Abstract
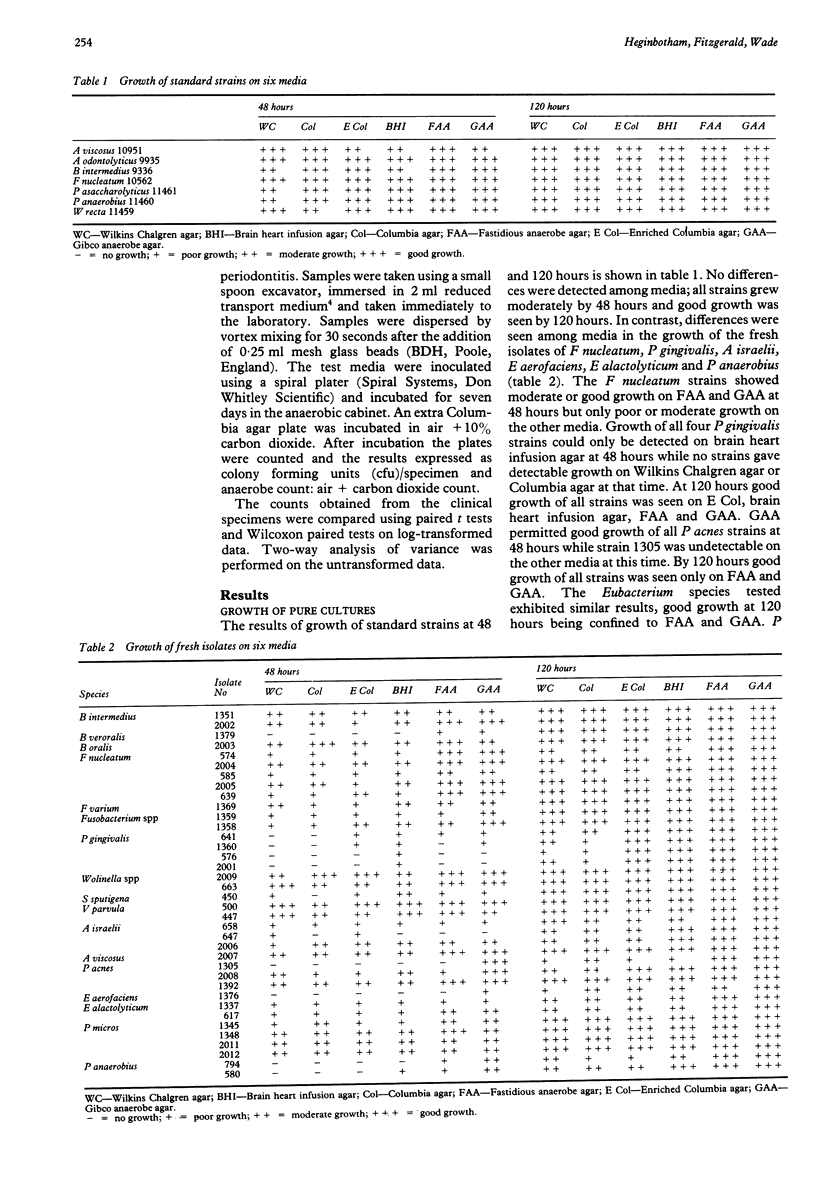
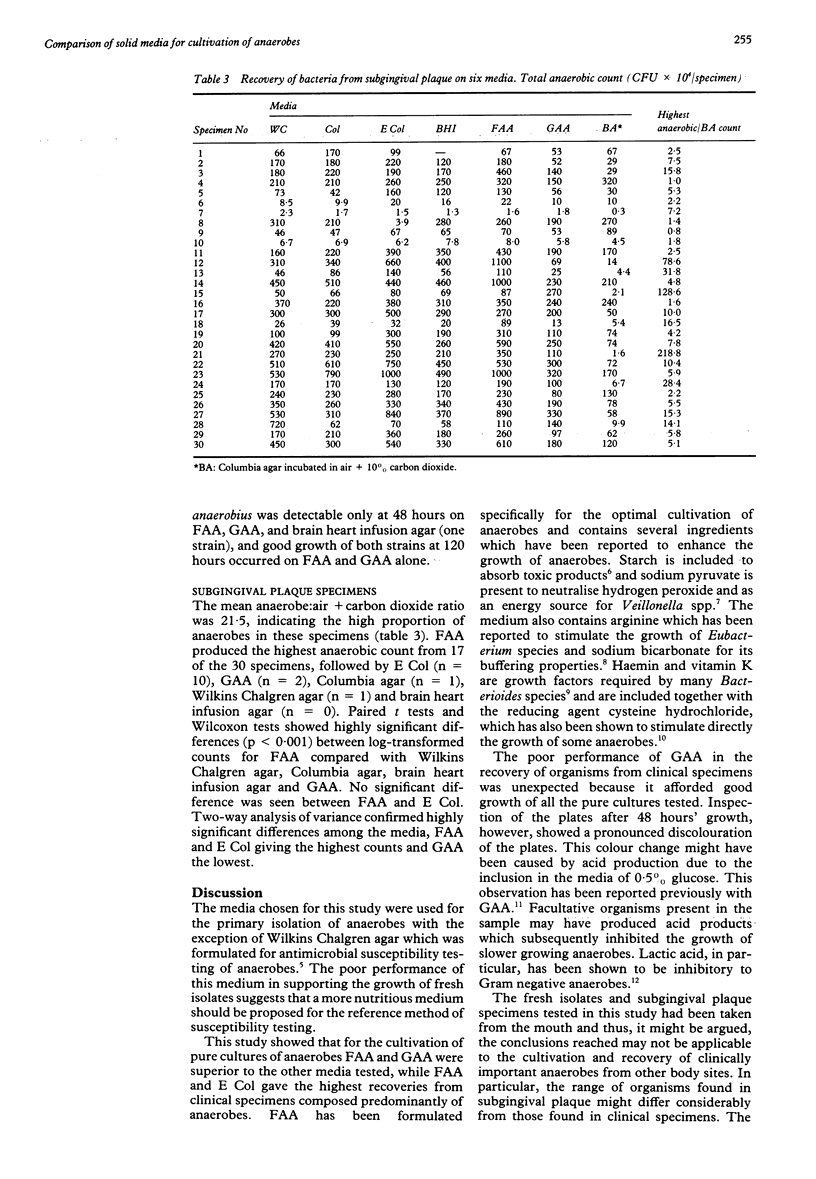
Two commercial agar media for the cultivation of anaerobes were compared with four other media for their ability to support the growth of a wide range of anaerobes from clinical specimens of subgingival plaque. Fastidious anaerobe agar (FAA, Lab M) and anaerobe agar (GAA, Gibco) allowed better growth of the pure cultures than the other media. FAA recovered the highest numbers of bacteria from subgingival plaque specimens which were composed predominantly of anaerobes. GAA performed poorly with these samples. It is concluded that FAA seemed to be superior to the other media tested for the cultivation and recovery of anaerobes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajello G. W., Feeley J. C., Hayes P. S., Reingold A. L., Bolan G., Broome C. V., Phillips C. J. Trans-isolate medium: a new medium for primary culturing and transport of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. J Clin Microbiol. 1984 Jul;20(1):55–58. doi: 10.1128/jcm.20.1.55-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. B., Ayers O. M., Kohan A. P. Characteristics and sites of infection of Eubacterium nodatum, Eubacterium timidum, Eubacterium brachy, and other asaccharolytic eubacteria. J Clin Microbiol. 1987 Aug;25(8):1540–1545. doi: 10.1128/jcm.25.8.1540-1545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo P. A., Yamamoto Y., Nakamura M., Reynolds H. S., Genco R. J. Lactic acid production by oral Streptococcus mitis inhibits the growth of oral Capnocytophaga. J Periodontol. 1985 Sep;56(9):548–552. doi: 10.1902/jop.1985.56.9.548. [DOI] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Nielsen P. A. Role of reduced sulfur compounds in nutrition of Propionibacterium acnes. J Clin Microbiol. 1983 Feb;17(2):276–279. doi: 10.1128/jcm.17.2.276-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanson D. C., Singh J. Effect of adding cysteine to brain-heart infusion broth on the isolation of Bacteroides fragilis from experimental blood cultures. J Clin Pathol. 1981 Feb;34(2):221–223. doi: 10.1136/jcp.34.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. F., Wilkins T. D. Arginine, a growth-limiting factor for Eubacterium lentum. J Bacteriol. 1976 Aug;127(2):780–784. doi: 10.1128/jb.127.2.780-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B., Brown F. V. Effect of the growth of anaerobic bacteria on the surface pH of solid media. J Clin Pathol. 1985 May;38(5):565–569. doi: 10.1136/jcp.38.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]



