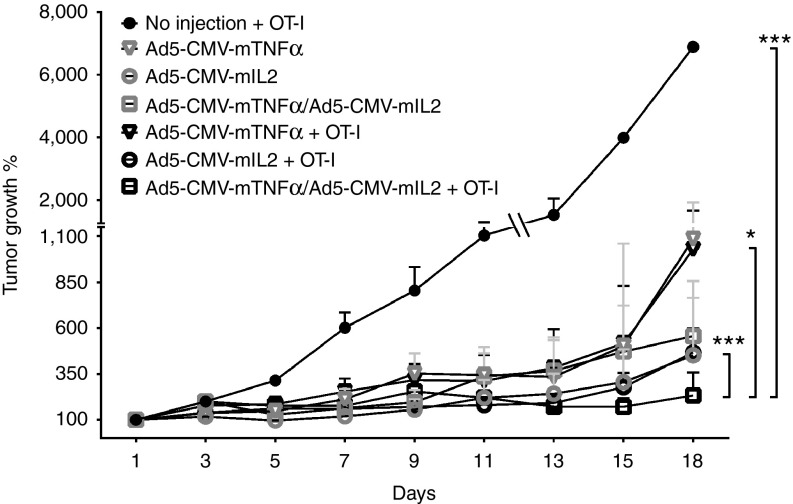
From initial experiments performed with single cytokine-armed adenoviruses (Figure 2), two cytokines emerged as the most promising candidates for further studies (mTNFα and mIL-2). The viruses coding for these cytokines were subsequently combined in a 1 to 1 ratio together with OT-I T-cell transfer for the assessment of antitumor efficacy. Interestingly, the double combination of Ad5-CMV-mTNFα/Ad5-CMV-mIL2 (0.5 × 109 VP each) with OT-I (1.5 × 106 cells) was superior to the mTNFa-armed virus combined with OT-I (Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I versus Ad5-CMV-mTNFα + OT-I, P < 0.05), the mIL2-armed virus combined with OT-I (Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I versus Ad5-CMV-mIL2 + OT-I, P < 0.001) and OT-I treatment alone (Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I versus OT-I, P < 0.001) (Figure 3).
Figure 3.
Ad5-CMV-mTNFα/Ad5-CMV-mIL2 dual virus combination together with adoptive T-cell transfer. Adenoviruses coding for mTNFα and mIL2 were combined in a 1 to 1 ratio (0.5 × 109 VP of each virus) to treat B16-OVA tumors together with adoptive transfer of 1.5 × 106 CD8-enriched OT-I T-cells. Virus treatments continued every 7 days. Error bars, mean + SEM, n = 8. *P < 0.05, ***P < 0.001.
Lymphocyte infiltrates in B16.OVA tumors following treatment with armed adenoviruses and OT-I transfer
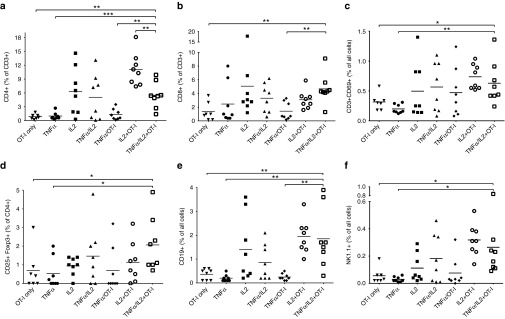
Next, we examined endpoint B16.OVA tumors for clues on the mechanism of action behind the enhanced antitumor efficacy (Figure 4). First, the percentage of CD4+ and CD8+ T-cells in tumors were analyzed by flow cytometry. The highest percentage (11%) of CD4+ T-cells was observed in tumors treated with Ad5-CMV-mIL2 + OT-I (Figure 4a). The treatment groups that received Ad5-CMV-mIL2 only, Ad5-CMV-mTNFα/Ad5-CMV-mIL2 only and Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I comprised 5–6% tumor-infiltrating CD4+ T-cells. The lowest CD4 levels (1% of all cells) were observed in the OT-I only, Ad5-CMV-mTNFα only and Ad5-CMV-mTNFα + OT-I groups. The highest percentage of CD8+ T-cells (5% of all cells) were observed in the Ad5-CMV-mIL2 and Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I groups (Figure 4b). The activation status of tumor-infiltrating T-cells after treatment was determined by CD69 staining of CD3-positive TIL (Figure 4c). Interestingly, the double cytokine combination (plus OT-I cells) resulted in a higher proportion of activated T-cells than seen in OT-I control group (Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I versus OT-I, P < 0.05). Regulatory T-cells (Treg) were moderately increased in tumors treated with the triple combination of Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I when compared with other treatment groups (Figure 4d). Besides T-cells, B-cell infiltration was increased in tumors treated with mIL2-coding viruses (Figure 4e). Increase in tumor-infiltrating natural killer cells (NK) was observed in the Ad5-CMV-mIL2 + OT-I and Ad5-CMV-mTNFα/Ad5-CMV-mIL2 + OT-I treatment groups (Figure 4f). Finally, low levels of immunosuppressive M2 macrophages were observed in tumors treated with combinations of cytokine-armed viruses and T-cells when compared with mTNFα and mIL2-coding viruses (Supplementary Figure S2a), and the number of mature dendritic cells (DC), as determined by cell-surface CD86 expression, was increased in the Ad5-CMV-mIL2 + OT-I-treated tumors (Supplementary Figure S2b).
Figure 4.
Tumor-infiltrating lymphocyte subsets following treatment with adenovirus and OT-I combination. Percentages of (a) CD4+ T-cells, (b) CD8+ T-cells, (c) CD3+CD69+ activated T-cells, (d) CD3+CD25+Foxp3+ regulatory T-cells, (e) CD19+ B-cells and (f) NK1.1+ Natural killer cells in B16.OVA tumors treated with 1.5 × 106 CD8-enriched OT-I T-cells and adenoviruses. Horizontal lines, mean values. *P < 0.05, **P < 0.01, ***P < 0.001.
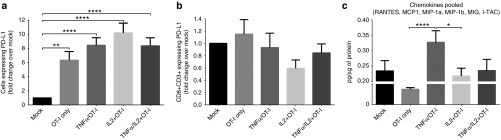
PD1/PD-L1 pathway in B16.OVA tumors is affected by adenovirus treatment
In further mechanistic studies, we examined the immune checkpoint pathway involving Programmed cell death 1 (PD1) and its ligand PD-L1 in B16.OVA. OT-I transfer alone resulted in significant upregulation of PD-L1 expression as compared with untreated mock tumors and this effect was further enhanced by the addition of cytokine-coding adenovirus injections (Figure 5a). Interestingly, Ad5-CMV-mIL2 + OT-I treatment resulted in a trend for PD1 downregulation on tumor-infiltrating CD8+ T-cells (Figure 5b).
Figure 5.
PD1/PD-L1 axis and chemokine expression in B16.OVA tumors. Tumor-bearing mice were killed on Day 7 after treatment with adenoviruses and OT-I T-cells, and the expression of (a) PD-L1 in the tumor and (b) PD1 on CD3+CD8+ TIL were analyzed by flow cytometry. Results are presented as fold change compared to mock treatment. (c) Homogenized tumor lysates were analyzed for the chemokines RANTES, MCP1, MIP-1a, MIP-1b, MIG, and I-TAC. Pooled values of chemokines were normalized to total protein content and presented as picogram per microgram of protein. Error bars, mean + SEM. *P < 0.05, **P < 0.01, ****P < 0.0001.
Virus-delivered mTNFα induces chemokine expression in tumors
The expression of the chemokines RANTES, MCP1, MIP-1a, MIP-1b, MIG, and I-TAC was studied in homogenized B16.OVA tumor lysates (Figure 5c). When individual chemokine results were pooled, the combination treatment with Ad5-CMV-mTNFα + OT-I showed significant upregulation of these molecules when compared with OT-I alone (P < 0.0001) and Ad5-CMV-mIL2 + OT-I (P < 0.05).
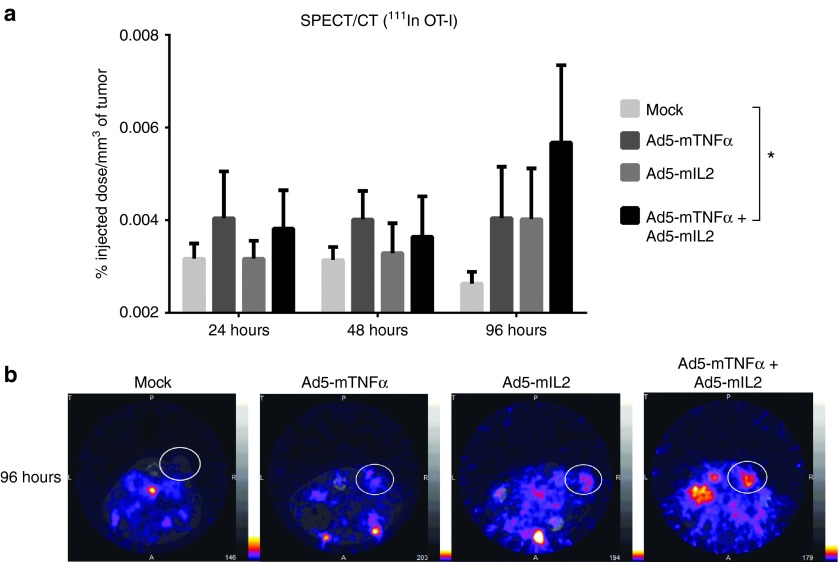
Adenovirus treatment increases tumor-infiltration of radiolabeled T-cells in SPECT/CT imaging
The increased chemokine expression observed in virus-treated tumors prompted us to study the migration dynamics of adoptively transferred OT-I T-cells further. To this end, 111In-labeled OT-I T-cells were administered to B16.OVA tumor-bearing mice with simultaneous adenovirus injections (Figure 6). Intriguingly, SPECT/CT imaging of the mice revealed a significant accumulation of radiolabeled T-cells in the tumors of Ad5-CMV-mTNFα/Ad5-CMV-mIL2-treated mice compared with mock-treated mice (P = 0.03) 96 hours after treatment start (Figure 6a). The viruses coding for either cytokine alone were also able to induce OT-I cell migration to tumors, but the differences were not statistically significant. Representative SPECT/CT images of 96 hour imaging data are shown in Figure 6b.
Figure 6.
SPECT/CT imaging of radiolabeled OT-I T-cells adoptively transferred to adenovirus-treated mice. B16.OVA tumor-bearing mice were administered 6 × 106 111Indium-oxine labeled OT-I T-cells intraperitoneally with simultaneous intratumoral injection of cytokine-coding adenoviruses. Mice were imaged on 24, 48, and 96 hours after treatment with a four-headed gamma camera with integrated CT system (nanoSPECT/CT). The results were calculated as percentage of the injected dose per tumor volume (%ID/mm3) (a). Representative SPECT/CT images of 96 hours timepoint with white circles indicating the locations of subcutaneous tumors (b). Error bars, mean + SEM. *P < 0.05, repeated measures analysis of variance.
Discussion
Adoptive T-cell transfer is a promising treatment modality for advanced cancer. However, given its low efficacy in solid tumors, especially if toxic pre-and postconditioning is not used, complimentary approaches that enable the full potential of the approach are needed. To this end, we constructed murine cytokine-armed adenoviruses that were used together with OT-I T-cell transfer to treat B16 melanoma.
Constitutive expression of murine cytokines from human serotype 5-based nonreplicating adenoviruses were confirmed in vitro and in vivo. TNFα was initially identified as one of the most potent molecules for destroying tumors.17 Subsequently, it has also been discovered that it can mediate important antitumor immunological effects.14,18 However, clinical trials with systemic recombinant TNFα have resulted in significant side effects, hepatotoxicity and hypotension being among the most common, while tumor responses have been rare.19 Thus, systemic delivery of TNFα results in significant off-target toxicities, which limit the concentration that can be achieved at the tumor, resulting in low efficacy. Therefore, recombinant TNFα is currently only used in isolated limb perfusion which physically restricts systemic exposure.20
A more sophisticated approach for improving local to systemic TNFα levels is vectored delivery. This can be effectively achieved with armed adenoviruses as we show with Ad5-CMV-mTNFα; cytokine production was restricted to the intratumorally injected tumors while serum levels of mTNFα were negligible. Since the prototype adenoviral vectors (coding for murine transgenes) used in this study have no specificity for tumor cells, expression of cytokines from non-tumor cells is conceivable. However, in the case of replication-competent oncolytic adenoviruses, which express human transgenes, and which selectively replicate in tumor cells defective in the p16/Rb pathway,21 cytokine expression has been shown to be tumor-restricted with no systemic exposure.22
Delivery from an oncolytic platform is an optimal approach for achieving high acute levels of TNFα in the tumor. Not only is transgene expression linked to virus replication, which occurs only in tumors, but the transgene production platform, the virus genome, is amplified locally as long as viable tumor cells remain, and then shut off. Sustained long-term expression of TNFα is associated with carcinogenesis23 while short term and dynamic expression can induce immune responses.18,24 Unfortunately, species-incompatibility issues prevent the use of replication-competent human adenoviruses in mice25 thus necessitating the development of nonreplicating and nonselective prototype vectors for preclinical studies such as performed in our paper.
IL-2 administration has been extensively studied as single agent therapy and in combination with adoptive TIL transfer in the treatment of melanoma.26 Systemically administered high-dose IL-2 together with preconditioning lymphodepletion and TIL infusion can lead to high response rates, but is accompanied by toxicity attributed to IL-2-induced vascular leakage syndrome.27 In contrast to toxicity, which occurs systemically at off-target organs, the useful effects if IL-2 on the T-cell graft occur locally at and near the tumor. Therefore, minimizing systemic exposure through local administration of IL-2 would seem preferable. As with Ad5-CMV-mTNFα, our data on intratumorally injected Ad5-CMV-mIL2 indicates that high local levels of cytokine can be achieved with minimal leakage to the circulation. With regard to clinical translation, the construction of replication-competent adenoviruses encoding human IL-2 (or other human cytokines) could yield a comparable safety profile due to tumor-restricted cytokine expression although this remains to be proven.
Antitumor effects of T-cell transfer combined with local virus injections were evaluated in the mouse B16.OVA melanoma model. We show that antitumor efficacy against highly aggressive and immunosuppressive melanoma—similar to many human melanomas—can be achieved with the combination OT-I T-cells and weekly injections of Ad5-CMV-mIL2 or Ad5-CMV-mTNFα without apparent toxicity. Previously, adenoviral vectors encoding IL-2 have shown efficacy in preclinical mouse models of breast cancer,28 mastocytoma,29 fibrosarcoma,30 and thyroid carcinoma.31 A phase 1 clinical trial with adenovirus-mediated IL-2 in metastatic breast cancer and melanoma did not induce clinical responses but the treatment was well tolerated.32 More recently, clinical activity was observed in 17% of patients treated intralesionally with IL-2-coding virus (TG1024), and the treatments were safe.33 Significant tumor regressions have also been observed with TNFα-coding adenoviruses preclinically14,34 and in patients with advanced solid tumors.35 Of note, recombinant TNFα and IL-2 seem attractive for enhancing adoptive cell therapy.36 Together with these previous reports, our data supports the notion that IL-2 and TNFα coding adenoviruses have translational potential in the context of enabling safe and effective T-cell therapy of humans with currently incurable solid tumors.
To further improve antitumor efficacy, we combined the two most promising cytokines from initial experiments, i.e., mIL-2 and mTNFα, with concurrent OT-I T-cell transfer. With Ad5-CMV-mIL2 and Ad5-CMV-mTNFα mixed together, the growth of B16.OVA tumors was significantly reduced when compared with single agent therapies. Moreover, no treatment related deaths were observed in the dual cytokine groups, indicating that the treatment was well-tolerated. Our data is in line with earlier studies which revealed a synergistic relationship between recombinant IL-2 and TNFα against various murine tumors.37,38 With regard to mechanism of action, our data suggests that TNFα has a role in recruiting T-cells (through induction of chemokine expression) that is further enhanced by the addition of IL-2 as shown in the SPECT/CT imaging experiment. Previous research supports our observations, as tumor-targeted TNFα has been shown to enhance lymphocyte infiltration and adoptive T-cell therapy.39,40 TNFα may also be important in counteracting immunosuppression, including M2 macrophages, when used in combination with IL-2 and T-cell therapy. It could also be of relevance that TNFα has been suggested to suppress regulatory T-cell function.41 In humans, however, no antitumor effects have been seen with systemically administered cytokines nor has the combination treatment been well-tolerated.42 The discrepancy between human and mouse data underscores the difficulty of translating rodent results directly into humans, one cause of which is the higher ability of the former to sustain doses which would be lethal in the latter. Moreover, these data emphasize the utility of local cytokine production, because it allows effective local concentrations to be obtained with limited systemic exposure. Clearly, only human data can ultimately determine the feasibility of combining IL-2/TNFα-coding adenoviruses with adoptive T-cell transfer.
The dual role of IL-2 as a promoter and inhibitor of antitumor immune responses is well-established.43 Concordantly, our previous research with recombinant murine IL-2 and OT-I in the B16.OVA model has revealed that simultaneous antitumor (i.e., dendritic cell activation) and immunosuppressive (i.e., Tregs) pathways appear to be induced with IL-2 (ref. 36). In line with these observations are reports where IL-2-transduced B16 tumors exhibited improved tumor growth control and increased NK cells and effector T-cells despite increases in infiltrating regulatory T-cells.44 In the experiments described here, B16.OVA tumors treated with Ad5-CMV-mIL2 and OT-I showed an increase in mature dendritic cells (CD86+CD11c+ cells) in the tumor, indicative of enhanced antigen presentation. On the other hand, higher percentages of Tregs (Foxp3+CD25+CD4+ cells) were observed in the mIL2/mTNFα combination groups when compared with single cytokines. This phenomenon may reflect a basic tenet in immunology: whenever there is an immune response, there is a reactionary immunosuppressive response. The stronger the immune response, the stronger the counter-response. Importantly, and in accord with the aforementioned tenet, increases in Tregs did not correlate with antitumor response, as the double cytokine combination resulted in superior efficacy.
The number or proportion of lymphocyte subsets may not tell the full story of immunological activity, since a smaller number of cells could result in more anti-tumor response if the cells are highly active.16 Therefore, it was promising that not only were key lymphocyte subtypes more frequent in tumors treated with IL-2, TNFα, and OT-I, but T-cells were also more active in the triple-treated tumors. In addition to T-cell infiltration we observed more CD19+ B-cells in B16.OVA tumors treated with IL-2-coding viruses. Interestingly, B-cell depletion in the B16 melanoma model has been shown to hamper CD4/CD8-mediated antitumor immune responses and result in enhanced tumor growth.45 Thus, IL-2 could have an important indirect role in augmenting antitumor immunity in the model described here.
Expression of PD-L1 in tumors was upregulated following combination treatment. This phenomenon could be explained by increased infiltration of T-cells (as shown in SPECT/CT imaging) secreting IFNg, a known inducer of PD-L1 (ref. 46). By contrast, CD8+ TIL expressing anergy marker PD1 were downregulated in IL-2-treated tumors, suggesting that tumor-induced immunosuppression could be countered with this approach. Of note, downregulation of PD1 on CD8+ TIL was observed in the same mouse model with recombinant IL-2 combined with OT-I transfer.36 Moreover, a recent phase 1 study with repeated intratumoral injections with oncolytic adenovirus ONCOS-102 resulted in CD8+ T-cell infiltration and upregulation of PD-L1 in solid tumors.47
Future studies could focus on replication-competent adenoviruses encoding human cytokines, used together with adoptive cell transfer. With regard to in vivo testing, we and others have utilized the Syrian hamster as a useful platform for studying human adenovirus vectors.48,49,50,51 Tumor-infiltrating lymphocytes from syngeneic hamster tumors were recently characterized and cultured ex vivo in our group52 allowing the study of human cytokine-coding oncolytic adenoviruses in the context of adoptive cell transfer and in a fully immunocompetent model. In conclusion, data presented here support the clinical translation of cytokine-armed adenoviruses combined with adoptive T-cell transfer for the treatment of solid tumors.
Materials and Methods
Cell lines and viruses. Mouse B16.OVA melanoma cell line was kindly provided by Professor Richard Vile (Mayo Clinic, Rochester, MN). L-929, CTLL-2 and 293 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). B16-Blue IFN-α/β cells were purchased from InvivoGen (San Diego, CA). All cell lines were cultured in recommended conditions. The non-replicating adenoviruses Ad5-CMV-mIFNg, Ad5-CMV-mIL2, Ad5-CMV-mIFNb and Ad5-CMV-mTNFα were constructed by inserting expression cassettes with murine cytokines interferon gamma (mIFNg), interleukin-2 (mIL2), interferon beta 1b (mIFNb) and tumor necrosis factor-α (mTNFα) into the multiple cloning site of the shuttle plasmid pDC315 (AdMax, Microbix Biosystems, Mississauga, Canada). Shuttle plasmids were recombined with pBHGloxdelE13cre (AdMax), which carries the whole adenovirus genome, and resulting rescue plasmids were transfected to 293 cells to generate the final virus constructs. Ad5Luc1 has been described previously.53
Cytokine functionality. 293 cells were infected with 100 viral particles/cell (VP/cell) with cytokine-coding viruses and Ad5luc1 (negative control) (8 replicate wells per virus). Supernatants were collected 48 hours later and filtered through 0.02 μm inorganic membrane filters (Whatman, Maidstone, UK). The bioactivity of mIFNb present in the supernatant was confirmed with B16-Blue IFN-α/β murine type I IFNs sensor cells following manufactureŕs instructions. TNFα-sensitive mouse L-929 cells were used to confirm the functionality of mTNFα by coculturing the supernatant with L-929 cells seeded on a 96-well plate (1 × 104 cells/well) for 24 hours before determining cell viability using the MTS assay (Promega, Madison, WI). The IL-2-dependent cell line CTLL-2 was used to examine the bioactivity of mIL-2 by culturing the cells with the filtered supernatant on a 96-well plate (2.5 × 104 cells/well) for 48 hours and determining cell viability. Functionality of mIFNg was confirmed by studying the ability of mIFNg to protect L-929 cells from vesicular stomatitis virus (VSV) infection. L-929 cells were seeded on a 96-well plate (3.5 × 104 cells/well) and supernatants were incubated with the cells for 6 hours. After incubation, supernatants were removed and the cells were infected with 1 × 104 VP/well of VSV strain M51 (kindly provided by Dr. Markus Vähä-Koskela, University of Helsinki). Cell viability was determined 96 hours later. Commercial cytokine products were used as positive controls in all functionality assays (R&D Systems, Minneapolis, MN). All functionality assays were performed in eight replicates.
Transgene expression. Cytokine production in vitro was measured from cell culture supernatants of virus-infected 293 cells (three replicate wells per virus) with ELISA kits: mIFNg (Abcam, Cambridge, UK), mIFNb (PBL InterferonSource, Piscataway, NJ), mIL-2 and mTNFα (IBL International, Hamburg, Germany). In vivo expression of cytokines was studied in C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) implanted subcutaneously with B16.OVA tumors and injected once with 1 × 109 VP intratumorally (n = 2–4). 72 hours later, the tumors were snap-frozen on dry ice and stored at −80 °C. Cytokine measurements were performed with CBA Flex Sets (BD, Franklin Lakes, NJ) as previously described36 and normalized to total protein content.
Virus treatments. 0.25 × 106 B16.OVA cells were injected subcutaneously into C57BL/6 mice (Harlan Laboratories) and tumors were allowed to develop until reaching a size feasible for injection. The mice were injected intratumorally with 1 × 109 VP/tumor (or 0.5 × 109 VP of each virus in TNFa/IL2 combination treatments) in 50 µl of PBS on the same day as CD8+ enriched OT-I T-cells were administered (described below). Virus treatments continued every 7 days and tumors were measured with digital calipers every 2–3 days. n = 5–6 animals per group.
Adoptive T-cell transfer. Splenocytes from OT-I TCR transgenic mice (The Jackson Laboratory, Bar Harbor, ME) were cultured in the presence of 160 ng/ml mIL-2 (R&D Systems) and 300 ng/ml anti-mouse CD3e (clone 145-2C11, eBioscience, San Diego, CA) for 3 days before enrichment of CD8a+ cells was perfomed with CD8a+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufactureŕs instructions. Enriched T-cells were cultured further in the presence of mIL-2 and anti-CD3e for 7 days before adoptive transfer into B16.OVA tumor-bearing mice. The mice received 1.5 × 106 CD8+ enriched OT-I T-cells intraperitoneally in 100 µl of plain RPMI. All animal protocols were reviewed and approved by the Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland.
Flow cytometry. Flow cytometric analyses were performed according to procedures established earlier in our group.36 Briefly, excised tumors were pushed through 70 µm cell strainers and cultured overnight in complete RPMI 1640 medium before storing the single-cell suspension at −80 °C for later analysis.
Chemokine expression. Established B16.OVA tumors were treated once with adenoviruses and OT-I cells. 7 days later the tumors were collected, snap-frozen and stored in −80 °C. Tumor homogenization and chemokine analysis was performed with BD CBA Flex Sets as previously described.36
OT-I radiolabeling and SPECT/CT imaging. 111In-oxine radiolabeling and SPECT/CT imaging was performed as previously described.16 Briefly, 6 × 106 CD8+ enriched OT-I T-cells were labeled with 111In-oxine for 15 minutes and injected intraperitoneally into recipient mice (5.98 ± 0.53 MBq per animal). On days 1, 2, and 4 after administration of radiolabeled cells, virus-treated (n = 3 per virus group) and mock-treated (n = 3) mice were imaged with a preclinical four-headed gamma camera with integrated CT system (nanoSPECT/CT, Bioscan). The results were calculated as percentage of activity in tumor from the injected dose divided by the tumor volume (mm3).
Statistical analyses. Statistics were performed with GraphPad Prism 6 (GraphPad Software, San Diego, CA) and SPSS version 21 (SPSS IBM, New York, NY). Student́s t-test, repeated measures analysis of variance and linear mixed-effects model on log-transformed relative tumor volume data were used; P values of <0.05 were considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Cytokine functionality. Figure S2. M2 macrophages and mature dendritic cells in B16.OVA tumors following combination treatment.
Acknowledgments
A.H. is shareholder in Targovax ASA. A.H. is employee and shareholder in TILT Biotherapeutics Ltd. M.S. and S.P. are employees of TILT Biotherapeutics Ltd. Other coauthors declare no potential conflict of interest. The authors wish to thank Susanna Grönberg-Vähä-Koskela, Marjo Vaha and Minna Oksanen for expert assistance.
Supplementary Material
References
- June, CH, Riddell, SR and Schumacher, TN (2015). Adoptive cellular therapy: a race to the finish line. Sci Transl Med 7: 280ps7. [DOI] [PubMed] [Google Scholar]
- Maude, SL, Frey, N, Shaw, PA, Aplenc, R, Barrett, DM, Bunin, NJ et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, SA, Yang, JC, Sherry, RM, Kammula, US, Hughes, MS, Phan, GQ et al. (2011). Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, LA, Morgan, RA, Dudley, ME, Cassard, L, Yang, JC, Hughes, MS et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, PF, Morgan, RA, Feldman, SA, Yang, JC, Sherry, RM, Dudley, ME et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, CH, Sleijfer, S, van Steenbergen, S, van Elzakker, P, van Krimpen, B, Groot, C et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, N, Brawley, VS, Hegde, M, Robertson, C, Ghazi, A, Gerken, C et al. (2015). Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 33: 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos, M and June, CH (2013). Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 39: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchelli, E, Eggermont, A, Fridman, WH, Galon, J, Zitvogel, L, Kroemer, G et al. (2013). Trial Watch: Immunostimulatory cytokines. Oncoimmunology 2: e24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo, V, Pesonen, S, Diaconu, I, Escutenaire, S, Arstila, PT, Ugolini, M et al. (2010). Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 70: 4297–4309. [DOI] [PubMed] [Google Scholar]
- Diaconu, I, Cerullo, V, Hirvinen, ML, Escutenaire, S, Ugolini, M, Pesonen, SK et al. (2012). Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 72: 2327–2338. [DOI] [PubMed] [Google Scholar]
- Kanerva, A, Nokisalmi, P, Diaconu, I, Koski, A, Cerullo, V, Liikanen, I et al. (2013). Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 19: 2734–2744. [DOI] [PubMed] [Google Scholar]
- Hemminki, O, Parviainen, S, Juhila, J, Turkki, R, Linder, N, Lundin, J et al. (2015). Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 6: 4467–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvinen, M, Rajecki, M, Kapanen, M, Parviainen, S, Rouvinen-Lagerström, N, Diaconu, I et al. (2015). Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum Gene Ther 26: 134–144. [DOI] [PubMed] [Google Scholar]
- Khammari, A, Nguyen, JM, Saint-Jean, M, Knol, AC, Pandolfino, MC, Quereux, G et al. (2015). Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients. Cancer Immunol Immunother 64: 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tähtinen, S, Grönberg-Vähä-Koskela, S, Lumen, D, Merisalo-Soikkeli, M, Siurala, M, Airaksinen, AJ et al. (2015). Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol Res 3: 915–925. [DOI] [PubMed] [Google Scholar]
- van Horssen, R, Ten Hagen, TL and Eggermont, AM (2006). TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 11: 397–408. [DOI] [PubMed] [Google Scholar]
- Balkwill, F (2009). Tumour necrosis factor and cancer. Nat Rev Cancer 9: 361–371. [DOI] [PubMed] [Google Scholar]
- Roberts, NJ, Zhou, S, Diaz, LA Jr and Holdhoff, M (2011). Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget 2: 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont, AM, de Wilt, JH and ten Hagen, TL (2003). Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 4: 429–437. [DOI] [PubMed] [Google Scholar]
- Fueyo, J, Gomez-Manzano, C, Alemany, R, Lee, PS, McDonnell, TJ, Mitlianga, P et al. (2000). A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19: 2–12. [DOI] [PubMed] [Google Scholar]
- Koski, A, Kangasniemi, L, Escutenaire, S, Pesonen, S, Cerullo, V, Diaconu, I et al. (2010). Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 18: 1874–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron, G, De la Fuente, M, Thuwajit, P, Thuwajit, C and Hermoso, MA (2014). Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014: 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers, W (1991). Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett 285: 199–212. [DOI] [PubMed] [Google Scholar]
- Blair, GE, Dixon, SC, Griffiths, SA and Zajdel, ME (1989). Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res 14: 339–346. [DOI] [PubMed] [Google Scholar]
- Rosenberg, SA (2014). IL-2: the first effective immunotherapy for human cancer. J Immunol 192: 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Otter, W, Jacobs, JJ, Battermann, JJ, Hordijk, GJ, Krastev, Z, Moiseeva, EV et al. (2008). Local therapy of cancer with free IL-2. Cancer Immunol Immunother 57: 931–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison, CL, Braciak, T, Ralston, R, Muller, WJ, Gauldie, J and Graham, FL (1995). Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA 92: 8522–8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier, L, Duffour, MT, Sabourin, JC, Lee, MG, Cabannes, J, Ragot, T et al. (1995). Complete recovery of mice from a pre-established tumor by direct intratumoral delivery of an adenovirus vector harboring the murine IL-2 gene. Gene Ther 2: 16–21. [PubMed] [Google Scholar]
- Toloza, EM, Hunt, K, Swisher, S, McBride, W, Lau, R, Pang, S et al. (1996). In vivo cancer gene therapy with a recombinant interleukin-2 adenovirus vector. Cancer Gene Ther 3: 11–17. [PubMed] [Google Scholar]
- Zhang, R, Baunoch, D and DeGroot, LJ (1998). Genetic immunotherapy for medullary thyroid carcinoma: destruction of tumors in mice by in vivo delivery of adenoviral vector transducing the murine interleukin-2 gene. Thyroid 8: 1137–1146. [DOI] [PubMed] [Google Scholar]
- Stewart, AK, Lassam, NJ, Quirt, IC, Bailey, DJ, Rotstein, LE, Krajden, M et al. (1999). Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: results of a phase 1 clinical trial. Gene Ther 6: 350–363. [DOI] [PubMed] [Google Scholar]
- Dummer, R, Rochlitz, C, Velu, T, Acres, B, Limacher, JM, Bleuzen, P et al. (2008). Intralesional adenovirus-mediated interleukin-2 gene transfer for advanced solid cancers and melanoma. Mol Ther 16: 985–994. [DOI] [PubMed] [Google Scholar]
- Wright, P, Braun, R, Babiuk, L, Littel-van den Hurk, SD, Moyana, T, Zheng, C et al. (1999). Adenovirus-mediated TNF-alpha gene transfer induces significant tumor regression in mice. Cancer Biother Radiopharm 14: 49–57. [DOI] [PubMed] [Google Scholar]
- McLoughlin, JM, McCarty, TM, Cunningham, C, Clark, V, Senzer, N, Nemunaitis, J et al. (2005). TNFerade, an adenovector carrying the transgene for human tumor necrosis factor alpha, for patients with advanced solid tumors: surgical experience and long-term follow-up. Ann Surg Oncol 12: 825–830. [DOI] [PubMed] [Google Scholar]
- Tähtinen, S, Kaikkonen, S, Merisalo-Soikkeli, M, Grönberg-Vähä-Koskela, S, Kanerva, A, Parviainen, S et al. (2015). Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS One 10: e0131242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake, JL, Stampfl, S and Zimmerman, RJ (1987). Synergistic effects of combination therapy with human recombinant interleukin-2 and tumor necrosis factor in murine tumor models. Cancer Res 47: 3948–3953. [PubMed] [Google Scholar]
- McIntosh, JK, Mulé, JJ, Merino, MJ and Rosenberg, SA (1988). Synergistic antitumor effects of immunotherapy with recombinant interleukin-2 and recombinant tumor necrosis factor-alpha. Cancer Res 48: 4011–4017. [PubMed] [Google Scholar]
- Calcinotto, A, Grioni, M, Jachetti, E, Curnis, F, Mondino, A, Parmiani, G et al. (2012). Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol 188: 2687–2694. [DOI] [PubMed] [Google Scholar]
- Johansson, A, Hamzah, J, Payne, CJ and Ganss, R (2012). Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci USA 109: 7841–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, H, Zheng, Y, Li, R and Zhang, J (2016). Reply to Suppressive activity of human regulatory T cells is maintained in the presence of TNF. Nat Med 22: 18–19. [DOI] [PubMed] [Google Scholar]
- Schiller, JH, Morgan-Ihrig, C and Levitt, ML (1995). Concomitant administration of interleukin-2 plus tumor necrosis factor in advanced non-small cell lung cancer. Am J Clin Oncol 18: 47–51. [DOI] [PubMed] [Google Scholar]
- Arenas-Ramirez, N, Woytschak, J and Boyman, O (2015). Interleukin-2: Biology, Design and Application. Trends Immunol 36: 763–777. [DOI] [PubMed] [Google Scholar]
- Gerber, SA, Sorensen, EW, Sedlacek, AL, Lim, JY, Skrombolas, D, Frelinger, JG et al. (2013). Local expression of interleukin-2 by B16 melanoma cells results in decreased tumour growth and long-term tumour dormancy. Immunology 138: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo, DJ, Yanaba, K and Tedder, TF (2010). B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol 184: 4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, SP and Kurzrock, R (2015). PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 14: 847–856. [DOI] [PubMed] [Google Scholar]
- Ranki, T, Pesonen, S, Hemminki, A, Partanen, K, Kairemo, K, Alanko, T et al. (2016). Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, MA, Spencer, JF, La Regina, MC, Dhar, D, Tollefson, AE, Toth, K et al. (2006). Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 66: 1270–1276. [DOI] [PubMed] [Google Scholar]
- Cerullo, V, Pesonen, S, Diaconu, I, Escutenaire, S, Arstila, PT, Ugolini, M et al. (2010). Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 70: 4297–4309. [DOI] [PubMed] [Google Scholar]
- Bramante, S, Koski, A, Kipar, A, Diaconu, I, Liikanen, I, Hemminki, O et al. (2014). Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans. Int J Cancer 135: 720–730. [DOI] [PubMed] [Google Scholar]
- Siurala, M, Bramante, S, Vassilev, L, Hirvinen, M, Parviainen, S, Tähtinen, S et al. (2015). Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int J Cancer 136: 945–954. [DOI] [PubMed] [Google Scholar]
- Siurala, M, Vähä-Koskela, M, Havunen, R, Tähtinen, S, Bramante, S, Parviainen, S et al. (2016). Syngeneic Syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. Oncoimmunol. 5: e1136046. [DOI] [PMC free article] [PubMed]
- Kanerva, A, Mikheeva, GV, Krasnykh, V, Coolidge, CJ, Lam, JT, Mahasreshti, PJ et al. (2002). Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 8: 275–280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.