Abstract
Ewing's sarcoma is a devastating rare pediatric cancer of the bone. Intense chemotherapy temporarily controls disease in most patients at presentation but has limited effect in patients with progressive or recurrent disease. We previously described preliminary results of a novel immunotherapy, FANG (Vigil) vaccine, in which 12 advanced stage Ewing's patients were safely treated and went on to achieve a predicted immune response (IFNγ ELISPOT). We describe follow-up through year 3 of a prospective, nonrandomized study comparing an expanded group of Vigil-treated advanced disease Ewing's sarcoma patients (n = 16) with a contemporaneous group of Ewing's sarcoma patients (n = 14) not treated with Vigil. Long-term follow-up results show a survival benefit without evidence of significant toxicity (no ≥ grade 3) to Vigil when administered once monthly by intradermal injection (1 × 10e6 cells/injection to 1 × 10e7 cells/injection). Specifically, we report a 1-year actual survival of 73% for Vigil-treated patients compared to 23% in those not treated with Vigil. In addition, there was a 17.2-month difference in overall survival (OS; Kaplan-Meier) between the Vigil (median OS 731 days) and no Vigil patient groups (median OS 207 days). In conclusion, these results supply the rational for further testing of Vigil in advanced stage Ewing's sarcoma.
Introduction
Ewing's sarcoma is a malignant cancer of the bones with rapid spread to the lungs. It is the second most frequently diagnosed primary malignant bone tumor in the United States with annual incidence of 1 in a million.1,2 Approximately 20–30% of Ewing's sarcoma cases are diagnosed in the first decade and 10% after age 20. The median age of diagnosis is 14–15 years old.3,4
Although less than 25% of patients present with overt metastatic disease, based on relapse patterns subclinical metastases are presumably present in up to 80% of children at diagnosis.4,5,6,7,8 The median time to relapse in unselected populations is 1.3 years.4,8 Patients who relapse have a marked reduction in 1- and 5-year survival.
Few recurrent patients respond to second-line therapy and even fewer achieve a second remission,4,8,9,10,11,12,13 particularly in those who relapse within 2 years of front-line treatment. In one large retrospective analysis of 714 patients from time of first relapse, the 1-year overall survival (OS) was 43%, 5-year OS was 13%, and 10-year OS was 9%.4 At time of first relapse, the most significant prognostic factors are time to relapse (<2 versus ≥2 years) and site(s) of recurrence (localized, metastatic, or combined localized and metastatic). For relapses that occur within the first 2 years after initial diagnosis, which make up 72% of relapses,4 the 2-year OS from relapse is 7%.10 In two other analyses, 5-year progression-free survival was 5%13 and 5-year OS 7%.4
Regarding the significance effect of metastatic disease as an independent factor related to response,14 in one assessment, metastatic disease at presentation in 24 patients (13 with lung metastases, 12 with distant bone metastases, and 1 with bone marrow involvement) the 5-year OS from presentation with metastatic disease was 27 versus 85% for patients with localized disease (n = 77, P < 0.0001) and the 5-year progression-free survival was 28 versus 73% (P < 0.001).
Second-line chemotherapy for relapsed Ewing's sarcoma generally shows limited efficacy with only 9 to 13% of patients achieving a second disease-free remission.11,13,15 Notably, the National Comprehensive Cancer Network guidelines do not provide standard of care recommendations for second-line treatment. Regimens such as topotecan/cyclophosphamide, irinotecan/temozolomide, or docetaxel/gemcitabine have been utilized in second-line or later treatments and may prolong life for those who respond.16,17,18,19,20,21,22,23,24,25,26,27,28,29 However, none of these regimens have been determined to have a significant advantage in randomized clinical assessments.30 The irinotecan/temozolomide regimen appears to be the most common second-line regimen utilized today.
Third-line treatment for Ewing's sarcoma is associated with cumulative toxicity (hematologic and neurologic) in addition to an even lower response rate. There is no standard of care treatment for Ewing's sarcoma in the third-line or greater setting.
Previously,31 we reported preliminary results of 12 patients with advanced disease (multiply recurrent or treatment failure within 2 years) Ewing's sarcoma treated with Vigil (formerly known as FANG) immunotherapy. Safety and immune responses were characterized and a preliminary 1-year survival of 75% was observed. We now report long-term follow-up of the previously reported 12 and an additional four patients with advanced Ewing's sarcoma. We compared the results in these 16 patients to 14 contemporaneous patients with advanced Ewing's sarcoma who fulfilled the same inclusion criteria and underwent similar surgical procedure and vaccine construction but did not receive Vigil.
Our observed preliminary results and now long-term follow-up of our expanded group of patients are a provocative observation in support of further randomized trial testing.
Results
Patient characterization
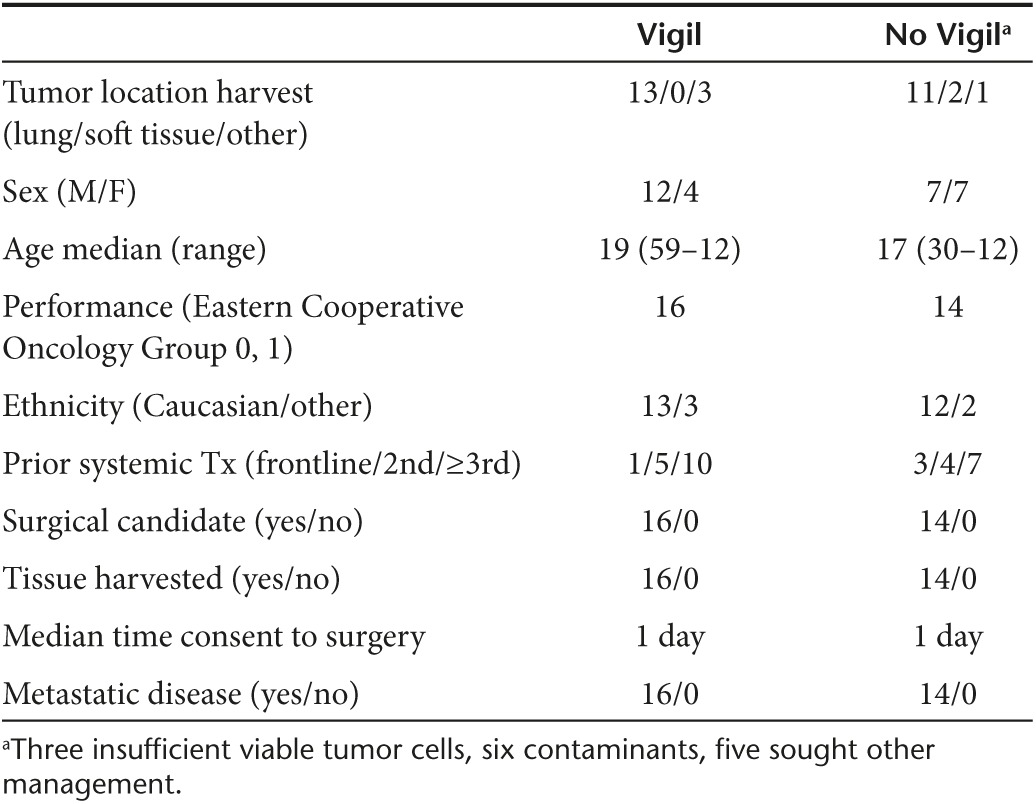
All 30 Ewing's sarcoma patients who signed MCCRC Institutional Review Board approved consent and qualified for phase 1 testing with Vigil were evaluated in this analysis. All had late stage (i.e., ≥ third-line chemotherapy; n = 17) or relapse < 2 years of front-line treatment (n = 13). One hundred sixty-three vaccine vials were successfully manufactured. Sixteen patients had successful vaccine construction and received Vigil. Median follow up at the time of reporting was 2 years 11 days. Median time from procurement to first treatment was 53 days. Five treated patients had between 104 and 294 days delay from procurement to treatment. An additional chemotherapy regimen was administered after procurement but prior to Vigil treatment in three of these five patients. Limited field palliative radiation therapy was administered to the other two patients with Vigil delivery. Three of the five remain alive and two passed away 37 days and 430 days after Vigil treatment. Fourteen patients in the comparative group did not receive Vigil after undergoing similar surgery and vaccine construction process. Nine of the latter were unable to have vaccine released (six contaminant, three insufficient viable tumor cells) and five chose other treatment management. One patient in the No Vigil group had progression and mortality before reaching the median time for vaccination in the treatment group. This patient fulfilled the same inclusion criteria as all other patients at time of procurement for surgical justification and study engagement. All vaccines constructed fulfilled release criteria of GMCSF production (one exception with insufficient material for GMCSF testing) and TGFβ1, β2 knockdown. Demographics of patients entered in the long-term follow up analysis are shown in Table 1. Though not statistically significant, there was a higher ratio of male patients in the Vigil arm.
Table 1. Ewing's sarcoma phase 1 demographics.
Safety
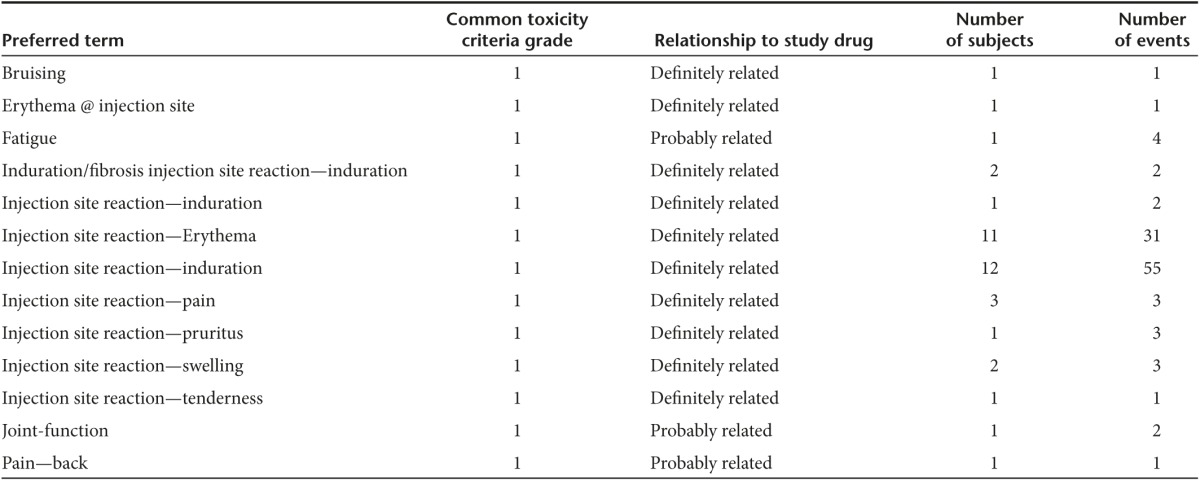
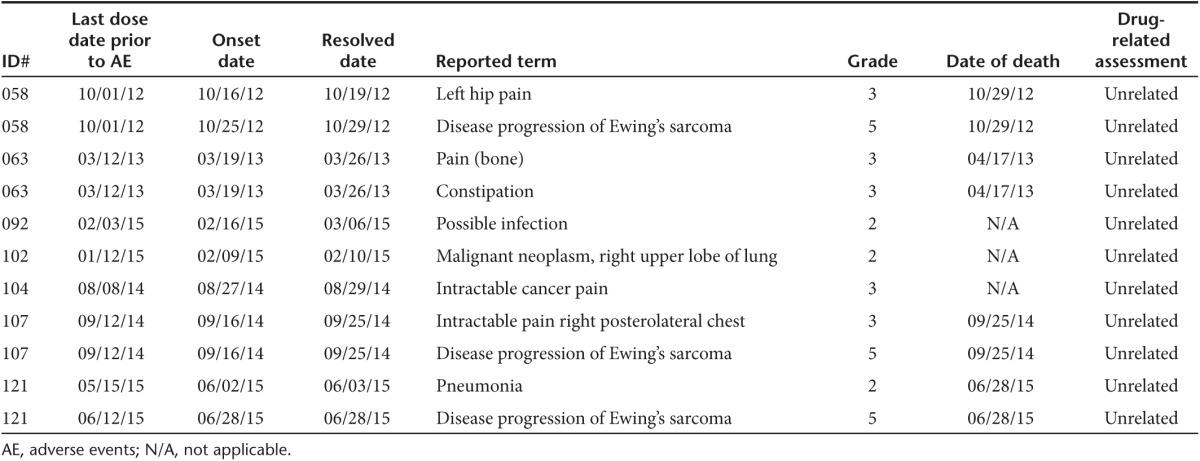
No significant toxicity was observed in the 16 Vigil-treated patients. Specifically no product related Grade 3, four toxic effects were demonstrated during the treatment course or in long-term follow-up. Ninety-three injections of Vigil have been administered to the 16 patients. Adverse events reported are shown in Table 2.
Table 2. Ewing's sarcoma patients who received Vigil, definitely or probably related adverse events and long-term follow up.
There were 11 serious adverse events reported involving 7 participants (See Table 3). None of the serious adverse events were related to Vigil. Seventeen deaths (7 Vigil, 10 No-Vigil) have occurred, none of which were determined to be related to Vigil.
Table 3. Phase 1 Ewing's sarcoma: serious adverse events reported and long-term follow up.
Response
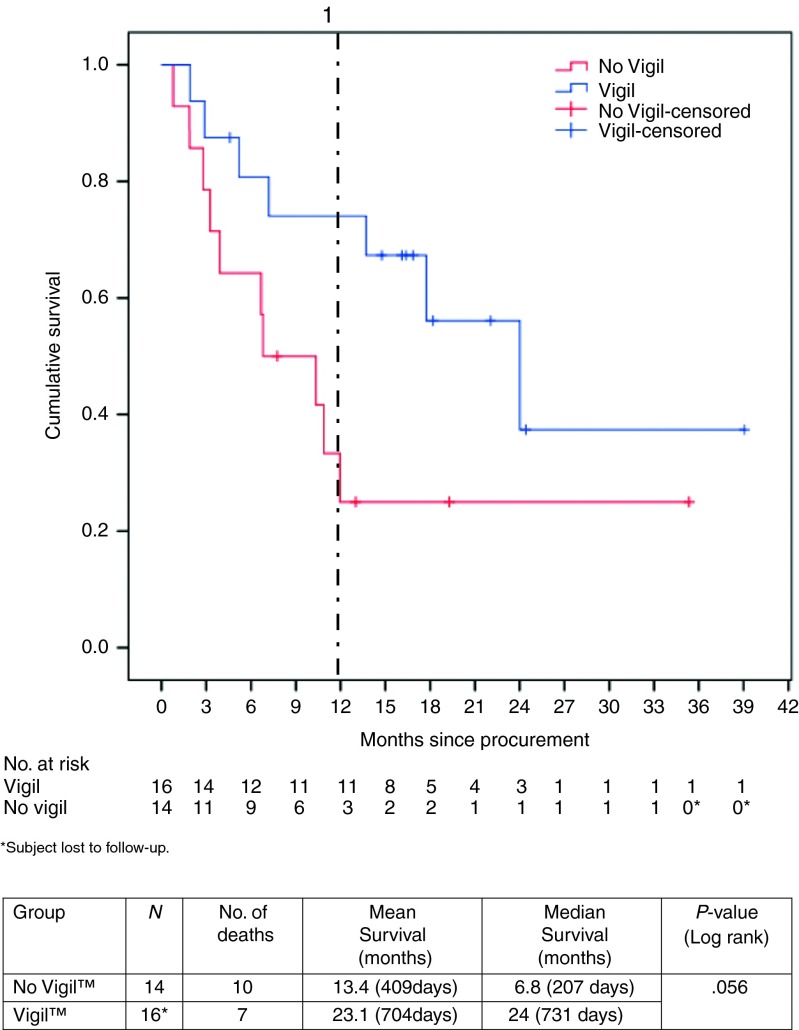
Figure 1 shows the results of the Kaplan-Meier analysis of the survival data. Overall survival from time of procurement revealed a 17.2-month improvement in survival in the Vigil-treated patients compared to the no Vigil group. The median survival for the Vigil-treated group from time of treatment was 22.7 months (689 days).
Figure 1.
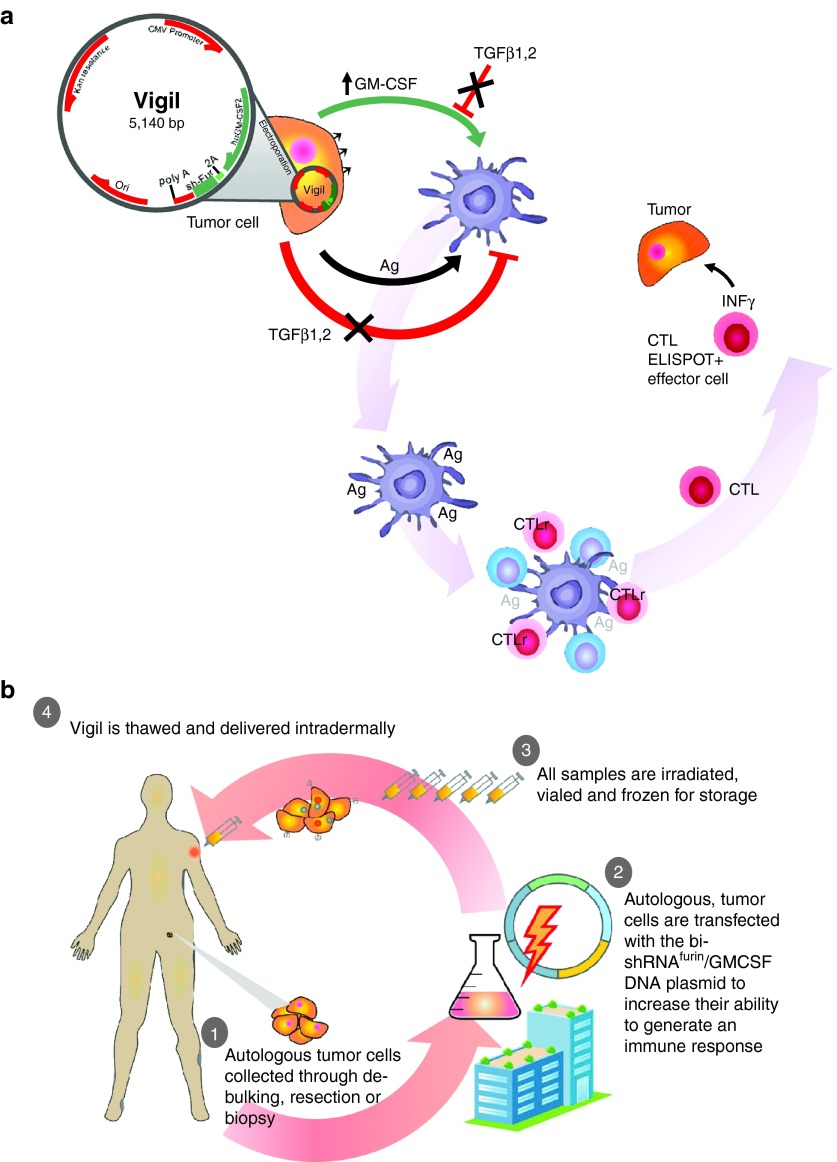
(a) Vigil is a 5,140 bp plasmid of a bifunctional shRNA-furin DNA sequence which prevents cleavage of TGFβ precursor into functional TGFβ1 & TGFβ2 and a GMCSF DNA sequence which stimulates antigen presentation and adaptive immune response when expressed after placement by electroporation into individual autologous tumor tissue which provides the full tumor antigen (Ag) profile and has demonstrated in phase 1, 2 testing induction of circulating cytotoxic T lymphocytes capable of specific lytic activity (ELISPOT and response) to autologous tumor. (b) Vigil constructing is portrayed.
The actual 1-year survival of patients who received Vigil (11/15, 73%) was higher than those who did not receive Vigil (3/13, 23%) (one in each of the Vigil and no Vigil have not yet achieved the 1-year survival time point).
One patient was previously reported as achieving partial response as per RECIST 1.1 criteria (at 2.7 months after immunotherapy initiation the sum of target lung tumor diameters had decreased from 9.3 to 5.3 cm; a 46% decrease) (#089). He progressed 20.6 months after procurement (17.9 months after treatment initiation). Six of the other 15 patients maintained stable disease ≥ 3 months. Another patient (#062), who demonstrated recurrent disease 20.4 months (622 days) after prolonged stable disease, underwent a second harvest to procure tumor for treatment for recurrent disease as previously described.31 She received no further systemic therapy since second treatment with Vigil harvested from recurrent disease and now no longer demonstrates evidence of Ewing's sarcoma by imaging 42.4 months (1,291 days) after initial procurement.
Discussion
The majority of relapsed Ewing's sarcoma patients who do not achieve a second remission, as well as those who go into a second remission but subsequently relapse, do not derive significant benefit from additional chemotherapy. Previously published results of 161 patients treated with second-line chemotherapy, comparing non-responding patients (SD/PD) to responding patients (PR/CR) revealed a 5-year survival of 4 versus 25% (2-year survival 10 versus 45%).15 Likewise, response versus no response to second-line chemotherapy was a significant (P = 0.0001) prognostic factor (5-year survival 48 versus 0% respectively) in another series of 55 patients.11 In a third analysis of 195 patients, 86% of the patients did not achieve second remission; 97% of these patients died with a median survival of 11.7 months and no patient achieved disease control. Of the 26 patients who did go into a second remission, 12 relapsed again and did not reach a third remission (10/12 died and in the first year and 2 were living with uncontrolled disease at 6 and 13 months).13 Several targeted and/or targeted plus chemotherapy combination studies are under early clinical or preclinical development (i.e., inhibition of insulin-like growth factor receptor I, midostaurin, YK-4–279, bevacizumab/topotecan/cyclophosphamide, olaparib, enzastaurin, zoledronic acid/ifosfamide) but none have thus far shown significant benefit.30 As such, there is a need for treatment development in patients with metastatic Ewing's sarcoma that is refractory to systemic therapy or has progressed after second-line treatment.
That the “No Vigil™” subset of patients followed was not a prospectively randomized group precludes ascribing significance to interpretation of results. However, insofar as all of these patients traveled to the treatment site, met all inclusion criteria, exhibited none of the exclusion criteria and underwent similar surgical harvest of tumor tissue for Vigil construction, we submit that comparison is justifiable albeit with interpretation of results as hypothesis-engendering at best. As shown in results, the demographics of the no Vigil versus Vigil groups appear well-balanced viv-a-vis prognostic factors that might be expected to affect survival based on historical data.
Regarding evaluation of the comparative findings; the results in the published literature that consistently reflect the severely limited survival of this population of Ewing's sarcoma patients do not necessarily apply to the selected subset of patients in the current report who, in addition to performance status, are also surgical candidates (particularly so for thoracic surgery that was performed in most of the Vigil/no Vigil patients). Thus, despite a perceived survival advantage of Vigil-treated patients when compared to historical study patients who received alternative systemic therapies, there could be an unintended bias toward a healthier patient population that could have produced skewed results. However, the comparison to the concurrently accrued “No Vigil™” population would strongly suggest that the reported survival prolongation is indeed meaningful. In addition, these results are also consistent with the data previously presented from the phase 1 study in which a prolongation in median survival was also shown in adult cancer patients treated with Vigil.32,33
Given these results of Vigil in advanced Ewing's sarcoma, our study group consensus under FDA IND support is that a randomized assessment of Vigil in this population is justified. To that end, a randomized, controlled, open-label phase 2 study of Vigil versus systemic chemotherapy in ≥ third-line treatment of patients with metastatic Ewing's sarcoma is now underway (NCT02511132).
Materials and Methods
The construction and current good manufacturing practice adherence of Vigil immunotherapy are summarized in Figure 2. Briefly, Vigil vector utilizes the pUMVC3 vector backbone in which the granulocyte-macrophage colony-stimulating factor (GMCSF)-encoding complementary DNA and the DNA encoding the furin bifunctional shRNA are under transcriptional control of the cytomegalovirus immediate-early promoter. The final construct was confirmed by bi-directional sequencing. Following protocol-specific informed consent, the tumor was excised, placed in sterile transport media, and brought to the Gradalis manufacturing facility (Carrollton, TX) for vaccine manufacture as previously described.32
Figure 2.
Survival from surgical procurement of advanced Ewing's patients successfully harvested for Vigil construction (n = 30). Comparison is made of those who received Vigil (n = 16) versus those who did not receive Vigil ((n = 14) as a result of construction failure or choice of other management). All patients are censored alive as of dates provided on 19 October 2015.
Study design. Briefly, each patient entered into study received monthly doses of Vigil the concentrations of which were based on cell yield from procured tumor (1 × 10e6 cells/injection, 4.0 × 10e6 cells/injection, 8.3 × 10e6 cells/injection, 1 × 10e7 cells/injection or 2.5 × 10e7 cells/injection in 1 ml volume). A minimum of 4 doses to a maximum of 12 doses were administered via intradermal injection, alternating between the right and left upper arms. Patients were discontinued from study for progressive disease or intolerable toxicity. The trial was performed after approval by a local Ethics Committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services.
Patient population. The study population involved patients with Ewing's sarcoma (n = 30) that was multiply recurrent or progressed within 2 years of treatment who, following study consent, underwent surgical tumor extraction and subsequent tumor processing for vaccine construction. These Ewing's sarcoma patients were a subset of the phase 1 solid tumor study previously published.32 Specific inclusion criteria included a histologically confirmed advanced or metastatic non-curable Ewing's sarcoma following completion of ≥1 disease appropriate standard of care therapy; recovery from all treatment-related toxicities to ≤ Grade 1 (except alopecia); availability of tumor in sufficient quantity (a minimum of 2–8 g of solid tumor tissue or at least 500 ml of pleural/ascites fluid) for vaccine processing; history of brain metastases allowed if treatment completed ≥2 months prior to enrollment with magnetic resonance imaging confirmation of no active disease; presence of ≥1 measurable or evaluable lesion; patient age ≥12 years; Eastern Cooperative Oncology Group performance status of 0–1; a signed, Institutional Review Board-approved, protocol-specific written informed consent document; a negative pregnancy test for women of child-bearing potential; and normal organ and marrow function defined as follows: absolute granulocyte count (>1,500/mm3), platelets (>100,000/ mm3), total bilirubin (<2 mg/dl), AST(SGOT)/ALT(SGPT) (<2× institutional upper limit of normal), and creatinine (<1.5 mg/dl).
Exclusion criteria included: surgery involving general anesthesia, chemotherapy, radiotherapy, or immunotherapy within 4 weeks of study entry; use of other investigational agents within 30 days prior to study entry; known immune compromised state or autoimmune disease; prior malignancy (excluding non-melanoma skin cancer) unless in remission for ≥2 years; uncontrolled intercurrent illness or psychiatric illness/social situations that would limit compliance with study requirements; confirmation that patient was pregnant or nursing; HIV; or chronic hepatitis B or C infection (except in patients with hepatocellular carcinoma).
All patients receiving vaccine (n = 16) were treated in the outpatient facilities of Mary Crowley Cancer Research Centers (MCCRC), Dallas, TX. Twelve of the 16 patients treated with Vigil in this analysis were previously described31 for early safety and response. Fourteen patients did not receive Vigil following procurement and vaccine manufacture (n = 14) and were followed as a comparative, nonrandomized but contemporarily alternatively treated group to the Vigil-treated patients. Thirteen of these patients were previously described31 as “consecutively harvested but not treated”. This analysis now describes, for the first time, comparison of survival through up to 3 years of both study groups (Vigil treated and non-Vigil treated).
Statistical analysis. Statistical analyses were performed using IBM SPSS Version 22 (IBM Corporation). Overall survival from time of surgical procurement or from time of treatment was analyzed using Kaplan-Meier curves. Actual 1-year survival difference between treatment groups was determined using the Fisher's Exact test. All patients were censored alive using the last known date alive.
Acknowledgments
We gratefully acknowledge the generous support of the Helen L. Kay Charitable Trust, the Jasper L. and Jack Denton Wilson Foundation, The Marilyn Augur Family Foundation, Michele Ashby, the Rutledge Foundation, the Speedway Children's Charities, Triumph Over Kid Cancer Foundation, Wipe Out Kids' Cancer, and the family and friends of Sam Day. The authors would like to acknowledge Michelle Watkins and Brenda Marr for their competent and knowledgeable assistance in the preparation of the manuscript. We also thank Lee Helman and Mark Thornton for critical review and comments regarding the database involved in this manuscript. The following authors are shareholders in Gradalis, Inc. and Strike Bio: Gladice Wallraven, Sam Whiting, Donald Rao, Neil Senzer, and John Nemunaitis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Esiashvili, N, Goodman, M and Marcus, RB Jr (2008). Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 30: 425–430. [DOI] [PubMed] [Google Scholar]
- http://www.cancer.gov/types/bone/hp/ewing-treatment-pdq. Revised 19 November 2015.
- Cotterill, SJ, Ahrens, S, Paulussen, M, Jürgens, HF, Voûte, PA, Gadner, H et al. (2000). Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol 18: 3108–3114. [DOI] [PubMed] [Google Scholar]
- Stahl, M, Ranft, A, Paulussen, M, Bölling, T, Vieth, V, Bielack, S et al. (2011). Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 57: 549–553. [DOI] [PubMed] [Google Scholar]
- Ozaki, T, Hillmann, A, Hoffmann, C, Rübe, C, Blasius, S, Dunst, J et al. (1996). Significance of surgical margin on the prognosis of patients with Ewing's sarcoma. A report from the Cooperative Ewing's Sarcoma Study. Cancer 78: 892–900. [DOI] [PubMed] [Google Scholar]
- Bacci, G, Picci, P, Ferrari, S, Mercuri, M, Brach del Prever, A, Rosito, P et al. (1998). Neoadjuvant chemotherapy for Ewing's sarcoma of bone: no benefit observed after adding ifosfamide and etoposide to vincristine, actinomycin, cyclophosphamide, and doxorubicin in the maintenance phase–results of two sequential studies. Cancer 82: 1174–1183. [DOI] [PubMed] [Google Scholar]
- Klingebiel, T, Pertl, U, Hess, CF, Jürgens, H, Koscielniak, E, Pötter, R et al. (1998). Treatment of children with relapsed soft tissue sarcoma: report of the German CESS/CWS REZ 91 trial. Med Pediatr Oncol 30: 269–275. [DOI] [PubMed] [Google Scholar]
- Leavey, PJ and Collier, AB (2008). Ewing sarcoma: prognostic criteria, outcomes and future treatment. Expert Rev Anticancer Ther 8: 617–624. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Galindo, C, Billups, CA, Kun, LE, Rao, BN, Pratt, CB, Merchant, TE et al. (2002). Survival after recurrence of Ewing tumors: the St Jude Children's Research Hospital experience, 1979-1999. Cancer 94: 561–569. [DOI] [PubMed] [Google Scholar]
- Shankar, AG, Ashley, S, Craft, AW and Pinkerton, CR (2003). Outcome after relapse in an unselected cohort of children and adolescents with Ewing sarcoma. Med Pediatr Oncol 40: 141–147. [DOI] [PubMed] [Google Scholar]
- Barker, LM, Pendergrass, TW, Sanders, JE and Hawkins, DS (2005). Survival after recurrence of Ewing's sarcoma family of tumors. J Clin Oncol 23: 4354–4362. [DOI] [PubMed] [Google Scholar]
- McTiernan, AM, Cassoni, AM, Driver, D, Michelagnoli, MP, Kilby, AM and Whelan, JS (2006). Improving outcomes after relapse in Ewing's sarcoma: analysis of 114 patients from a single institution. Sarcoma 2006: 83548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci, G, Ferrari, S, Longhi, A, Donati, D, De Paolis, M, Forni, C et al. (2003). Therapy and survival after recurrence of Ewing's tumors: the Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann Oncol 14: 1654–1659. [DOI] [PubMed] [Google Scholar]
- Hamilton, SN, Carlson, R, Hasan, H, Rassekh, SR and Goddard, K (2015). Long-term outcomes and complications in pediatric Ewing sarcoma. Am J Clin Oncol (epub ahead of print). [DOI] [PubMed]
- Rasper, M, Jabar, S, Ranft, A, Jürgens, H, Amler, S and Dirksen, U (2014). The value of high-dose chemotherapy in patients with first relapsed Ewing sarcoma. Pediatr Blood Cancer 61: 1382–1386. [DOI] [PubMed] [Google Scholar]
- Navid, F, Billups, C, Liu, T, Krasin, MJ and Rodriguez-Galindo, C (2008). Second cancers in patients with the Ewing sarcoma family of tumours. Eur J Cancer 44: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, TE, Kushner, BH, Sheldon, JM, LaQuaglia, M and Healey, JH (1999). Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 33: 65–70. [DOI] [PubMed] [Google Scholar]
- Saylors, RL 3rd, Stine, KC, Sullivan, J, Kepner, JL, Wall, DA, Bernstein, ML et al.; Pediatric Oncology Group. (2001). Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 19: 3463–3469. [DOI] [PubMed] [Google Scholar]
- Wagner, LM, McAllister, N, Goldsby, RE, Rausen, AR, McNall-Knapp, RY, McCarville, MB et al. (2007). Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 48: 132–139. [DOI] [PubMed] [Google Scholar]
- Wagner, LM, Crews, KR, Iacono, LC, Houghton, PJ, Fuller, CE, McCarville, MB et al. (2004). Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res 10: 840–848. [DOI] [PubMed] [Google Scholar]
- Wagner, LM (2010). Oral irinotecan for treatment of pediatric solid tumors: ready for prime time? Pediatr Blood Cancer 54: 661–662. [DOI] [PubMed] [Google Scholar]
- Wagner, LM, Perentesis, JP, Reid, JM, Ames, MM, Safgren, SL, Nelson, MD Jr et al. (2010). Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children's Oncology Group phase I consortium study. Pediatr Blood Cancer 54: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, L, Turpin, B, Nagarajan, R, Weiss, B, Cripe, T and Geller, J (2013). Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr Blood Cancer 60: 1447–1451. [DOI] [PubMed] [Google Scholar]
- Casey, DA, Wexler, LH, Merchant, MS, Chou, AJ, Merola, PR, Price, AP et al. (2009). Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer 53: 1029–1034. [DOI] [PubMed] [Google Scholar]
- Raciborska, A, Bilska, K, Drabko, K, Chaber, R, Pogorzala, M, Wyrobek, E et al. (2013). Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 60: 1621–1625. [DOI] [PubMed] [Google Scholar]
- McGregor, LM, Stewart, CF, Crews, KR, Tagen, M, Wozniak, A, Wu, J et al. (2012). Dose escalation of intravenous irinotecan using oral cefpodoxime: a phase I study in pediatric patients with refractory solid tumors. Pediatr Blood Cancer 58: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navid, F, Willert, JR, McCarville, MB, Furman, W, Watkins, A, Roberts, W et al. (2008). Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer 113: 419–425. [DOI] [PubMed] [Google Scholar]
- Mora, J, Cruz, CO, Parareda, A and de Torres, C (2009). Treatment of relapsed/refractory pediatric sarcomas with gemcitabine and docetaxel. J Pediatr Hematol Oncol 31: 723–729. [DOI] [PubMed] [Google Scholar]
- Rapkin, L, Qayed, M, Brill, P, Martin, M, Clark, D, George, BA et al. (2012). Gemcitabine and docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer 59: 854–858. [DOI] [PubMed] [Google Scholar]
- Gaspar, N, Hawkins, DS, Dirksen, U, Lewis, IJ, Ferrari, S, Le Deley, MC et al. (2015). Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol 33: 3036–3046. [DOI] [PubMed] [Google Scholar]
- Ghisoli, M, Barve, M, Schneider, R, Mennel, R, Lenarsky, C, Wallraven, G et al. (2015). Pilot trial of FANG immunotherapy in Ewing's sarcoma. Mol Ther 23: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer, N, Barve, M, Kuhn, J, Melnyk, A, Beitsch, P, Lazar, M et al. (2012). Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther 20: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer, N, Barve, M, Nemunaitis, J, Kuhn, J, Melnyk, A,Beitsch,P et al. (2013). Long term follow up: phase I trial of “bi-shRNA furin/GMCSF DNA/Autologous Tumor Cell” immunotherapy (FANG™) in advanced cancer. J Vaccines and Vaccination 4: 209. [Google Scholar]