Abstract
Background
Vitamin D status, as measured by serum 25-hydroxyvitamin D (25(OH)D), has been shown in some studies to be inversely associated with gestational diabetes risk. Recently, it has been suggested that maternal smoking status may modify this relationship. We explored the association between 25(OH)D concentration and gestational diabetes and determined if there was an interaction between smoking and 25(OH)D.
Methods
A nested case–control study was conducted in Halifax, Nova Scotia and Quebec City, Quebec. Women were recruited before 20 weeks gestation and 25(OH)D was measured. Cases were women who developed gestational diabetes and controls were frequency matched to cases on study site, gestational age at blood draw, and season and year of blood draw. Logistic regression models estimated adjusted odds ratios (aOR) and 95% confidence intervals (CI). Models were tested for multiplicative and additive interaction, which was estimated by relative excess risk due to interaction (RERI).
Results
The study included 395 gestational diabetes cases and 1925 controls. Women who smoked during pregnancy and had 25(OH)D concentrations <30 nmol/L had an aOR = 3.73 [95% CI 1.95, 7.14] compared to non-smokers with 25(OH)D concentrations ≥50 nmol/L. Additive interaction was detected between smoking status and 25(OH)D [RERI = 2.44, 95% CI 0.03, 4.85].
Conclusion
Our study supports the inverse association of vitamin D status with gestational diabetes risk, particularly among women who smoke during pregnancy. More research is needed to confirm this finding and, if confirmed, to determine the mechanism by which the combined effect of smoking and low vitamin D status increases the risk of developing gestational diabetes.
Keywords: 25-hyroxyvitamin D, pregnancy, gestational diabetes, smoking
Vitamin D is obtained exogenously through diet and endogenously with dermal exposure to ultraviolet B rays from sunlight. In Canada, with most of the population living above the 42nd parallel, much of the population is unable to achieve vitamin D adequacy in winter months from sunlight alone, thereby requiring vitamin D to be obtained from exogenous sources much of the year.1 The most common indicator of vitamin D status is its major circulating form, 25-hydroxyvitamin D (25(OH)D). Consensus has not been achieved regarding criteria of insufficient/sufficient concentrations of 25(OH)D for pregnant women. While the Institute of Medicine does not have specific recommendations for pregnant women, their updated report in 2011, commissioned by the United States and Canada, suggests that achieving 25(OH)D concentrations of at least 30 nmol/L will prevent deficiency with respect to bone health and that 50 nmol/L will ensure sufficiency in most people.2 In contrast, the Canadian Paediatric Society defines optimal as 25(OH)D concentrations of 75–<225 nmol/L for pregnant and lactating women.3 Results of the Canadian Health Measures Survey (2007–09) found that among women of reproductive age, the mean concentration of 25(OH)D was 64.2 nmol/L in winter months, but that only 36% were above 75 nmol/L, the target suggested by the Canadian Paediatric Society.4
Gestational diabetes mellitus (GDM), defined as impaired glucose tolerance with onset or first detection in pregnancy, has important short and long-term consequences for the health of the mother and the fetus.5 Three recent meta-analyses have reviewed the evidence from observational studies looking at vitamin D status and pregnancy outcomes, including GDM.6–8 All of the meta-analyses concluded that a significant inverse association exists between 25(OH) D concentration and GDM, although some individual studies did not observe a significant association. Two large studies published after these meta-analyses did not observe significant inverse associations between 25(OH)D concentration and GDM.9,10 Another recent study suggested that maternal smoking may modify the relationship between 25(OH)D and maternal hyperglycaemia (measured instead of gestational diabetes because of small numbers), such that in smokers a significant inverse association was observed between 25(OH)D concentration and hyperglycaemia, but no association was found in non-smokers.11 A potential mechanism, proposed by Tomadi et al., suggests that vitamin D may work to reduce the risk of hyperglycaemia in smokers, because of increased inflammation, a risk factor for GDM.11 Although these mechanisms are speculative, if interactions between smoking and 25(OH)D are verified, a large public health impact could be realised if it were possible to identify such at-risk women for targeting interventions. For the same purpose, it was of interest to explore potential interaction effects between pre-pregnancy body mass index (BMI) and vitamin D status on GDM risk, although this has not been explored previously.
Improving vitamin D status may offer a simple preventive measure, if the association with GDM is con-firmed. This study was conducted to quantify the association between concentration of 25(OH)D and development of GDM and to determine whether smoking or BMI interacted with this association.
Methods
Study design
We conducted a nested case–control study from two separate cohort studies conducted in Halifax, Nova Scotia and Quebec City, Quebec, which included a total of 9208 pregnant women. In Halifax, recruitment took place between October 2002 and July 2005 at the IWK Health Centre, which is the only hospital in the city providing obstetrical services. Pregnant women who presented to the Blood Collection Services Laboratory at the IWK for routine prenatal blood work were invited to participate, as previously described.12 In Quebec City, recruitment took place from March 2005 to April 2010 and women were recruited at the CHU de Québec, the only hospital centre providing obstetrical services in the Québec metropolitan area, at their first prenatal visit between gestational ages 14–17 weeks or at the dating ultrasound (8–12 weeks gestation).
Participants
In both sites, pregnant women were recruited before 20 weeks’ gestation and asked to provide a blood sample. Blood samples were processed immediately and serum and plasma aliquots were prepared and frozen. At approximately 20 weeks’ gestation, women completed a questionnaire that collected information on demographics, life style factors and past medical history. After delivery, the medical charts were reviewed and information on pregnancy outcomes was collected. The larger nested case–control study was designed to evaluate vitamin D status in relation to several outcomes, including pregnancy loss, GDM, pre-eclampsia, and neonatal outcomes (preterm birth, low birthweight, and small for gestational age).
For this study, singleton, livebirths were included and women with pre-existing diabetes (as ascertained by self-report questionnaire) were excluded. Cases were women who developed GDM, as determined by chart review and diagnosed according to Canadian Diabetes Association guidelines and the Society of Obstetricians and Gynaecologists of Canada.13,14 In both sites, screening for GDM was recommended for all pregnant women, and not based on presence of risk factors.
Controls were frequency matched to women with any of the study outcomes by study site (Halifax or Quebec City), gestational age at the time of blood collection (<10, 10–13, 14–15, 16–17, 18–19 weeks), and season (October–December, January–March, April–September) and year of blood collection. Since women in the other case groups could potentially develop GDM, for this analysis, the control group also included women with the other case conditions (e.g. pre-eclampsia, preterm birth, low birthweight, and small for gestational age), selected at random.
25(OH)D measurement
The samples were stored at their respective sites in −80°C freezers. Concentrations of 25(OH)D are known to be stable after long-term freezing.15,16 The serum samples were shipped on dry ice to McGill University and analysed for total 25(OH)D concentration using an automated chemiluminescence immunoassay (Diasorin Liason, Stillwater, MN, USA). The laboratory follows state of the art quality assurance procedures and is certified by the National Institute for Standards and Technology (NIST) for 25(OH)D testing. An inter-assay coefficient of variation of 5.65% and an intra-assay coefficient of variation of 3.9% was observed at the time of the assays. In the absence of standard criteria for 25(OH) D deficiency, insufficiency, and adequacy, we used the Institute of Medicine recommendations. Levels of 25(OH)D were categorised as <30 nmol/L (deficiency with respect to bone health), 30–<50 nmol/L, and ≥50 nmol/L (sufficiency in most people).
Statistical analysis
Covariates included study site, gestational age at blood sampling, and season and year of blood sample, maternal age, education, pre-pregnancy BMI, marital status, income, parity, smoking during pregnancy, and infant sex. Maternal smoking was converted to a binary variable that was defined as any, or no, smoking during pregnancy. Women who quit smoking during pregnancy were included as smokers during pregnancy. Covariates were compared between GDM cases and controls using chi-square tests. Adjusted logistic regression models were developed to estimate the association between 25(OH)D and GDM and included maternal age as well as the frequency matching variables (study site, gestational week at blood sampling, season, and year of blood sample collection). Additionally, other covariates were tested for inclusion as confounding factors by determining whether they changed the odds ratio (OR) for GDM in any 25(OH)D category by 5% or more. Multiplicative interactions between 25(OH)D and with maternal smoking and with pre-pregnancy BMI were tested by a likelihood ratio test comparing models with and without the interaction term. Additive interaction was assessed using ORs to estimate the relative excess risk due to interaction [RERI and 95% CI] and the attributable proportion due to interaction [AP and 95% CI].17 Statistical analyses were conducted using SAS software package, Version 9.2.
Results
There were 7172 and 2036 eligible women in the Quebec City and Halifax cohorts respectively. Of the 412 women diagnosed with GDM, 395 women had a serum sample adequate for determining 25(OH)D concentration. There were 1925 women included in the control group. Table 1 shows the distribution of covariates according to case–control status. Factors significantly associated with GDM status were education, pre-pregnancy BMI, maternal age, and foetal sex. The median value (and interquartile range) of 25(OH)D was 51.9 nmol/L (40.6–62.4 nmol/L) for controls and 45.5 nmol/L (35.9–56.7 nmol/L) for GDM cases. Of the women with GDM, 12.9% had 25(OH)D concentrations <30 nmol/L compared to 8.6% of the control women. Fifty-four per cent of the control women had 25(OH)D concentrations ≥50 nmol/L compared to 38% of the women with GDM (data not shown).
Table 1.
Study population characteristics by case–control status
| Controls n (%) |
Cases n (%) |
P-value | |
|---|---|---|---|
| Maternal age, years | |||
| <25 | 225 (11.7) | 32 (8.1) | <0.0001 |
| 25–<30 | 726 (37.3) | 117 (29.6) | |
| 30–<35 | 691 (35.9) | 154 (39.0) | |
| ≥35 | 282 (14.7) | 92 (23.3) | |
| Missing (n = 1) | |||
| Pre-pregnancy BMI, kg/m2 | |||
| Underweight or normal (<25) | 1218 (66.2) | 144 (36.9) | <0.0001 |
| Overweight (25–<30) | 370 (20.1) | 102 (26.2) | |
| Obese (≥30) | 251 (13.7) | 144 (36.9) | |
| Missing (n = 91) | |||
| Parity | |||
| Nulliparous | 888 (46.2) | 172 (43.5) | 0.33 |
| Parous | 1034 (53.8) | 223 (56.5) | |
| Missing (n=3) | |||
| Education | |||
| High school or less | 380 (21.7) | 123 (33.6) | <0.0001 |
| Some or all college/trade school | 566 (32.4) | 118 (32.2) | |
| Some or all university degree | 802 (45.9) | 125 (34.2) | |
| Missing (n = 206) | |||
| Household Income ($CAD) | |||
| <40 000 | 374 (22.7) | 82 (24.8) | 0.41 |
| ≥40 000 | 1275 (77.3) | 249 (75.2) | |
| Missing (n = 340) | |||
| Marital status | |||
| Married/partnered | 1648 (91.5) | 340 (92.9) | 0.88 |
| No partner | 153 (8.5) | 26 (7.1) | |
| Missing (n = 153) | |||
| Smoking in pregnancy | |||
| Never or quit before pregnancy | 1456 (80.4) | 279 (76.2) | 0.07 |
| Smoker in pregnancy | 354 (19.6) | 87 (23.7) | |
| Missing (n = 144) | |||
| Infant sex | |||
| Female | 936 (49.0) | 166 (42.5) | 0.02 |
| Male | 975 (51.0) | 225 (57.5) | |
| Missing (n = 18) | |||
Table 2 shows the unadjusted and adjusted ORs for GDM in each category of 25(OH)D relative to women with concentrations ≥50 nmol/L. The adjusted models, though attenuated, still showed significantly increased odds of GDM for both categories of 25(OH)D concentration [aOR = 1.57, 95% CI 1.04, 2.37 for <30 nmol/L and aOR = 1.41, 95% CI 1.09, 1.81 for 30–<50 nmol/L]. Although only 13 (3.3%) GDM cases had 25(OH)D concentrations above 75 nmol/L (compared with 163 (8.5%) in controls), we conducted an analysis to see if the odds were increased in the 50–<75 nmol/L group compared to those with concentrations ≥75 nmol/L recognising the limitations of the small referent group. In this analysis, the aOR for women with concentrations of 50–<75 nmol/L was 1.58 [95% CI 0.86, 2.92] compared to women with concentrations ≥75 nmol/L (data not shown).
Table 2.
Unadjusted and adjusted odds ratios between 25(OH)D concentrations and gestational diabetes
| (25(OH)D) | Controls na (%) |
GDM cases na (%) |
Unadjusted OR (95% CI) |
Adjustedb OR (95% CI) |
|---|---|---|---|---|
| All | ||||
| <30 nmol/L | 146 (7.9) | 48 (12.3) | 2.18 (1.51, 3.15) | 1.57 (1.04, 2.37) |
| 30–<50 | 684 (37.2) | 190 (48.7) | 1.84 (1.46, 2.33) | 1.41 (1.09, 1.81) |
| ≥50 | 1008 (54.8) | 152 (39.0) | 1.00 (Reference) | 1.00 (Reference) |
Numbers represent sample size included in the adjusted model (i.e. women with missing values for covariates are not included in the unadjusted analysis).
Adjusted for maternal age, body mass index, gestational week at blood collection, study site, season, and year of blood collection
Table 3 shows the results from models showing the interactions between BMI and 25(OH)D concentration and the odds of developing GDM. The multiplicative interaction term between 25(OH)D concentration and maternal pre-pregnancy BMI was not statistically significant (P = 0.86). Due to small numbers, BMI was categorised into two groups; overweight and obese combined (BMI ≥25 kg/m2) vs. BMI <25 kg/m2. The aORs for aBMI ≥25 kg/m2 were all statistically significant, irrespective of 25(OH)D concentration. Significant additive interaction was seen in the category of 30–<50 nmol/L [RERI = 1.57, 95% CI 0.14, 2.99], but not in the category with 25(OH)D <30 nmol/L [RERI = 0.36, 95% CI −0.02, 0.71].
Table 3.
Adjusted odds ratios and 95% confidence intervals for development of GDM and RERI and AP for interactions between BMI and 25(OH)D concentration
| BMIa | 25(OH)D category
|
||
|---|---|---|---|
| ≥50 nmol/L | 30–<50 nmol/L | <30 nmol/L | |
| N cases/N controls | N cases/N controls | N cases/N controls | |
| OR (95% CI)b | OR (95% CI)b | OR (95% CI)b | |
| <25 | 73/731 | 58/408 | 13/78 |
| 1.00 (Reference) | 1.31 (0.90, 1.90) | 1.61 (0.83, 3.09) | |
| ≥25 | 79/277 | 132/276 | 35/68 |
| 3.13 (2.19, 4.48) | 5.01 (3.60, 6.96) | 5.80 (3.51, 9.59) | |
| RERI [95% CI] | – | 1.57 (0.14, 2.99) | 2.06 (−0.78, 4.90) |
| AP [95% CI] | – | 0.31 (0.07, 0.55) | 0.36 (−0.02, 0.71) |
P-value for multiplicative interaction = 0.86.
Adjusted for maternal age, body mass index, gestational week of blood collection, study site, season, and year of blood collection.
The multiplicative interaction term between maternal smoking and 25(OH)D concentration was statistically significant (P = 0.04). In non-smokers, 25(OH)D concentration <30 nmol/L was not associated with an increased odds of developing GDM (Table 4). In smokers, however, a significant increased odds of developing GDM was seen among women with 25(OH)D concentration <30 nmol/L [aOR = 3.73, 95% CI 1.9, 7.14] compared to non-smokers with 25(OH)D concentration ≥50 nmol/L. When assessing additive interaction between smoking and 25(OH)D, a significant RERI was observed [2.44, 95% CI 0.03, 4.85] between smoking and 25(OH)D concentration <30 nmol/L, indicating an OR that is 2.4 times higher as a result of the interaction. The corresponding AP was 0.65 indicating that 65% of the combined effect of smoking and low 25(OH)D concentration is due to the interaction effect of these exposures. There was no evidence of significant additive interaction between smoking and 25(OH)D concentrations 30–<50 nmol/L on GDM risk.
Table 4.
Adjusteda odds ratios and 95% confidence intervals for development of GDM and RERI and AP for interactions between maternal smoking and 25(OH)D concentration
| Smoking in pregnancyb | 25(OH)D category
|
||
|---|---|---|---|
| ≥50 nmol/L | 30–<50 nmol/L | <30 nmol/L | |
| N cases/N controls | N cases/N controls | N cases/N controls | |
| OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | |
| No | 117/808 | 134/517 | 27/104 |
| 1.00 (Reference) | 1.34 (1.00, 1.81) | 1.14 (0.66, 1.87) | |
| Yes | 29/163 | 37/137 | 20/35 |
| 1.18 (0.73, 1.89) | 1.48 (0.95, 2.31) | 3.73 (1.95, 7.14) | |
| RERI [95% CI] | – | −0.04 (−0.87, 0.79) | 2.44 (0.03, 4.85) |
| AP [95% CI] | – | −0.03 (−0.59, 0.54) | 0.65 (0.36, 0.94) |
Adjusted for maternal age, body mass index, gestational week of blood collection, study site, season, and year of blood collection.
P-value for multiplicative interaction = 0.04.
Discussion
The results of our study suggest that 25(OH)D concentrations of <50 nmol/L are associated with increased risk of developing GDM. Investigation of the additive interaction between smoking and 25(OH)D concentration suggests that smoking and low 25(OH)D concentration (<30 nmol/L) may act synergistically to increase the risk of developing GDM, with an estimated 65% of the combined effect of smoking and low 25(OH)D due to the interaction between these exposures. As well, the joint OR for smoking and 25(OH)D <30 nmol/L is 3.73 [95% CI 1.95, 7.14) when compared to non-smokers with 25 (OH)D ≥50 nmol/L.
The idea that smoking status could modify the association between 25(OH)D concentration and GDM was initially proposed by Tomedi et al.11 In their study, they were not able to look at GDM per se because of the low prevalence of this condition, but instead evaluated hyperglycaemia. The population studied by Tomedi et al. was unique in that the rates of smoking were very high (45%) and the prevalence of 25(OH)D levels below 50 nmol/L was also high (67%).11 If the finding of a stronger effect of 25(OH)D in smokers is real, differences in the proportion of smokers in study populations could contribute to the inconsistencies that have been observed in the literature with respect to the association between 25(OH)D concentration and GDM. Since the overall effect size is determined by an average effect among smokers and non-smokers combined, the proportion of smokers would have an influence of the magnitude of the ORs estimated in a study, assuming there was a differential effect by smoking status.
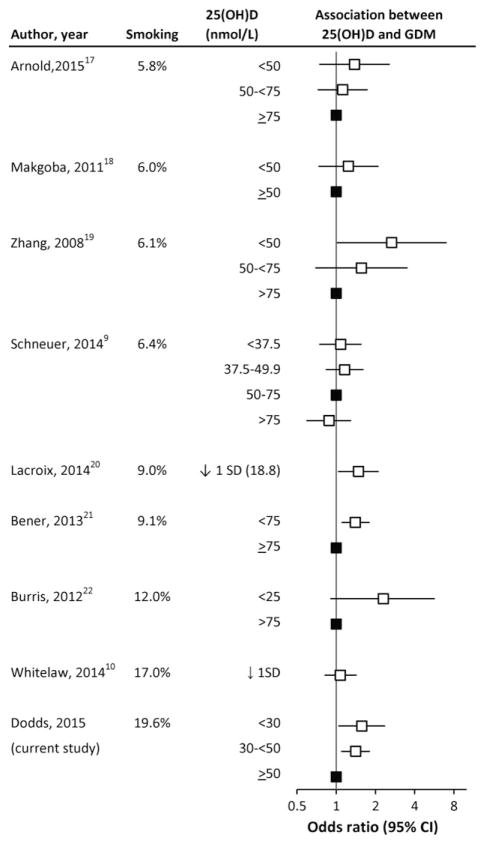
To examine this possibility, Figure 1 shows the results of previous studies ordered by the smoking prevalence in the population being studied (among those studies that reported smoking prevalence).9,10,18–23 As can be seen in the Figure, a slight shift towards a higher OR is seen as smoking prevalence increases. Schneuer et al. conducted a well-powered study with 376 women with gestational diabetes and 5109 controls in Australia where the maternal smoking rate was relatively low (6.4%).9 No effect was observed for 25(OH)D concentration among women within any of the categories (<37.5, 37.5–49.9 and >75 nmol/L) compared to women with concentrations of 50–75 nmol/L.9 Similarly, Makgoba et al. studied women in the United Kingdom where the overall smoking rate was 6%, and found no association between 25(OH)D concentration and GDM.19 However, not all of the studies support the hypothesis of effect modification by smoking. A study from northern England, with a smoking rate of 17%, which is similar to the smoking rate in our cohort, did not find a significant association between GDM and 25(OH)D concentration.10 However, the median 25(OH)D concentration was about 25 nmol/L, which was substantially lower than the median in the present study (50.6 nmol/L). The study by Zhang et al. showed a significant inverse relationship with 25(OH)D concentration in Washington State with a smoking rate of only 6.1% in the controls.20 Although the Figure suggests that the relative risk estimates may differ with prevalence of smoking, we are not able to conclude that smoking causally interacts with the vitamin D and GDM relationship. As well, the potential heterogeneity of the subjects in the studies (such as differences in amount smoked) also make it difficult to provide evidence for, or against, interactions between smoking and 25(OH)D. In our study, we were not able to look at the effect of amount of smoking, because of small numbers in these categories. More precise smoking information might help to reduce the inconsistencies that have been observed between studies.
Figure 1.
The association between 25(OH)D concentration and gestational diabetes ranked by smoking status in study populations. (filled boxes represent the reference group).
There is some rationale for expecting a more pronounced effect of vitamin D status on GDM among smokers than non-smokers. Tomadi et al.11 provided a potential explanation related to oxidative stress and inflammation, which was associated with the development of type 2 diabetes.24 Smokers have increased oxidative stress25 and inflammation26 and since it is thought that vitamin D may have antioxidant27 and anti-inflammatory effects,28 vitamin D status may have a differential effect in smokers and non-smokers. In a recent study of elderly subjects, the association between vitamin D deficiency and c-reactive protein (a biomarker of systemic inflammation) was stronger in smokers compared to non-smokers.29 Since higher c-reactive protein levels are positively associated with markers of insulin resistance and type 2 diabetes,30 the possibility of a differential effect of GDM among smokers for GDM risk is plausible. Although smoking could also be a confounder rather than an interaction variable, in our study, smoking was not a confounder in the relationship between 25(OH)D and GDM, as inclusion of smoking in the model did not change the OR for 25(OH)D by >5%.
While our study cannot infer causality or mechanisms, another possible explanation for the elevated risk of GDM in women who smoked and had low 25(OH)D is altered parathyroid hormone (PTH). Using insulin clamp studies in non-pregnant females, 25(OH)D positively and independently associates with insulin sensitivity, whereas PTH inversely explained insulin sensitivity.31 In pregnancy, higher PTH is independently associated with increased risk of GDM.32 Normally in pregnancy, PTH values decline by the third trimester,33 possibly due to elevations in 1,25(OH)2D from placental and renal origin which would suppress PTH in a negative-feedback loop. It is possible that the degree or timing of decline in PTH or reductions in the activation of 1,25(OH)2D is different among those who smoke. One study of pregnant women suggests that smokers have lower PTH well into the third trimester,34 suggesting that smoking may reduce stimulus for renal 1,25(OH)2D production or that circulating 1,25(OH)2D is elevated above normal and suppresses PTH. However, studies of smokers show that 25(OH)D, 1,25(OH)2D and PTH are all low compared to non-smokers.35 Future studies in pregnancy should include measurement of 25(OH)D along with PTH, 1,25(OH)2D and ionised calcium as well as smoking and thorough dietary assessment to better understand the endocrine aberrations responsible for elevated risk of GDM.
The additive interaction observed with BMI and the middle category of 25(OH)D concentration, but not the lowest category, is more difficult to interpret because of the expected dose–response relationship. The significant ORs for BMI ≥25 kg/m2 across all levels of 25(OH)D suggest that the independent effect of BMI on GDM is important, with a modest added contribution from 25(OH)D concentration.
Our study is among the largest to examine the association between 25(OH)D concentration and GDM. The availability of information on smoking and BMI allowed us to examine both the additive and multiplicative interaction on their effects with 25(OH)D concentration on development of GDM. However, several limitations to our study are noted. We did not have information on diet or supplements, which may be related to smoking. However, since we are using a biomarker of vitamin D status, intake through diet or supplements will be reflected in the 25(OH)D concentrations that we obtained. Although liquid chromatography coupled with mass spectrometry (LC-MS/MS) is often considered the gold standard for assessing vitamin D status, the chemiluminescence immunoassay method is most frequently used in research studies. Of the automated assays, the Diasorin Liaison Autoanalyzer used in this study has been shown to be accurate and precise compared to an established reference standard36 and compared to LC-MS/MS methods.37 At the McGill University laboratory where our samples were assayed, 25(OH)D concentrations were compared for 40 samples using chemiluminescence immunoassay and LC-MS/MS methods. The mean difference was 3 nmol/L (higher when using the chemiluminescence immunoassay method), suggesting that the results using this method are valid compared to the LC-MS/MS method. As well, the inter-assay coefficient of variation of 5.65% and the intra-assay coefficient of variation of 3.9% observed in our data were within acceptable limits. Ethnicity information was collected only for the Quebec City cohort and >90% of the women reported being Caucasian. Although ethnicity was not collected for the Halifax cohort, census data suggest that about 93% of the Halifax population was Caucasian during the years of recruitment. Therefore, the results of this study are more applicable to a Caucasian population than other ethnicities.
Our study supports the suggestion that vitamin D status is inversely associated with gestational diabetes among women who smoke during pregnancy, but not in non-smokers. The OR for women who smoke with a 25(OH)D concentration of <30 nmol/L have a 3.7 increased odds of developing GDM compared to the non-smokers with 25(OH)D concentrations of ≥50 nmol/L. The RERI suggests that the odds of GDM in this group are 2.4 times higher as a result of the interaction between smoking and 25(OH)D concentration <30 nmol/L. New guidelines for the diagnosis of GDM have been adopted by the International Association of Diabetes and Pregnancy Study Groups38 and the Canadian Diabetes Association39 and will increase the prevalence, because of the relaxed criteria for diagnosis. Studies of 25(OH)D, to date, have not used this new definition of GDM.
Despite the challenges in drawing causal conclusions from observational studies and the conflicting results, the potential for a low-risk, low-cost intervention for prevention of GDM is appealing. Establishment of a causal relationship between 25(OH)D concentration and GDM will need to be based on well-conducted randomised controlled trials. Confirmation of the effect of 25(OH)D concentration by smoking status is important, before randomised controlled trials are conducted, to determine if they should be stratified by smoking status. Results of these randomised controlled trials can then be used to support the development of public health guidelines for screening or for supplementation.
Acknowledgments
We thank Nathalie Bernard, Myléne Badeau for their professional assistance with the project, and our research nurses for the recruitment of participants and retrieval of data from the medical records. We thank Madonna Achkar and Sherry Agellon for conducting the 25(OH)D assays. We also thank all study participants. This study was funded by the Canadian Institutes of Health Research (Operating Grant 244113).
References
- 1.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. The Journal of Clinical Endocrinology and Metabolism. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 2.Ross AC, Taylor CL, Yatkine AL, Del Valle HB Committee to Review Dietary Reference Intakes for Vtiamin D and Calcium Food and Nutrition Board, Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Canadian Pediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatrics & Child Health. 2007;12:583–589. [PMC free article] [PubMed] [Google Scholar]
- 4.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whitting S. Vitamin D status of Canadians as measured in the 2007–2009 Canadian Health Measures Survey. Health Reports. 2010;21:47–55. [PubMed] [Google Scholar]
- 5.Joergensen JS, Lamont RF, Torloni MR. Vitamin D and gestational diabetes: an update. Current Opinion in Clinical Nutrition and Metabolic Care. 2014;17:360–367. doi: 10.1097/MCO.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 6.Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. European Journal of Internal Medicine. 2012;23:465–469. doi: 10.1016/j.ejim.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. Journal of Maternal-Fetal and Neonatal Medicine. 2013;26:889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 8.Aghajafari F, Naqulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. British Medical Journal. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 9.Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in first trimester on subsequent pregnancy outcomes in an Australian population. The American Journal of Clinical Nutrition. 2014;99:287–295. doi: 10.3945/ajcn.113.065672. [DOI] [PubMed] [Google Scholar]
- 10.Whitelaw DC, Scally AJ, Tuffnell DJ, Davies TJ, Fraser WD, Bhopal RS, et al. Association of circulating calcium and 25-hydroxyvitamin D with glucose metabolism in pregnancy: a cross-sectional study in European and South Asian women. The Journal of Clinical Endocrinology and Metabolism. 2014;99:938–946. doi: 10.1210/jc.2013-2896. [DOI] [PubMed] [Google Scholar]
- 11.Tomedi LE, Simhan HN, Bodnar LM. Early-pregnancy maternal vitamin D status and maternal hyperglycemia. Diabetic Medicine: A Journal of the British Diabetic Association. 2013;30:1033–1039. doi: 10.1111/dme.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodds L, Fell DB, Dooley KC, Armson BA, Allen AC, Nassar BA, et al. Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clinical Chemistry. 2008;54:326–334. doi: 10.1373/clinchem.2007.097469. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Canadian Journal of Diabetes. 2008;32(Suppl 1):S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Berger H, Crane J, Farine D, Armson A, De La Ronde S, Keenan-Lindsay L, et al. Screening for gestational diabetes mellitus. Journal of Obstetrics and Gynaecology Canada. 2002;24:894–912. doi: 10.1016/s1701-2163(16)31047-7. [DOI] [PubMed] [Google Scholar]
- 15.Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutrition and Cancer. 2010;62:51–57. doi: 10.1080/01635580903191460. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JG, Elder PA. Serum 25-OH vitamin D2 and D3 are stable under exaggerated conditions. Clinical Chemistry. 2008;54:1931–1932. doi: 10.1373/clinchem.2008.111526. [DOI] [PubMed] [Google Scholar]
- 17.VanderWeele TJ, Knol MJ. A tutorial on interaction. [last accessed October 9, 2015];Epidemiol Methods. 2014 3:33–72. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/603/2013/03/InteractionTutorial.pdf. [Google Scholar]
- 18.Arnold DL, Enquobahrie DA, Qiu C, Huang J, Grote N, VanderStoep A, et al. Early pregnancy maternal vitamin D concentrations and risk of gestational diabetes mellitus. Paediatric and Perinatal Epidemiology. 2015;29:200–210. doi: 10.1111/ppe.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makgoba M, Nelson SM, Savvidou M, Messow CM, Nicolaides K, Sattar N. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care. 2011;34:1091–1093. doi: 10.2337/dc10-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS ONE. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacroix M, Battista MC, Doyon M, Houde G, Ménard J, Ardilouze JL, et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetologica. 2014;51:609–616. doi: 10.1007/s00592-014-0564-4. [DOI] [PubMed] [Google Scholar]
- 22.Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcomes: global comparisons. International Journal of Women’s Health. 2013;5:523–531. doi: 10.2147/IJWH.S51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burris HH, Rifas-Shiman SL, Kleinman K, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Vitamin D deficience in pregnancy and gestational diabetes mellitus. American Journal of Obstetrics and Gynecology. 2012;207:182e1–8. doi: 10.1016/j.ajog.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradham AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 25.Isik B, Ceylan A, Isik R. Oxidative stress in smokers and non-smokers. Inhalation Toxicology. 2007;19:767–769. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- 26.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35:1002–1010. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shab-Bidar S, Neyestani TR, Djazayery A. The ineractive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. European Journal of Clinical Nutrition. 2015;69:216–222. doi: 10.1038/ejcn.2014.240. [DOI] [PubMed] [Google Scholar]
- 28.Hendryx M, Luo J. A test of vitamin D benefits on respiratory health mediated through inflammatory markers. Chronic Respiraotry Disease. 2015;12:24–30. doi: 10.1177/1479972314556086. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Kim KN, Lim YH, Hong YC. Interactions of vitamin D and smoking on inflammation markers in the urban elderly. Journal of Preventive Medicine and Public Health. 2015;48:249–256. doi: 10.3961/jpmph.15.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. The American Journal of Clinical Nutrition. 2010;92:1344–1349. doi: 10.3945/ajcn.110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Connelly PW, Sermer M, et al. Vitamin D and parathyroid hormone status in pregnancy: effect on insulin sensitivity, β-cell function, and gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 2014;99:4506–4513. doi: 10.1210/jc.2014-2341. [DOI] [PubMed] [Google Scholar]
- 33.Møller UK, Streym S, Mosekilde L, Heickendorff L, Flyvbjerg A, Frystyk J, et al. Changes in calcitropic hormones, bone markers and insulin-like growth factor I (IGF-I) during pegnancy and postpartum: a controlled cohort study. Osteoporosis International. 2013;24:1307–1320. doi: 10.1007/s00198-012-2062-2. [DOI] [PubMed] [Google Scholar]
- 34.Díaz-Gómez N, Mendoza C, González-González NL, Barroso F, Jimánz-Sosa A, Domenech E, et al. Maternal smoking and the vitamin D-parathyroid hormone system during the perinatal period. Journal of Pediatrics. 2007;151:618–623. doi: 10.1016/j.jpeds.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Brot C, Rye Jørgensen N, Helmer Sørensen O. The influence of smoking on vitamin D status and calcium metabolism. European Journal of Clinical Nutrition. 1999;53:920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 36.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clinical Biochemistry. 2009;42:1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clinical Chemistry. 2012;58:531–542. doi: 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- 38.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Thompson D, Berger H, Feig D, Gagnon R, Kader T, et al. Diabetes and pregnancy. Canadian Journal of Diabetes. 2013;37(Suppl 1):S168–S183. doi: 10.1016/j.jcjd.2013.01.044. [DOI] [PubMed] [Google Scholar]