Abstract
Neuropathic pain, the most debilitating of all clinical pain syndromes, may be a consequence of trauma, infection or pathology from diseases that affect peripheral nerves. Here we provide a framework for understanding the spinal mechanisms of neuropathic pain as distinct from those of acute pain or inflammatory pain. Recent work suggests that a specific microglia response phenotype characterized by de novo expression of the purinergic receptor P2X4 is critical for the pathogenesis of pain hypersensitivity caused by injury to peripheral nerves. Stimulating P2X4 receptors initiates a core pain signaling pathway mediated by release of brain-derived neurotrophic factor, which produces a disinhibitory increase in intracellular chloride in nociceptive (pain-transmitting) neurons in the spinal dorsal horn. The changes caused by signaling from P2X4R+ microglia to nociceptive transmission neurons may account for the main symptoms of neuropathic pain in humans, and they point to specific interventions to alleviate this debilitating condition.
Acute pain is an evolutionary protective mechanism. We reflexively withdraw from painful stimuli to protect ourselves from further damage. We do inevitably damage ourselves throughout life, and tissue damage initiates inflammation and consequent inflammatory pain hypersensitivity. Again this can be considered ‘good’ pain in that we instinctively protect and guard the painful area from potential further damage. The insistent inflammatory pain is a constant nagging reminder to be careful and allow the damaged tissue to heal. Inflammatory pain may be chronic, depending on the damage or disease state, but normally subsides with the resolution and healing of the damage or disease. However, chronic pain can also be induced by explicit nerve damage: neuropathic pain. In contrast to the beneficial role of acute pain, neuropathic pain can persist long after the initial injury has healed and offers no advantage to the sufferer. On the contrary, neuropathic pain can become devastatingly disabling for the individual, and it exists and persists with such a high prevalence in the community as to be a major socioeconomic problem.
Extensive and converging lines of research have made it clear that chronic pain is not simply acute pain that lasts a long time. Rather, the underlying neurobiological mechanisms of the two are fundamentally different: acute pain is a physiological function of the normal nervous system, whereas chronic pain, in particular chronic neuropathic pain, is the manifestation of pathological alterations to the peripheral and central nervous systems. Acute pain arises from activation of nociceptive neurons in the periphery with information processed in the nociceptive networks of the dorsal horn of the spinal cord. This complex spinal circuitry consists of opposing excitatory and inhibitory influences that effectively filter the input before transmitting information to the brain, where it is disseminated to the sensory, emotional, autonomic and motor centers to produce the nociceptive brain network activity we experience as pain. In the case of neuropathic pain, there is a change in the balancing excitatory and inhibitory influences within the spinal cord nociceptive network that may underlie the three cardinal symptoms of neuropathic pain: hyperalgesia, allodynia, and spontaneous pain. Responses to stimuli that normally produce pain are pathologically amplified, causing hyperalgesia, and innocuous sensory inputs that normally do not evoke an output of the spinal nociceptive network are pathologically transformed, causing aberrant activation of this network leading to allodynia and spontaneous pain.
The transmission pathways from the periphery to the brain nociceptive network are neuronal ones. Peripheral terminals of primary sensory nociceptors are activated by adequate noxious stimuli leading to encoded action potential input to the spinal cord. Neurons in the spinal cord that project to the brain transmit action potentials representing the processed sensory input. And likewise, encoded information is transmitted throughout the brain nociceptive network. Consequently, neuron-to-neuron signaling and transmission has long been considered the sole site of the pathological amplification and transformation of nociceptive input underlying chronic pain. However, it is now clear that within the spinal cord local glia-neuron interactions are key to establishing and maintaining chronic neuropathic pain states, and in particular it is the influence of microglia that is critical. Microglia are well known as the principal immune cells of the CNS and respond to pathological insults by adopting a reactive state1. There are several potential responsive phenotypes, reflecting the functional diversity of reactive microglia to diverse pathologies, such that they can mediate both neuroprotective and neuroinflammatory effects. Here we argue for a specific functional state of microglia in the spinal dorsal horn as critical for neuropathic pain, and we develop a framework describing the key microglia-neuron interactions. We focus on a microglial phenotype characterized by de novo expression of the purinergic P2X4 receptor, as there are now converging lines of evidence that this P2X4R+ state is essential for pain hypersensitivity following peripheral nerve damage2–4.
Spinal microglia respond to peripheral nervous system damage
Microglia in the dorsal horn of the spinal cord (Fig. 1), as elsewhere in the CNS, exist in a lattice-like pattern of adjacent, non-overlapping microdomains, ideal for a surveillance function keeping vigilant for disruptions in CNS homeostasis. How this network of microglia interacts and modulates the cellular circuitry of the CNS is a key factor in understanding the mechanisms underlying the pathophysiology of neuropathic pain. Microglia adopt a stimulus modality–dependent and appropriate phenotype in response to injury or disease. Their phenotypic catalog includes proliferative, migrational and phagocytic responses, although how much these phenotypes are distinct, with discrete molecular fingerprints, is not clear1. The phenomenon of reactive microglia was first described by Nissl in 1899, who coined the term “rod cells” for a population of cells supposed to be responsive to various brain pathologies5. The link between this phenotypic change and response to damage to a peripheral nerve was shown by Cammermeyer in 1965, who noted perineuronal microglial proliferation around axotomized motor neurons following facial nerve transection6. Microglia proliferation around the central endings of primary sensory afferents was first reported for the projections to the dorsal horn of the spinal cord and gracile nucleus of the medulla following injury to the sciatic nerve7. It is tempting to consider the response of the microglia as a being a consequence of a transganglionic degenerative response8–10, an example of microglia as “sensors of pathology”11. However, the degree of microglia response is markedly less after dorsal rhizotomy than after lesions distal to the dorsal root ganglia12. Thus, the changes of microglia in the somatosensory area of the spinal cord are active responses at a distance from the site of neuronal injury rather than simply the response to direct neuronal damage or degeneration.
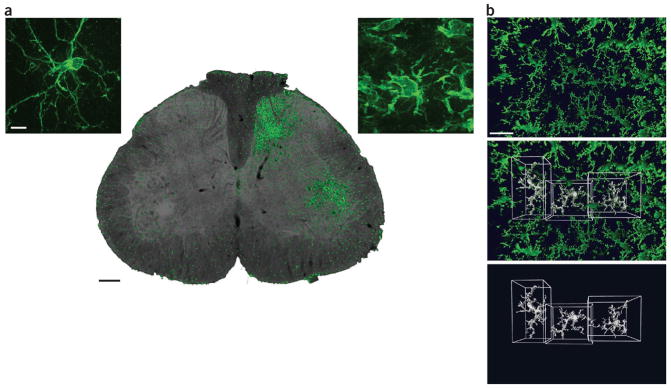
Figure 1.
Microglia; microdomains and proliferation after nerve injury. (a) Transverse section of the lumbar spinal cord showing the characteristic proliferation of microglia (immunostained with iba1, green) around the central terminals of primary afferents whose peripheral axons have been severed (right side). Scale bar, 100 μm. Insets: confocal images showing the characteristic morphology in the absence of peripheral nerve injury (left) and the morphological change that occurs after injury (right). Scale bar, 10 μm. (b) In the normal brain and spinal cord, microglia have a grid-like distribution. Two-photon microscopy image (top) shows the distribution in vivo of microglia in the parenchyma of the CNS of a CX3CR1GFP mouse (in which the Cx3cr1 gene, expressed specifically in microglia, has been replaced with a gene encoding green fluorescent protein). Three microglia have been surface-rendered using Volocity software (PerkinElmer) to show the adjacent, non-overlapping microdomains of individual cells (bottom); center, overlay. Scale bar, 30 μm.
Microglial proliferation, and also the stereotyped changes in morphology and in the repertoire of genes expressed, are reliably induced by damage to peripheral sensory nerves in rodent models regardless of the type of injury13. The microglial responses to peripheral nerve injury are induced so consistently that all schemes for pain hypersensitivity after nerve injury envisage some requisite degree of spinal microglial reactivity13,14. In contrast, these are typically not observed in inflammatory and chemotoxic models of pain15–17, although they have been seen by some investigators. Thus, we focus here on pain hypersensitivity after nerve injury, although some considerations may extend to other chronic pain conditions.
Microglial P2X4 receptors in pain from nerve injury
The response of microglia to peripheral nerve injury was largely ignored by most investigating pain mechanisms and at best was considered an epiphenomenon unrelated to the neuronal and behavioral sequelae of nerve injury. The initial observations that identified P2X4 receptors (P2X4Rs) as a critical molecular element underlying neuropathic pain were that blocking P2X4R function pharmacologically or suppressing its expression with antisense RNA transiently reverses pain behaviors, indicating that ongoing activation of P2X4Rs is required to maintain nerve injury–induced pain hypersensitivity2. The development of mechanical allodynia correlates temporally with an increase in spinal P2X4R expression, with the unexpected twist that this increase is confined to microglia in the ipsilateral spinal dorsal horn. Moreover, building on these initial findings, subsequent studies in P2X4R null mutant mice reported a dramatic reduction in pain behaviors after peripheral nerve injury3,18.
These findings on the one hand argue for the necessity of P2X4Rs as an active component in neuropathic pain. On the other hand, sufficiency of microglial P2X4Rs in producing neuropathic pain was definitively shown by adoptive microglia transfer experiments in which P2X4R-stimulated microglia injected intrathecally into naive rats elicit tactile allodynia similar to that seen in neuropathic rats2. This pharmacological, genetic and behavioral battery of findings provides converging lines of evidence that activity of P2X4Rs on spinal microglia is both necessary to produce the behavioral manifestations of neuropathic pain arising from peripheral nerve injury and sufficient to convert the response to normally non-painful peripheral inputs from innocuous to nociceptive. These findings are thus the basis for the concept that the microglial phenotype characterized by dramatic upregulation of P2X4Rs—the P2X4R+ state—is critical in the etiology of neuropathic pain (Fig. 2).
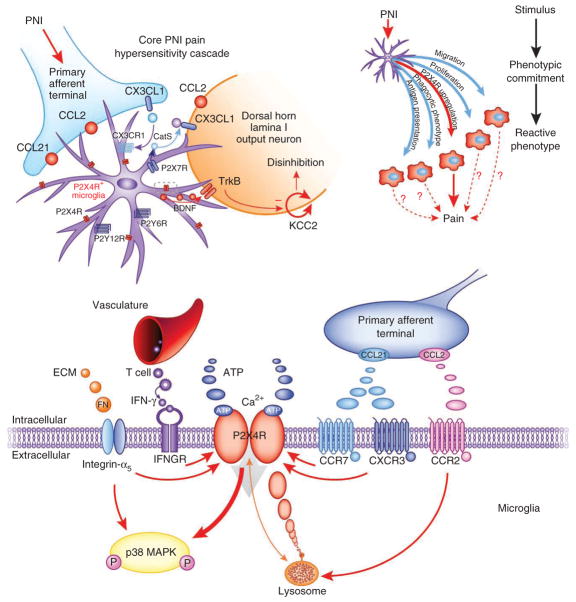
Figure 2.
The P2X4R+ microglial phenotype mediates a core pain hypersensitivity cascade following peripheral nerve injury. Chronic pain due to peripheral nerve injury is caused by amplification of input from the damaged nerve (blue) by the nociceptive network in the spinal cord. Neuronglia interactions in the spinal cord contribute to the resulting enhanced output of this network. Peripheral axotomy or nerve damage (PNI) stimulates changes in spinal microglia (purple) such that they adopt a phenotype characterized by increased expression of P2X4Rs (top left). CCL2, chemokine (C-C motif) ligand 2; CX3CL1, chemokine (C-X3-C motif) ligand 1; P2X7R, P2X purinoceptor 7; P2Y12R, P2Y purinoceptor 12; P2Y6R, P2Y purinoceptor 6; CatS, cathepsin S. The dotted rectangle is expanded in the bottom panel. This is one of several reactive phenotypes that microglia can adopt under pathological conditions (top right). Whether other phenotypes contribute to the induction and maintenance of neuropathic pain is not fully understood. Peripheral nerve injury modulates P2X4R expression by the release of various factors from the extracellular matrix, infiltrating T cells and injured primary afferents (bottom). P2X4Rs are localized predominantly to lysosomes and are trafficked to the cell surface (orange arrow). P2X4R activation by ATP leads to calcium inflow, phosphorylation (P) of p38 MAPK, and p38-mediated synthesis and release of BDNF from the microglia. BDNF then binds neuronal TrkB receptors in the spinal nociceptive network and in turn causes a downregulation of the potassium–chloride cotransporter KCC2, disturbing the chloride homeostasis of the neuron. The outcome is a net disinhibition of the intrinsic inhibitory system in the dorsal horn. ECM, extracellular matrix; IFN, interferon; IFNGR, interferon-γ receptor; CXCR3, chemokine (C-X-C motif) receptor 3; CCR7, C-C chemokine receptor type 7. Red arrows indicate increased expression and activation.
What drives conversion of microglia to the P2X4R+ state?
Given the body of evidence that upregulation of P2X4Rs by spinal microglia is key for pain hypersensitivity after nerve injury, a central issue has been to determine the cellular and molecular mechanisms mediating the switch to the P2X4R+ state. As the changes in microglia in the spinal dorsal horn are responses to distant nerve injury, there must be a series of signaling steps culminating in a direct or indirect effect of the injured primary afferents on the surveillance mode microglia. Although action potential discharge evoked by the nerve injury appears critical, mere enhancement of sensory neuron activity is not sufficient, as discharge in response to peripheral inflammation does not evoke the P2X4R expression. Thus, the damaged afferents may release signaling molecules that upregulate P2X4R expression.
Signaling molecules that have been implicated include the chemokine CCL2 (also known as monocyte chemoattractant protein 1, MCP-1), whose cognate receptor, CCR2, is present on microglia19,20. Activation of CCR2 promotes cell surface expression of P2X4Rs21. CCL21 is another neuronally released chemokine critical for nerve injury–induced upregulation of P2X4Rs22–24. The cytokine interferon-γ has been shown to transform quiescent spinal microglia into a P2X4R-expressing phenotype25, and P2X4R expression levels are regulated by the extracellular matrix molecule fibronectin26–28. Lyn kinase signaling is the intracellular mediator modulating the transcriptional and post-transcriptional levels of microglial P2X4R expression29. Other non-neuronal cells can also be involved: mast cells modulate P2X4R expression through the release of the protease tryptase, activating proteinase-activated receptor 2 (PAR2) in microglia30. Thus, a number of signaling molecules are implicated in converting microglia from surveillance mode to the P2X4R+ state, and a key step for the future will be determining whether the signaling is best understood as a linear pathway or an interconnected network.
BDNF from P2X4R+ microglia disinhibits pain output neurons
Because it is long-range neuronal signaling through nociceptive output neurons to the brain that ultimately spans the anatomical and perceptual bridge from nociception to pain, it was clear from the initial discovery of the role of P2X4R+ microglia that these cells must signal to neurons in the nociceptive network in the spinal cord. At that time, it had been found that nociceptive signaling in the spinal cord after nerve injury is enhanced by weakening of GABAA receptor– and glycine receptor–mediated inhibition31. This disinhibition is mediated by a rise in the intracellular concentration of chloride through downregulation of the principal neuronal potassium–chloride cotransporter KCC2 (refs. 31,32). The rise in intracellular chloride concentration is sufficient to suppress inhibition in the majority of neurons, but in a minority of neurons the rise is large enough to switch GABAA and glycine receptor responses from inhibition to excitation. Nevertheless, overall the net effect of GABAA and glycine receptor activation remains inhibition32, with sufficient inhibition remaining to explain the anti-allodynic effects of GABAA agonists in neuropathic pain33.
Intrathecal administration of P2X4R-stimulated microglia to naive, uninjured rats mimics the nerve injury–induced depolarizing shift in anion reversal potential in spinal lamina I neurons. Three lines of evidence established the action of BDNF in producing this disinhibition: first, direct application of BDNF to the spinal cord produces mechanical allodynia and depolarizes the anion reversal potential in naive rats. Second, blocking BDNF signaling through its cognate receptor TrkB prevents pain evoked by intrathecally administered P2X4R-stimulated microglia and pain following peripheral nerve injury. Third, and key, targeted knockdown of microglial BDNF expression by short interfering RNA abolishes both the disinhibitory effects of intrathecally administered ATP-stimulated microglia and consequent pain behaviors. Taken together, these data provide compelling evidence that BDNF from microglia is a critical signaling molecule mediating the central pathophysiological effects of peripheral nerve injury. Consistent with these observations, P2X4R-deficient mice have impaired microglial BDNF release and altered BDNF signaling in the spinal cord in vivo3. The intracellular signaling by which stimulating P2X4Rs drives synthesis and release of BDNF has now been elucidated4.
Disinhibition transforms input-output of ‘pain’ neurons
The neurons that are disinhibited by nerve injury, or by BDNF from P2X4R+ microglia, are in lamina I of the dorsal horn. Normally lamina I neurons respond to noxious peripheral stimuli, and thus hyperalgesia can be readily explained by an increase in responses of nociceptive relay neurons such that the same nociceptive input generates a larger action potential output. However, as the majority of lamina I neurons respond to noxious input only, a quantitative change in nociceptive responsiveness is insufficient to explain allodynia. Rather, allodynia implies a qualitative change in processing, a miscoding of information such that innocuous inputs are converted to a nociceptive message. Similarly, spontaneous pain requires ongoing or episodic activity in nociceptive pathways, and lamina I nociceptive output neurons have little or no spontaneous activity. After peripheral nerve injury, however, the output of lamina I neurons is transformed such that they become able to relay innocuous mechanical input, increase discharge in response to a noxious stimulus and display spontaneous activity34,35.
Disrupting Cl− homeostasis acutely in vivo produces a switch in the phenotype of lamina I projection neurons indistinguishable from that caused by nerve injury by unmasking tactile afferent input to the previously nociception-specific circuitry35. P2X4R-stimulated microglia applied to the spinal cord produce the same effect35. Thus, we infer that induction of the P2X4R+ state of spinal microglia and the disinhibition resulting from the downregulation of KCC2 mediated by BDNF and TrkB produces the cellular and nociceptive network alterations necessary to account for the three cardinal symptoms of neuropathic pain in humans: mechanical allodynia, hyperalgesia and spontaneous pain.
What is the source of ATP that activates P2X4Rs?
The finding that mechanical hypersensitivity is reversed rapidly by pharmacological blockade of P2X4Rs implies that neuropathic pain hypersensitivity depends on ongoing signaling through these receptors. After peripheral nerve injury, ATP in the cerebrospinal fluid is not elevated2. Whether the lack of change in cerebrospinal fluid ATP reflects a lack of change of extracellular ATP locally within the dorsal horn is unknown. But the very low basal P2X4R expression would not be expected to drive the signaling necessary to produce mechanical hypersensitivity. Thus, the most parsimonious explanation is that pain hypersensitivity following nerve injury depends on the markedly enhanced expression of P2X4Rs, which are then activated by the constitutive level of the endogenous ligand ATP.
In the dorsal horn, ATP may be released from many types of cell, including terminals of primary sensory neurons36, dorsal horn neurons and astrocytes37. Recently, it has been found that pain hypersensitivity is unaltered in mice in which astrocytic release of ATP is blocked by expression of a dominant-negative SNARE (soluble NSF attachment protein receptor) protein38. Thus astrocytes can be eliminated, but the source of ATP that activates P2X4Rs remains to be determined. Release of ATP from the central terminals of large-diameter, myelinated primary sensory neurons39 could account for P2X4R-dependent p38 MAP kinase (mitogen-activated protein kinase) activation in microglia. Certainly activity in large myelinated fibers is important for these changes, as only a full local anesthetic blockade of the peripheral nerve before injury is sufficient to prevent subsequent spinal microglial proliferation and p38 MAP kinase activation, whereas selectively blocking small-diameter, unmyelinated nociceptive fibers is ineffectual40. Although still unproven, involvement of large-diameter sensory afferents would also account for the nerve injury–evoked responses of microglia in the gracile nucleus, as well as in the dorsal horn, both sites of termination of large-diameter fibers.
Microglial ‘markers’, proliferation and pain
We focus here on the P2X4R+ response state of dorsal horn microglia because of the marked upregulation in the expression of these receptors after peripheral nerve injury and the many lines of evidence indicating that these receptors are necessary and sufficient for inducing pain hypersensitivity. However, molecules other than P2X4Rs are commonly used as markers of one or more reactive phenotypes of microglia. These markers are often iba1 or CD11b (also known as CR3), specifically expressed on innate immune cells. Iba1 and CD11b increase in the spinal cord after peripheral nerve injury, but whether the increase is necessary for microglia to be able participate in pain hypersensitivity is not known, having never been tested directly. By contrast, it is clear that the increased expression of iba1 and CD11b is not sufficient to produce pain hypersensitivity: for example, peripheral nerve injury in P2X4R-null (P2rx4−/−) mice results in a robust increase in iba1 and CD11b in the spinal cord, indistinguishable from that in the wild type, but the P2X4R-null mice exhibit no pain hypersensitivity3. Similarly, expression of these proteins is robustly increased by nerve injury in mice lacking the matabotropic P2Y12 receptor (P2ry12−/− mice), but they lack pain hypersensitivity and show no increase in P2X4Rs41. Determining whether marker proteins, such as iba1, CD11b or others, are necessary in the signaling cascades for pain hypersensitivity is key. If so, then assessment of such proteins may be justified as surrogate endpoints. But if not, then it will be critical to assess microglial responses in terms of elements that are known to be involved in pain hypersensitivity, such as P2X4Rs.
By similar reasoning, the function of the proliferative response of microglia must be questioned. This response might be a distinct microglial phenotype that marks the initial response of the CNS to peripheral injury, which is presumably initiated with an afferent barrage and associated release of various factors and culminates in a phagocytic activity to remove axonal debris. But whether microglia proliferation per se is necessary or sufficient for pain hypersensitivity remains to be determined. Without knowing this, microglia proliferation should not be considered a proxy for pain.
Therapeutic targeting of P2X4R+ state–dependent pathway
It is being increasingly recognized that discrete response states for microglia, such as the P2X4R+ state, may have specific functions in pathological processes, and presumably in physiological ones as well11,42. Nevertheless, the now passé concept of microglia being either ‘at rest’ or ‘activated’ persists, in no small part owing to the relative ease of measuring expression of microglial ‘markers’, assessing morphology and counting cell numbers. However, we would caution against developing therapeutic strategies that rely on microglial markers, morphology or proliferation without definitive evidence that any of these cause the disease process being addressed. Undertaking such a ‘microglia inhibitor’ strategy would seem to be perilous especially as contradictory roles of microglia—both protective and pathological—have been demonstrated in neuroinflammatory responses to CNS injury43,44. A case in point may be the recent failure of propentofylline, purported to have nonspecific glial modulating properties, to reduce pain intensity in postherpetic neuralgia45,46.
A further level of complexity is emerging within the microglial involvement in neuropathic pain, with evidence for sex differences47 and priming effects of early injury on subsequent adult pain responses48. Furthermore, genetic variability in microglial responses to nerve injury has been observed between inbred mouse strains49, raising the possibility that differences in microglial responses may contribute to interindividual variability in neuropathic pain in humans.
Rather than attempting to inhibit microglia nonspecifically, we propose that a rational approach is to target the core signaling pathway using the framework described above. Even with this relatively simple pathway, there are several potential approaches: (i) suppressing P2X4R expression or function (regardless of the effect on microglial shape, proliferation or expression of iba1 or CD11b), (ii) blocking transcriptional upregulation or release of BDNF, (iii) blocking TrkB and signaling to downregulate KCC2 or (iv) directly upregulating KCC2 expression or function. Additionally, given that GABAA channels are permeable to HCO3- as well as to Cl-, one may overcome the rise in Cl− by lower HCO3-through inhibiting carbonic anhydrase50.
The P2X4R+ state and the microglia-neuronal signaling framework represents the unification of a body of evidence demonstrating sufficiency and necessity in the initiation and expression of pain hypersensitivity following peripheral nerve injury. Although this response state is characterized by de novo expression of P2X4Rs and BDNF, other microglial effectors have been implicated, and it is therefore necessary to determine whether they fit into this schema or whether additional microglial pathways are also critical for maintaining behavioral sensitivity. Neuropathic pain is necessarily a result of explicit nerve damage and can occur as a consequence of traumatic injury to peripheral nerves or the spinal cord, from disease states such as diabetic neuropathy or postherpetic neuralgia, or even from treatment regimens such as chemotherapy and morphine. Elucidation of the core pathway by which nerve injury evokes ATP, microglial and BDNF-dependent changes in spinal cord responsiveness following experimental neuropathy opens the possibility that this signaling cascade, or its components, may be a common mechanism underlying pathological pain states in addition to neuropathic pain.
Acknowledgments
The work of the authors is supported by grants from the Canadian Institutes of Health Research (MT-11219), the Krembil Foundation and the Ontario Research Fund Research Excellence Program. M.W.S. is supported by a Canada Research Chair (Tier I) in Neuroplasticity and Pain, and the Anne and Max Tanenbaum Chair in Molecular Medicine at the Hospital for Sick Children. T.T. was supported by a Canadian Institutes of Health Research fellowship.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 3.Ulmann L, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissl F. Ueber einige Beziehungen zur Nervenzellenerkrankungen und gliosen Erscheinungen bei verschiedenen Psychosen. Arch Psychiatr. 1899;32:656–676. [Google Scholar]
- 6.Cammermeyer J. Juxtavascular karyokinesis and microglia cell proliferation during retrograde reaction in the mouse facial nucleus. Ergeb Anat Entwicklungsgesch. 1965;38:1–22. [PubMed] [Google Scholar]
- 7.Gehrmann J, Monaco S, Kreutzberg GW. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor Neurol Neurosci. 1991;2:181–198. doi: 10.3233/RNN-1991-245605. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson NP, et al. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res. 1993;96:19–27. doi: 10.1007/BF00230435. [DOI] [PubMed] [Google Scholar]
- 9.Graeber MB, Tetzlaff W, Streit WJ, Kreutzberg GW. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988;85:317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- 10.Svensson M, Eriksson P, Persson J, Liu L, Aldskogius H. Functional properties of microglia following peripheral nerve injury. Neuropathol Appl Neurobiol. 1994;20:185–187. [PubMed] [Google Scholar]
- 11.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Persson JK, Svensson M, Aldskogius H. Glial cell responses, complement, and clusterin in the central nervous system following dorsal root transection. Glia. 1998;23:221–238. [PubMed] [Google Scholar]
- 13.Rezaie P, Male D. Mesoglia & microglia—a historical review of the concept of mononuclear phagocytes within the central nervous system. J Hist Neurosci. 2002;11:325–374. doi: 10.1076/jhin.11.4.325.8531. [DOI] [PubMed] [Google Scholar]
- 14.Penfield W. Cytology & Cellular Pathology of the Nervous System. Hafner; 1965. [Google Scholar]
- 15.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Honore P, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 17.Lin T, et al. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192:40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda M, et al. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbadie C, et al. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyomitsu E, et al. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012;8:301–310. doi: 10.1007/s11302-011-9288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biber K, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30:1864–1873. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong EK, et al. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jong EK, et al. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. FASEB J. 2008;22:4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- 25.Tsuda M, et al. IFN-γ receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci USA. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuda M, et al. Fibronectin/integrin system is involved in P2X4 receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 27.Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M, Toyomitsu E, Kometani M, Tozaki-Saitoh H, Inoue K. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. J Cell Mol Med. 2009;13:3251–3259. doi: 10.1111/j.1582-4934.2009.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda M, et al. Lyn tyrosine kinase is required for P2X4 receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 30.Yuan H, et al. Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res. 2010;88:1348–1354. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- 31.Coull JAM, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 32.Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain. Mol Pain. 2006;2:32. doi: 10.1186/1744-8069-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 34.Coull JAM, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 35.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- 37.Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foley JC, McIver SR, Haydon PG. Gliotransmission modulates baseline mechanical nociception. Mol Pain. 2011;7:93. doi: 10.1186/1744-8069-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993;41:125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 40.Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tozaki-Saitoh H, et al. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 43.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 44.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landry RP, Jacobs VL, Romero-Sandoval EA, Deleo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340–350. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Hutchinson MR, Johnson KW. Commentary on Landry et al : “Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages”. Exp Neurol. 2012;234:351–353. doi: 10.1016/j.expneurol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Sorge RE, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135:404–417. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suleman S, Beggs S, Mogil JS, Salter MW. Genetic correlation of microglial activation-associated proteins and pain-related behaviours across 12 inbred mouse strains. Proc. 12th World Congress on Pain; IASP Press; 2008. [Google Scholar]
- 50.Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148:302–308. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]